INTRODUCTION
Lenke type 1 and 2 curves in adolescent idiopathic scoliosis (AIS) consist of structural major thoracic curves and nonstructural minor thoracolumbar curves. They are the most common types of AIS curves accounting for 51% and 20% in prevalence, respectively [
1]. Thoracic fusion is a common strategy used for surgical treatment of Lenke 1 and 2 curves with the goals of achieving surgical correction of thoracic curves while preserving as many lumbar motion segments as possible [
2-
4]. The proper selection of the lower instrumented vertebra (LIV) remains a challenging problem for spine surgeons. There is currently no universally accepted method of choosing the LIV, but various studies have demonstrated that fusions stopped too short often lead to suboptimal deformity correction along with the distal ŌĆ£adding-onŌĆØ (AO) phenomenon. Distal AO is the progressive loss of correction distal to the instrumentation after thoracic fusion for AIS. It is defined as an increase in the Cobb angle of > 5┬░ and extension of the end vertebra (EV) to a more distal segment, or a change in the angulation of the disc below the LIV of at least 5┬░ or more on standing radiographs during the first 2 years postoperative [
5,
6]. Distal AO can often cause pain due to deformity progression and may require additional surgery adding the associated morbidity and healthcare costs of distal fusion extension [
7].
To minimize the risk of AO, several authors had reported various methods to determine the LIV during AIS correction. In 2003, Suk et al. [
8] reported using the preoperative neutral vertebra (NV) and EV as landmarks for LIV selection. The authors proposed that if the NV was at the same level or one level distal to the EV, the NV should be chosen as the LIV; if the NV was 2 or more levels distal to the EV, the NV-1 segment should be chosen as the LIV. This method usually saves one or 2 motion segments compared with using the stable vertebra (SV) as the LIV [
8]. Subsequently, Cao et al. [
9] and Matsumoto et al. [
10] proposed using the last touched vertebra (LTV), defined as the last cephalad vertebra touched by the central sacral vertical line (CSVL), as the LIV with good clinical results. Cho et al. [
5] also proposed the concept of the substantially touched vertebra (STV), defined as the most proximal vertebra where the CSVL either intersected the pedicle outline or was medial to the pedicle outline. In addition, Qin et al. [
11] further divided the LTV into the STV and nonsubstantially touched vertebra (nSTV, the most proximal vertebra touched by the CSVL lateral to the pedicle) and suggested selecting the STV or nSTV+1 as the LIV could yield a promising outcome. No consensus to date can be drawn regarding the best LIV landmark and a relatively small number of patients were included in these studies.
The aim of our study was to examine the pooled data from the existing literature regarding LIV selection in Lenke type 1A and 2A curves to determine the odds ratios (ORs) and the absolute risks of developing AO for each of the LIV selection methods.
MATERIALS AND METHODS
1. Search Strategy and Study Selection
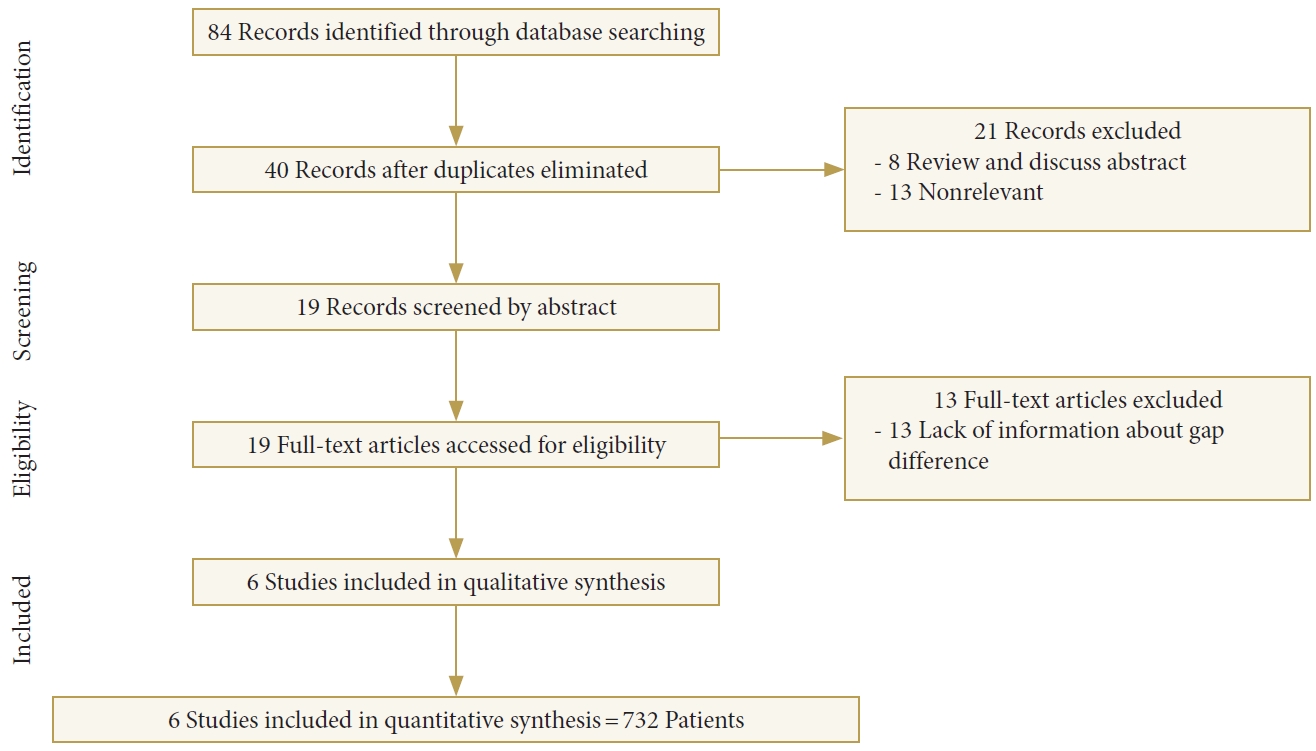
Using electronic databases including PubMed, MEDLINE, Embase, and the Cochrane Library Databases (including The Cochrane Database of Systematic Reviews, the Database of abstracts of Reviews of effects, The Cochrane Controlled Trials Register, and The Health Technology Assessment Databases) dating from inception to September 30, 2016, studies reporting on LIV selection and its effect on the risk of ŌĆ£adding-onŌĆØ in Lenke type 1A and 2A curves were identified using the following search parameters: ŌĆ£Adding-onŌĆØ AND ŌĆ£Lenke.ŌĆØ All articles included in the present study were clinical human studies and written in English.
We focused the literature search primarily on randomized controlled trials (RCTs). However, in the absence of any existing RCTs, we included non-RCTs that had Ōēź 30 patients with Lenke type 1A or 2A curves who underwent a thoracic spinal fusion. We excluded studies that could not provide information about the outcome differences in the AO and non-AO groups. Review articles, letters, and case reports were excluded.
2. Outcome Measures
The primary focus of our study was to determine the OR of using each LIV selection method; a secondary focus was on the difference in the absolute risk of AO for each method. Slightly different definitions of ŌĆ£adding-onŌĆØ were used in the existing literature. The first definition was provided by Wang et al. [
6], as a distal extension of the primary curve combined with either an increase > 5 mm in deviation from the CSVL of the first vertebra below the instrumentation, or an increase > 5┬░ in angulation of the first disc below the instrumentation at 1-year follow-up. Another definition by Cho et al. [
5] defined AO as an increase in the Cobb angle of Ōēź 5┬░ along with distal extension of the EV or a change of Ōēź 5┬░ in disc angulation below the LIV from the first erect to the 2-year follow-up radiographs. We accepted both definitions of ŌĆ£adding-onŌĆØ in our study.ŌĆÖ
3. Data Extraction
Data were searched and extracted by 2 authors independently. Any disagreement was resolved by discussion until a consensus was reached. Data on the following measures were extracted and recorded: study design, inclusion criteria, sample size, surgical approaches, and authorsŌĆÖ conclusion (
Table 1). Information regarding Cobb angle and gap differences related to LIV selection method were also extracted and recorded (
Table 2).
We defined the gap difference as the actual LIV used minus the location of the selected landmark for LIV selection, where a positive value corresponded to a LIV distal to the landmark, and a negative value corresponded to a LIV proximal to the landmark. The landmarks used in various methods of LIV selection such as SV-1, NV, EV+1, LTV, STV/(nSTV+1) were selected based on the results of our systematic review of the current literature [
5,
9-
13]. These boundaries were set to maximize the number of patients that could be included in our study.
4. Data Analysis
For all included studies, we performed a pooled estimate of AO rates for thoracic-only fusion surgery according to the abovementioned landmarks. We calculated the ORs of AO when the chosen LIV was proximal to, at, or distal to the landmark. The results were expressed as the OR with a 95% confidence interval (CI). Homogeneity testing was performed using the I2 test. A fixed-effects model with inverse variance methods was used if there was lack of heterogeneity. If I2 was > 60%, a random-effects model was used. When the number of cases of AO was 0, we added 0.5 to each cell for calculation purposes. All calculations were performed using MetaXL (ver. 5.3, Epigear International Pty Ltd., Sunrise Beach, Queensland, Australia).
DISCUSSION
The ultimate goal for AIS correction is to achieve a globally balanced spine in both the coronal and sagittal planes while minimizing surgical comorbidities. The surgery should correct/halt deformity progression while maintaining as many motion segments as possible while providing a long lasting and stable alignment. Despite the importance of LIV selection, there is no universally accepted method for determining the LIV for AIS correction. In 1983, King et al. [
16] retrospectively reviewed 405 patients who underwent AIS correction and recommended extending the fusion down to ŌĆ£the vertebra that is neutral and stable.ŌĆØ However, this recommendation was based on the Harrington rod construct that was widely used at the time.
In 2003, Suk et al. [
8] conducted a retrospective review of 42 AIS patients with a main thoracic curve who underwent scoliosis correction. The authors found that when the NV is at the same level or one level distal to the EV, fusion extending to the NV provided satisfactory deformity correction with minimal risk of AO. When the NV is 2 or more levels distal to the EV, fusion extending to NV-1 provided adequate results. However, when the fusion was too short, i.e., fusion extending to NV-2 or shorter, a high percentage of patients (73.7%) had unsatisfactory results postoperatively and had an increased risk of developing AO. Unfortunately, both NV and EV have poor interobserver reliability [
17], therefore determining the LIV based on the NV and EV can be inconsistent amongst different surgeons thus producing variable results.
In 2011, Wang et al. [
13] reviewed a series of 45 patients with Lenke type 1A curves who underwent surgical correction and found 51.1% with AO during a minimum of 1-year follow-up. They found that the incidence of AO increased significantly when the preoperative LIV+1 deviation from the CSVL was more than 10 mm, thus they recommended choosing the first vertebra cranially that deviates from the CSVL > 10 mm as the LIV. However, this method is more cumbersome and has not been validated in a larger group of patients.
To overcome the inconsistencies of using SV, NV, and EV as methods of LIV selection, several authors [
10,
13,
14] have demonstrated that using the LTV and STV is a more reliable method of LIV selection for thoracic fusions.
In 2013, Matsumoto et al. [
15] studied 112 patients who had undergone posterior thoracic fusion surgery for Lenke type 1A curves where 18.8% of patients had AO at > 2-year follow-up. The authors found that patients with fusions ending proximal to the LTV were at a much higher risk for AO after surgery (OR, 6.7; 95% CI, 1.9ŌĆō23.9; p = 0.003). Thus, the authors suggested choosing the LIV at or distal to the LTV for Lenke type 1A curves.
In 2014, Cao et al. [
14] studied 116 patients with Lenke type 2A curves and found 14% of the patients had AO at 2-year follow-up based on their criteria. Patients with a LIV proximal to the LTV had a much higher incidence of AO in their study. Thus, these authors concluded that the LIV should be at the LTV or LTV+1.
In 2016, Qin et al. [
11] studied a series of 104 patients with Lenke type 1A curves who underwent posterior thoracic fusion with a minimum of 2-year follow-up. They defined the STV as the vertebra where the CSVL was between the pedicles or touching the pedicle, and the nSTV as the vertebra where the CSVL was only touching the corner of the vertebra lateral to the pedicle. They found that the nSTV group was at a significantly higher risk than the nSTV+1 or the STV groups (66.7% vs. 11.6% vs. 10.0%, p < 0.01). Therefore, the authors recommended choosing the STV or nSTV+1 as the LIV for patients with Lenke type 1A curves.
In our study, we summarized the occurrence and absolute risk of AO with various methods of LIV selection (
Tables 2,
3). Surprisingly, we found that using the LTV as the LIV, which is one of the commonly used landmarks for AIS correction, produced the highest absolute risk of AO (14.6%), while using the STV/nSTV+1 yielded the lowest (11.3%). It is interesting that these 2 landmarks (LTV vs. STV/nSTV+1) adapted from a similar concept are at 2 ends of the risk spectrum from the available data. Our data showed that fusing shorter than the STV/nSTV+1 also gave the statistically highest OR of AO [
4,
5] compared to using the LTV, SV-1, EV+1, and NV; this means that there was a more significant reduction in risk if the instrumentation extended at least equal or distal to the STV or nSTV+1 as compared with other landmarks. Interestingly, fusion beyond the STV/nSTV+1 did not provide further statistically significant risk reduction of AO.
Beside the coronal landmarks, other important factors such as sagittal landmarks [
17], surgical techniques and patient-specific variables can also contribute to the difference in clinical outcomes. Various risk factors have been associated with the distal AO phenomenon including young age [
13], skeletal immaturity [
12], preoperative coronal C7-CSVL distance > 2 cm [
13], right tilt of the L4 disc [
5], as well as improper selection of the LIV [
5,
6,
8-
15,
18]. In 2008, Miyanji et al. [
18] noted a distinct difference within the Lenke 1A subtype based on the direction of the L4 vertebral tilt, grouping the patients as 1A-L if L4 is tilted down on the left side and 1A-R if the right side is lower (
Fig. 3). Cho et al. [
5] suggested fusing distally to 1 level above the NV or 1 to 2 levels above the SV in 1A-R curves to prevent AO. Murphy et al. [
12] further suggested choosing the last STV (LSTV) as the LIV in Lenke 1 and 2 curve patterns with an A-R lumbar modifier which significantly decreased the risk of distal AO. In our meta-analysis, no RCTs were published in the current literature regarding LIV selection for AIS correction, however, we have included publications with the highest evidence. The landmarks in the study and the extraction of associated information were limited by the published data.
The definition of distal AO varied in the existing literature. Cho et al. [
5] defined distal AO as an increase in the Cobb angle of at least 5┬░ and distalization of the EV of the thoracic curve or a change in disc angulation of 5┬░ or greater below the lowest instrumented vertebra from the first erect to 2-year postoperative radiographs. Wang et al. [
6] defined distal AO as a progressive increase in the number of vertebrae included distally within the primary curve combined with either an increase of more than 5 mm in deviation of the first vertebra below instrumentation from the CSVL, or an increase of more than 5┬░ in the angulation of the first disc below the instrumentation. These slight discrepancies in definitions may contribute to some errors in our analysis. Another limitation of the study is fact that there will always be some interobserver errors with selecting the landmarks, thus creating some variability on the final LIV selection. However, all the landmarks have fairly objective imaging criteria, we believe the interobserver error is relatively small, and does not significantly affect the results. In addition, ideally Lenke type 1A and 2A curves should be analyzed separately given the potential influence of shoulder balance on distal AO. However, due to the limited number of studies, these were grouped together for the purpose of this study.