 |
 |
- Search
| Neurospine > Volume 18(4); 2021 > Article |
|
|
Abstract
Objective
To summarize the vertebral artery (VA) pattern of 96 “sandwich” atlantoaxial dislocation (AAD) patients and to describe the strategies of reducing the injury of VA during surgery.
Methods
From 2009 to 2020, we retrospectively reviewed the 3-dimensional computed tomography angiography data of 96 AAD patients combined with atlas occipitalization and C2–3 fusion, which were diagnosed as “sandwich” AAD and 96 patients as control group patients who were without atlas occipitalization, C2–3 fusion and any other cervical bone deformity at our institution. The variations of each side of VA were described in 3 different parts (C0–1, C1–2, and C2–3) according to the characteristics of the 3-part pathological structures in “sandwich” subgroup.
Results
One hundred ninety-two sides of VAs in every group of patients were analyzed and every VA was described separately at 3 different level regions. There were different variations in these 3 different regions: 4 variations in the upper fusion region, 5 variations in the sandwiched region, and 6 variations in the lower fusion region in sandwich AAD patients. And the rate of VA deformity in sandwich AAD patients was much higher and more types of VA variations existed.
Conclusion
In “sandwich” AAD patients, deformities of vertebral arteries in craniovertebral junction are more common, and the same VA may have deformities at different levels that severely affect surgical procedures. Therefore, preoperative imaging examination of VA for “sandwich” AAD patients is vital of guiding surgeons to avoid injury of VA during surgery.
Vertebral artery (VA) injury is one of the most serious complications during cervical spine surgeries, which may result in problems such as cerebral vascular disturbance, neurological deficit, and even death [1-7]. When atlantoaxial dislocation (AAD) is combined with atlas occipitalization and C2–3 fusion, we consider it as a special subgroup of AAD cases with prominent clinical features including younger onset age, increased probability of concomitant Chiari deformity, syringomyelia and cranial neuropathy, and a lower rate of myelopathy improvement compared with typical AAD patients, which have been reported as “sandwich” AAD [8]. The 3 deformities included in “sandwich” AAD were classified into 3 parts: the upper fusion region (C1 occipitalization), the sandwiched region (AAD), and the lower fusion region (C2–3 fusion) (Fig. 1). Neurologic deterioration in such patients commonly presents in the third or fourth decade of life, which requires surgical treatment. However, such osseous deformities as atlas occipitalization and C2–3 fusion are often accompanied by severe VA variations. To understand VA malformations are particularly important in the treatment of sandwich AAD patients.
A normal VA is divided into 4 segments: V1 segment (It originates from the subclavical artery and enters the C6 transverse process), V2 segment (walks through C6–2 transverse process), V3 segment (the segment courses from C2 to the foramen magnum), and V4 segment is coursing from entering the cranium to attending the basilar artery [9]. Wang et al. [10] has previously reported anatomical V3 segment variations of VA in patients with atlas occipitalization. The bone deformity of “sandwich” AAD patients was more serious than mere atlas occipitalization, and the combined VA deformities may be more complex, which have not been reported yet. In this current group, due to the existence of 3 deformities ranging from occipital bone to C3, we would like to focus on summarizing VA variations of segment from C3 level to the foramen magnum, to help the planning for surgical strategies.
Here, we summarized the VA pattern of 96 sandwich AAD patients to describe their variations and to set up strategies of how to reduce the injury of VA during surgery.
This study was a retrospective study and no formal ethics approval was required as ruled by the ethics committee of Peking University Third Hospital. From 2009 to 2020, we retrospectively reviewed 3-dimensional computed tomography angiography (3D-CTA) data of 96 AAD patients combined with atlas occipitalization and C2–3 fusion at our institution, which were diagnosed as “sandwich” AAD. Based on the dynamic radiographs (flexion and extension) and computed tomography (CT) scan evidence, the diagnosis of AAD was defined as an abnormal local relationship between the atlas and axis, with an atlantodental interval > 3 mm in adults and > 5 mm in children (< 18 years of age). Patients were excluded if adequate radiographs were unavailable or they did not undergo surgical treatment. All of the patients’ 3D-CTA data were evaluated to identify the VA variations from C3 level to the foramen magnum before surgery. The average age of the patients was 39.6 years (range, 5–67 years), with 45 males and 51 females. Nonionic contrast material was intravenously administered to all patients via the antecubital vein. Then 3D-CTA were performed on all 96 cases, using a 64-slice scanner (lightspeed VCT, GE Healthcare Systems, Chicago, IL, USA) with the scanning parameters: 120 kV, 500 mA, 0.6 seconds/rotation, table speed with 0.516 mm/rotation and 0.625 mm slice thickness. Three-dimensional images were obtained and analyzed by 2 spine surgeons.
According to the characteristics of the 3-part pathological structures in “sandwich” subgroup, we described the variations of each VA in 3 different parts. In the upper fusion region, the classification of VA variation was based on the position of VA entering the cranial or spinal canal and the relationship with atlas; in the sandwiched region, the VA variations were described according to the morphology of VA in this region; in the lower fusion region—the VA variations were classified for the shape of VA in the transverse foramen of C2 and C3 and its relationship with adjacent bone. Besides, the diameter of bilateral VA in each patient was measured at C2 level, then a dominant VA was defined if it was at least 30% larger than the other side.
Then, we also retrospectively reviewed 3D-CTA data of 96 patients as control group patients who were without atlas occipitalization, C2–3 fusion, and any other cervical bone deformity at our institution at the same time (from 2009 to 2020). The average age of the patients was 42.3 years (range, 11–69 years), with 47 males and 49 females. And there was no statistical difference in the age and sex distribution between the 2 patient groups using chi-square test (p>0.05). Their 3D-CTA data were evaluated to identify the VA variations following the 3-part classification method. Chi-square test was also performed for statistical analyses using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA) to compare the difference of the rate of VA deformity between these 2 different groups of patients.
In our study, 192 sides of VAs were analyzed. The VA variations of sandwich AAD patients in 3 regions were summarized separately and presented in Table 1.
Type I: The VA passes through the transverse foramen of the C1 and enters an extraosseous canal created in the assimilated atlas before reaching the cranium (101 sides, 52.6%) (Fig. 2A, a); Type II: The VA enters the spinal canal directly under the posterior arch of atlas without passing through the transverse foramen of C1 (69 sides, 35.9%) (Fig. 2B, b; red arrow); Type III: The VA passes through the transverse foramen of the C1 and is exposed partly when passing through the VA sulcus due to incomplete occipitalization of the C1 posterior arch (17 sides, 8.9%) (Fig. 2C, c); Type IV: Absent VA (5 sides, 2.6%) (Fig. 2B; yellow arrow).
Type I: The VA goes through the C2 transverse foramen and ascends directly into the C1 transverse foramen without tortuosity (113 sides, 58.9%) (Fig. 3A, a); Type II: The VA courses above the axis facet or makes a curve below the atlas lateral mass and then turns directly medially towards the spinal canal after leaving the C2 transverse foramen without going through the C1 transverse foramen (first intersegmental artery [FIA]) (66 sides, 34.4%) (Fig. 3B, b); Type III: The VA is duplicated after emerging from the C2 transverse foramen; one branch passes through the C1 transverse foramen; the other branch enters the spinal canal between C1 and C2 before joining the former (fenestration of the VA above and below C1 [FEN]) (6 sides, 3.1%) (Fig. 3C, c). One special type of them is that the 2 branches converge under the C1 posterior arch and then goes in spinal canal (Fig. 3D, d); Type IV: Absent VA (5 sides, 2.6%); Type V: The posterior inferior cerebellar artery (PICA) originates from the level C1/2 (2 sides, 1.0%) (Fig. 3E, e).
Type I: The VA travels normally in the C2 and C3 transverse foramen (138 sides, 71.9%) (Fig. 4A, a); Type II: Tortuosity or medial loop formation can be seen in the C2 or C3 transverse foramen (loop) (21 sides, 10.9%) (Fig. 4B, b); Type III: The VA is anomalously located too medially, too posteriorly, and/or too high with a C2 isthmus height of ≤ 5 mm (HRVA) (20 sides, 10.4%) (Fig. 4C, c); Type IV: Loop and HRVA are concurrent at the same time (7, 3.6%) (Fig. 4D, d); Type V: Absent VA (5 sides, 2.6%); Type VI: The VA emerges from the C3 transverse foramen and ascends directly without passing through C2 transverse foramen (1, 0.5%) (Fig. 4E, e).
Forty-six cases of 96 patients had unilateral-side VA dominance, accounting for 48.0%. Twenty-three cases were left-side VA dominance (including 1 right-side absence), while 23 cases were right-side VA dominance (including 4 left-side absence).
Following the 3-part classification method, 4 sides of VA were abnormal (3 sides of type II, 1 absent side of type IV) in U-region, 8 sides were abnormal (3 sides of type II, 3 sides of type III, 1 side of type IV and 1 side of type V) in S-region and 6 sides (3 sides of type II, 2 sides of type III and 1 side of type V) were abnormal in L-region in control group patients. The specific rate of VA deformity between sandwich AAD patients and control group patients was shown in Table 2. The rate of VA deformity in sandwich AAD patients was much higher than that in control group patients (p<0.001). Besides, 48 cases of 96 patients had unilateral-side VA dominance (24 cases of left-side dominance, 24 cases of right-side dominance) in control group patients (50.0% vs 48.0%, p=0.77, no statistically significant difference).
VA variations in different patients series have been reported. Wakao et al. [11] reported 387 cases of VA variations at C1–2 level without osseous deformities in craniovertebral junction in 2014, including high-riding VA (HRVA, 10.1%), persistent FIA (1.8%), fenestration of the VA above and below C1 (FEN 1.3%), PICA from C1/2 (PICA, 1.3%) and the ponticulus posticus (6.2%). Wang et al. [10] have reported the variations of V3 segment of VA in 36 patients with atlas occipitalization in 2009, which was divided into 4 categories: Type I (8.3%), the VA enters the spinal canal below the C1 posterior arch, and courses below the occipitalized C1 lateral mass. Type II (25%), the VA enters the spinal canal below the C1 posterior arch, and the course of VA is on the posterior surface of the occipitalized C1 lateral mass, or makes a curve on it. Type III (61.1%), VA ascends laterally after leaving the C2 transverse foramen, enters an osseous foramen created between the atlas and occiput, then reaches the cranium. Type IV (5.6%), the VA is absent. Here, we regarded types I and II in the article of Wang et al. [10] as different subtypes of FIA as reported by Wakao et al. [11], but their proportion is much higher than that of Wakao’s in normal people, and the proportion of type III of entering an osseous foramen in Wang’s article was also high. This result might mean that different bone deformities may lead to an increased incidence of associated VA deformities. From the 2 different group of patients in our research, the rate of VA deformity in sandwich AAD patients was much higher and more types of VA variations existed.
In our case series, due to the existence of the upper and lower fusion regions in sandwich AAD patients, we first proposed a 3-part formula to describe the variations of VA in more detail. In this article, we further divided the VA segment at craniovertebral junction of sandwich AAD patients into 3 regions—U, S, and L regions. Since there were different surgical operations in different regions, this division of the VA was conductive to the operators to make detailed preoperative planning.
As can be seen from our results, due to the atlas occipitalization in the U-region, the highest incidence of deformity was the type I of VA entering an osseous foramen, with proportion as high as 52.6%; Type III of coursing an incomplete osseous canal has not been reported before, and their occurrence was due to incomplete ossification of C1 posterior arch. So, the electronic exposure needs to be careful with this type during the posterior procedure, to protect the partial lie-open VA.
The highest occurrence of VA malformation in the S-region was type II of VA making a curve and turning directly medially towards the spinal canal below the C1 posterior arch (34.4%), and it was mostly tortuous on the surface of C1-2 lateral mass joint. As for duplicated type III, 5 of the 6 cases was that one branch ascended under the posterior arch of C1 and merged with another branch passing through the transverse foramen of C1, while 1 case was unique that both branches merged and entered the spinal canal directly under the posterior arch of C1 without passing through the C1 transverse foramen (Fig. 3D, d). This unique duplicated variation has not been reported before.
In the L-region, high-riding VA and VA loop were high incidence, accounting for 10.4% and 10.9% respectively, and these 2 deformities both occurred simultaneously in 7 cases, which was vital to guide our selection of surgical methods. To our knowledge, VA Loop has been rarely reported in the upper cervical spine previously. Ekşi et al. [12] have mentioned in his article that VA loops were more commonly observed at V2 segment (90.5 %), while they were less common at V1 (7.6%) and V3 (1.9%) segments in the literature. And in his report, the occurrence segments of Loop were C4–5 (31.4%), C3–4 (20%), and C5–6 (18.1%), respectively.
As for the surgical treatment, VA malformations in different parts also lead to different risks.
In the U-region, the risk of injury to the VA during the posterior C1 lateral mass screw (C1LMS) was highlighted. Hong et al. [13] has suggested that C1 lateral mass screw insertion can be dangerous in cases of a persistent FIA where the VA courses abnormally below the C1 posterior arch. But Wang et al. [10] proposed that not all FIAs were at risk during C1LMS placement, and the high risk of VA injury was only for those FIAs that were tortuous on the surface of the C1 lateral mass, and the FIAs that went under the C1 lateral mass were relatively safe during placement of the C1LMS. Li et al. [14] reported 120 cases of VA malformations in patients with BI and atlas occipitalization in 2018. In his classification, 2 types of variations that would increase the risk of VA injury with C1LMS were type of entering an osseous foramen and FEN, and he proposed to reduce the risk of VA injury by changing the entry point and direction of C1MS. In our classification of the upper fusion region, we believed that type I of passing through an osseous foramen and type III of being exposed partly due to incomplete occipitalization of the C1 posterior arch had an increased risk of injury during C1LMS placement. In type I, it was important to evaluate the shape of VA in the osseous foramen and the location of the osseous foramen preoperatively. In type III, due to the incomplete ossification of the posterior arch of the atlas, there is no bone protection on part of the surface of the VA, so special care should be taken during the exposure of the posterior arch of C1. For type II of VA entering the spinal canal directly under the posterior arch of atlas, in our cases, since they did not perforate the C1 transverse foramen, most of their course was tortuous on the joint surface of the lateral mass joint of C12, so the risk of injury with C1LMS was relatively small.
In the S-region, VA injury is mainly caused during the operation of the lateral mass joint. Li et al. [14] have mentioned that VA could be much more easily injured during the surgical manipulations around the facets, such as the placement of spacers or Titanium Cages between the C1–2 facets. In our classification, we have similar opinions. Type II of VA in this region is tortuous on the surface of the C1–2 lateral mass facets, so the risk of VA injury is greatly increased when handling the lateral mass joint. In addition, type III and type V of this region have the same risk in the lateral mass joint operation, especially type V with C1–2 level PICA which may lead to serious consequences such as postoperative cerebellar infarction after injury.
In the L-region, we should focus on the presence of high-riding VA and VA loop during C2 pedicle screw (C2PS) insertion. The risk of injury to VA caused by C2PS insertion due to high-riding VA has been reported [15], but the risk of VA loop in upper cervical spine during surgery have not been reported before. Park et al. [16] reported the incidence of VA loop as 0.6% in patients with vertebral bone erosion or widened transverse foramen. Then, the enlargement of the transverse foramen leads to the hypoplasia pedicle, which is too thin to accommodate C2PS. Moreover, when the VA loop curves inwards the spinal canal, it results in a significantly increased risk of injury during posterior surgical procedures. Therefore, either of these 2 VA deformities increases the risk of injury to the VA during surgery at the lower fusion region, especially for the VA with these 2 deformities together. Besides, in this region, we found a case where the VA went from the C3 transverse foramen to the C1 transverse foramen without passing through the C2 transverse foramen, which might increase the risk of VA injury during the operation of the exposure of the C2 pedicle.
For the dominance of VA, this type of malformation was through 3 regions. The diameters of the VA are of equal size in only 6%–26% of patients in angiographic reported in post studies, and the left VA is often larger than the right VA, this is called the VA dominance (VAD) because of asymmetric VA [17,18]. Individual with VAD usually do not have symptoms of Vertebrobasilar insufficiency [19]. Smith and Bellon [20] have reported that the diameter of the dominant VA should be at least 30% larger than the other side. We followed this standard and considered the unilateral absence type as the contralateral dominant type in our cases. And we found that there was no statistically significant difference in the rate of unilateral-side VAD between the 2 group patients. But there might be a relationship between the side of VA variation and the VAD. We chose a subgroup including 31 patients with VAD and unilateral VA variation from the sandwich AAD patients to analyze if there was a relationship between the side of VA variation and VAD using chi-square test. The statistical results showed that the rate of variation in the dominant side of VA was significantly higher than in the recessive side (p<0.05) (Table 3). Although VA variation may occur in the case without VAD and there were bilateral VA variations or bilateral regular VA in the case with VAD, we still found that the dominant side of VA has a higher rate of VA variation, which reminded us to pay high attention to the dominant VA during surgery. Besides, the VAD was same at the risk of injury as unilateral absence type VA during the surgery. Then we have to evaluate the dominant VA carefully preoperatively to avoid the occurrence of disastrous results.
In sandwich AAD patients, deformities of vertebral arteries in the upper cervical spine region are more common, and the same VA may have deformities at different levels that severely affect surgical procedures. Therefore, for these patients, preoperative imaging examination of VA is very important, which is essential to guide us to avoid injury of VA during surgery.
Fig. 1.
Illustration of the 3 parts of vertebral artery (VA) in the “sandwich” atlantoaxial dislocation.
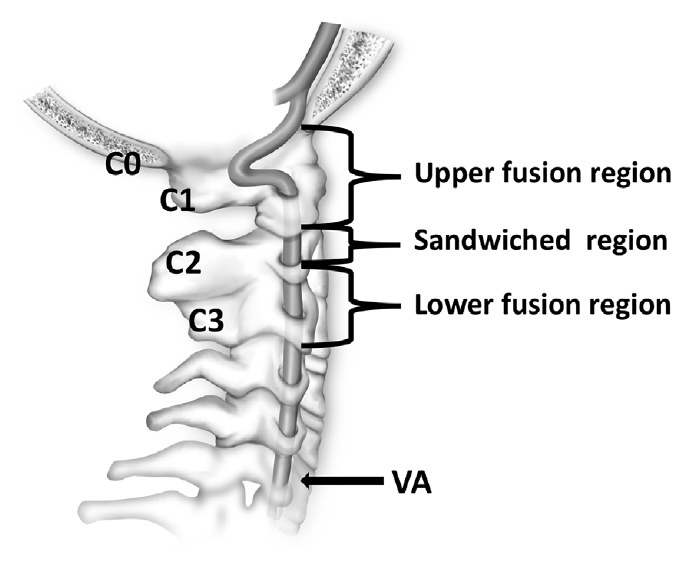
Fig. 2.
Vertebral artery (VA) variations in U-region. (A, a) Type I of passing through an extrasseous canal created by the assimilated atlas (red arrow). (B, b) The red arrow shows the type II of entering the spinal canal directly under the C1 posterior arch; The yellow arrow shows the absent VA. (C, c) Type III of exposed partly by the incomplete occipitalization of the C1 posterior arch (red arrow).
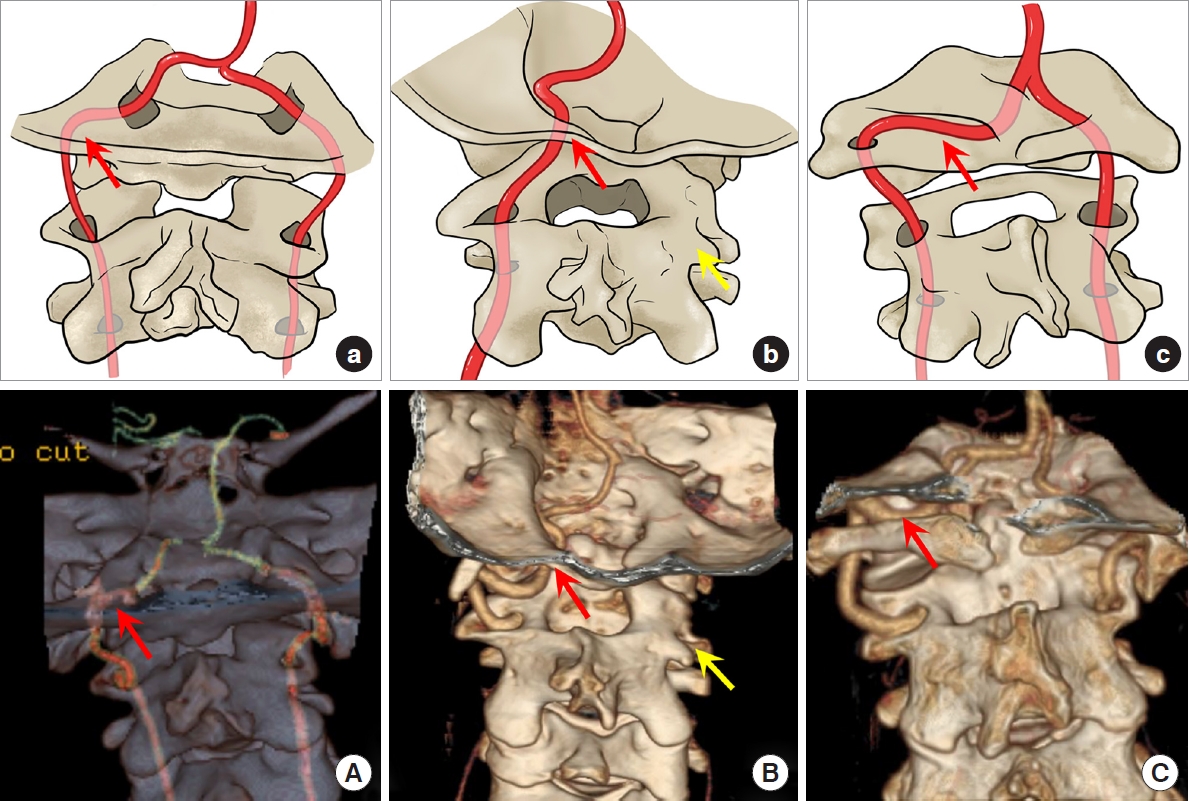
Fig. 3.
Vertebral artery (VA) variations in S-region. (A, a) Type I in this region of going through the C2 and C1 transverse foramen without tortuosity. (B, b) Type II of coursing above the axis facet or making a curve below the atlas lateral mass illustrated by the red arrow. (C, c) Type III of duplicated VA. (D, d) A special subtype of type III of the 2 branches converge under the C1 posterior arch and then goes in spinal canal. (E, e) Type V of the posterior inferior cerebellar artery originating from the level C1/2. All the variations were marked by the red arrow.
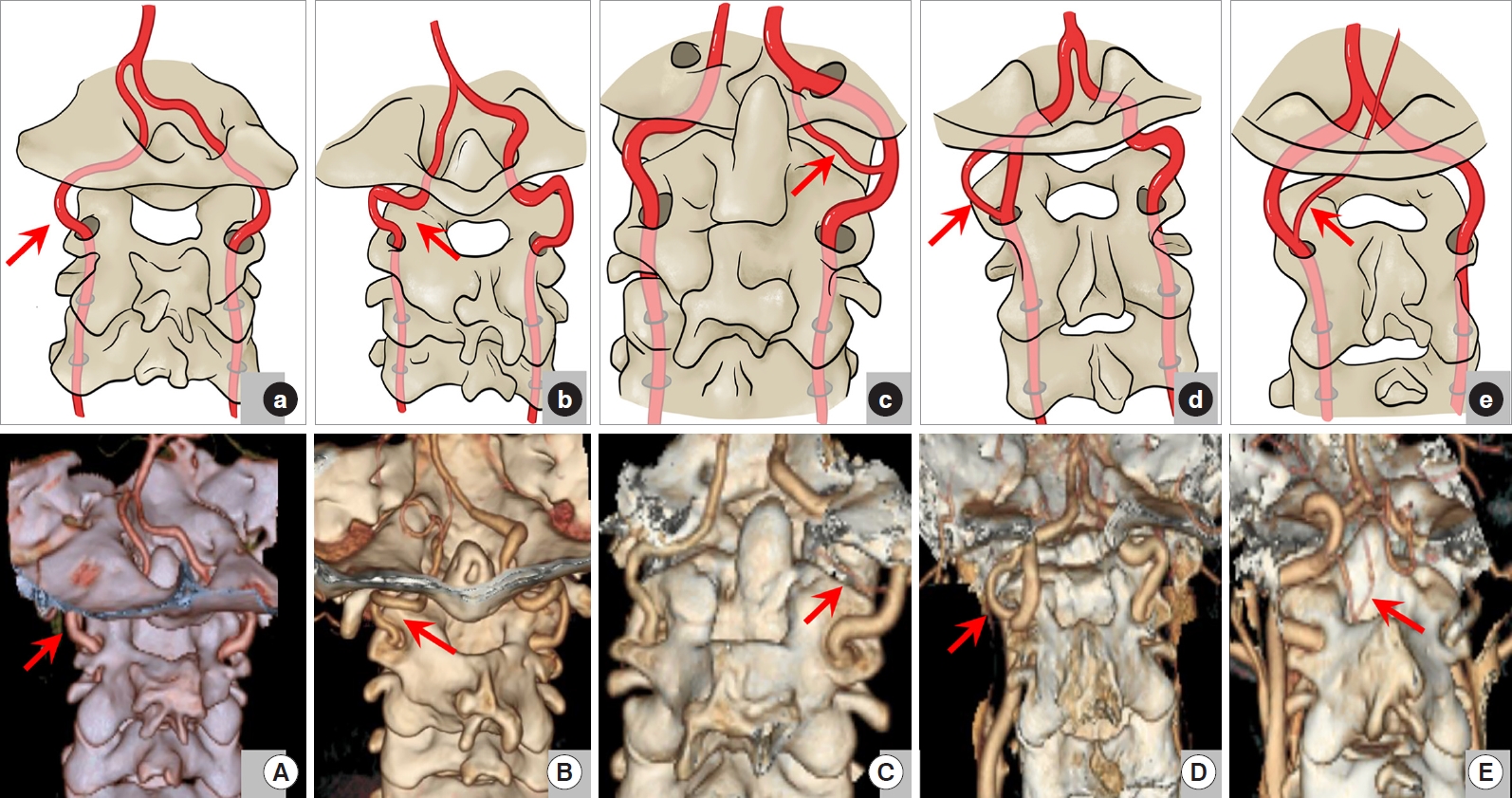
Fig. 4.
Vertebral artery (VA) variations in L-region. (A, a) Type I in this region of traveling normally in the C2/3 transverse foramen. (B, b) Type II of tortuosity or medial loop formation in the C2 or C3 transverse foramen. (C, c) Type III of high-riding VA (HRVA). (D, d) Type IV of Loop and HRVA existing at the same time. (E, e) Type VI of emerging from the C3 transverse foramen and ascending directly without passing through C2 transverse foramen. All the variations were marked by the red arrow.
Table 1.
The VA variations of sandwich AAD patients in 3 regions
REFERENCES
1. Burke JP, Gerszten PC, Welch WC. Iatrogenic vertebral artery injury during anterior cervical spine surgery. Spine J 2005 5:508-14.


2. Garcia Alzamora M, Rosahl SK, Lehmberg J, et al. Life-threatening bleeding from a vertebral artery pseudoaneurysm after anterior cervical spine approach: endovascular repair by a triple stent-in-stent method. Case report. Neuroradiology 2005 47:282-6.


3. Smith MD, Emery SE, Dudley A, et al. Vertebral artery injury during anterior decompression of the cervical spine. A retrospective review of ten patients. J Bone Joint Surg Br 1993 75:410-5.


4. Tumialan LM, Wippold FJ 2nd, Morgan RA. Tortuous vertebral artery injury complicating anterior cervical spinal fusion in a symptomatic rheumatoid cervical spine. Spine (Phila Pa 1976) 2004 29:E343-8.


5. Wang S, Ren WJ, Zheng L, et al. Anatomical variations of the vertebral artery: analysis by three-dimensional computed tomography angiography in Chinese POPULATion. Orthop Surg 2021 13:1556-62.



6. Siedlecki Z, Szostak M, Nowak K, et al. Atypical course of vertebral artery outside the cervical spine: case report and review of the literature. World Neurosurg 2021 145:405-8.


7. Ramamurti P, Weinreb J, Fassihi SC, et al. Vertebral artery injury in the cervical spine: anatomy, diagnosis, and management. JBJS Rev 2021 9:e20.00118.

8. Tian Y, Xu N, Yan M, et al. Atlantoaxial dislocation with congenital "sandwich fusion" in the craniovertebral junction: a retrospective case series of 70 patients. BMC Musculoskelet Disord 2020 21:821.



9. Magklara EP, Pantelia ET, Solia E, et al. Vertebral artery variations revised: origin, course, branches and embryonic development. Folia Morphol (Warsz) 2021 80:1-12.


10. Wang S, Wang C, Liu Y, et al. Anomalous vertebral artery in craniovertebral junction with occipitalization of the atlas. Spine (Phila Pa 1976) 2009 34:2838-42.


11. Wakao N, Takeuchi M, Nishimura M, et al. Vertebral artery variations and osseous anomaly at the C1-2 level diagnosed by 3D CT angiography in normal subjects. Neuroradiology 2014 56:843-9.


12. Ekşi MŞ, Toktaş ZO, Yılmaz B, et al. Vertebral artery loops in surgical perspective. Eur Spine J 2016 25:4171-80.


13. Hong JT, Lee SW, Son BC, et al. Analysis of anatomical variations of bone and vascular structures around the posterior atlantal arch using three-dimensional computed tomography angiography. J Neurosurg Spine 2008 8:230-6.


14. Li T, Yin YH, Qiao GY, et al. Three-dimensional evaluation and classification of the anatomy variations of vertebral artery at the craniovertebral junction in 120 patients of basilar invagination and atlas occipitalization. Oper Neurosurg (Hagerstown) 2019 17:594-602.


15. Roosen K, Trauschel A, Grote W. Posterior atlanto-axial fusion: a new compression clamp for laminar osteosynthesis. Arch Orthop Trauma Surg 1982 100:27-31.


16. Park SB, Yang HJ, Lee SH. Medial loop of v2 segment of vertebral artery causing compression of proximal cervical root. J Korean Neurosurg Soc 2012 52:513-6.



17. Jeng JS, Yip PK. Evaluation of vertebral artery hypoplasia and asymmetry by color-coded duplex ultrasonography. Ultrasound Med Biol 2004 30:605-9.


18. Zhu W, Wang YF, Dong XF, et al. Study on the correlation of vertebral artery dominance, basilar artery curvature and posterior circulation infarction. Acta Neurol Belg 2016 116:287-93.






























