|
 |
- Search
| Neurospine > Volume 19(2); 2022 > Article |
|
|
Abstract
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a chronic relapsing disease of unknown aetiology. The diagnosis of this disease is still very complicated. The treatment is medical but, in some cases, a surgical decompression might be required. In rare cases it develops a radicular hypertrophy that can cause a cervical myelopathy; this pathology should be put in differential diagnosis with neurofibromatosis 1 and Charcot-Marie-Tooth (CMT) syndromes. The cases of CIDP cervical myelopathy reported in the literature are rare and even more rarely a surgical decompression was described. Here we report a first and unique case of CIDP cervical myelopathy treated with an open-door laminoplasty technique with 10-year postoperative follow-up (FU). The surgical decompression revealed to be effective in stopping the progression of myelopathy without destabilizing the spine. The patient that before surgery presented a severe tetraparesis could return to walk and gain back his self-care autonomy. At 10-year FU he did not complain of neck pain and did not develop a cervical kyphosis. In case of cervical myelopathy caused by radicular hypertrophy, CIDP should be kept in mind in the differential diagnosis and an open-door laminoplasty is indicated to stop myelopathy progression.
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a chronic relapsing disease that chiefly affects the limbs. It is characterised by symmetric sensorimotor impairment with the most common clinical signs being muscular weakness of the limbs, distal sensory impairment, and decreased tendon reflexes. Slowing or blocking in nerve conduction velocities can be seen on electrophysiological examinations [1].
Although the causes of this disease are unknown, a predominant role is ascribed to immune-mediated inflammation with local cytokine production. Repeated demyelination-regeneration results in onion-bulb formation, leading to high-intensity signal in short tau inversion recovery sequences and thickening of the roots on magnetic resonance imaging (MRI) [2-4]. Some authors have advocated a correlation of CIDP with diabetes [5,6].
CIDP is one of the chief causes of hypertrophic neuropathy and should be put in differential diagnosis with neurofibromatosis 1 (NF-1) and Charcot-Marie-Tooth (CMT) diseases [3,7]. It affects the peripheral nerves, and in rare cases, radicular hypertrophy leads to medullary compression [3]. The main treatment consists of immune-modulating agents but in rare cases of severe spinal roots enlargements with medullary compression, a surgical decompression might be needed [8].
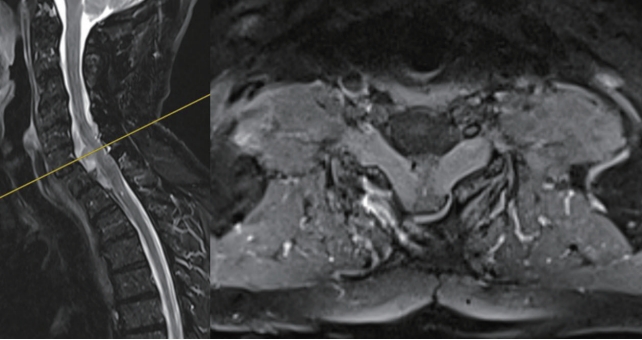
Herein we report the case of a 58-year-old male presenting CIDP complicated by cervicomedullary compression resulting from radicular hypertrophy; the patient was followed-up for 10 years with periodical MRI evaluations. The patient was first diagnosed with CIDP in 1987 and the initial clinical signs consisted of paraesthesia and weakness of the hands and feet. Clinical examination during the early phase revealed tendon hyporeflexia and superficial hypersensitivity of the lower limbs. The patient exhibited normal muscle tone, coordination, and cranial nerve status. Electromyography performed at that point showed an increase in distal motor latency, increased latency of sensory response, conduction block in the median and ulnar nerves, and increased F-wave latency. The analysis of cerebrospinal fluid (CSF) revealed albumin-cytological dissociation. No familiar history was reported. The patient was successfully treated with plasmapheresis and corticosteroids, and clinical remission was achieved in 1990. In 1991, he experienced major worsening consisting of cervical myelopathy signs such as muscular hypertonia and ataxic gait, which led to a change of the treatment to Tegeline (Human Immunoglobulins, Vidal, Paris, France) and Cellcept (Mycophenolate Mofetil, Roche, Boulogne-Billancourt, France). These 2 drugs were discontinued in 2008 following further worsening of the disease. Between November 2008 and April 2009, treatment consisting of 5 courses of cyclophosphamide proved ineffective. In May 2009, the patient presented with a tetraparesis, which continued to advance and rendered him virtually bedridden, with triple reflex response in the lower limbs and hypoesthesia extending to T10. Urinary retention of central origin resulting from spasticity of the bladder neck and the striated urinary sphincter was demonstrated. Plasmapheresis was started again as well as bolus corticosteroids, resulting in partial but transient (2 weeks) improvement. An initial stay at a physiotherapy center resulted in improved walking, autonomy in self-care, as well as improved prehension. Because of a following worsening of central nervous system signs (spinal myoclonus, spasticity of the lower limb, acute urinary retention), a cervical MRI was performed in August 2010 and revealed major hypertrophy of the nerve roots after gadolinium injection. T2 medullary hypersignal was also noted at C5-C6-C7 in the cervical spinal canal (Fig. 1). A CSF sample performed at this moment exhibited albumin-cytological dissociation with massive impairment of the blood-brain barrier. Surgical decompression was indicated in view of the mechanical medullary compression resulting from hypertrophy of nerve tissue, and the patient underwent surgery the 31st of August 2010.
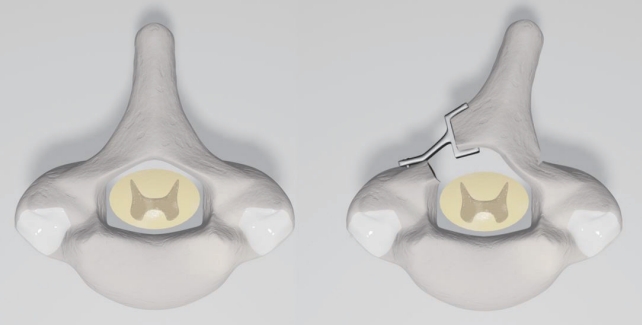
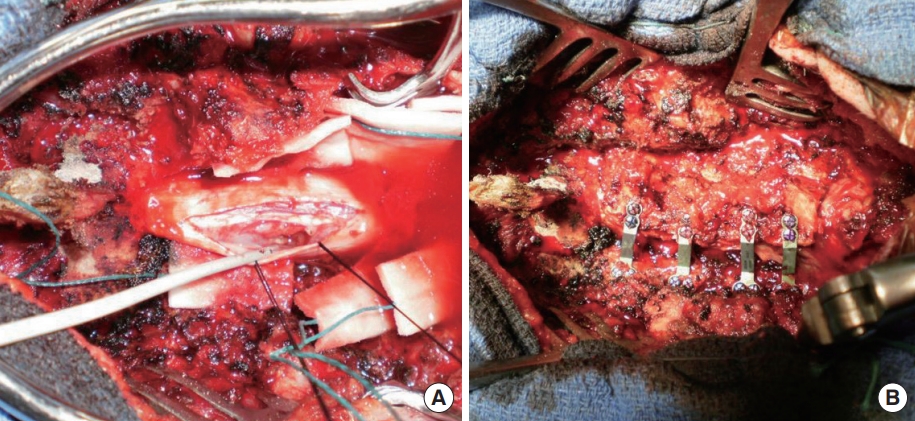
The surgical procedure consisted of open-door laminoplasty with insertion of a Centerpiece Plate Fixation System (Medtronic, Memphis, TN, USA) to achieve widening of the canal. The patient was placed in ventral decubitus position and a Mayfield pin head-holder was used to allow moderate neck flexion. The incision extended from C3 to T1 with posterior stripping to the facets. Following splitting of the spinous processes, laminoplasty was carried out with thinning of the left side of the lamina at the lamina-facet junction and an opening was made on the right side of the lamina. The spinal cord was exposed via an open-door technique (Fig. 2). The dura mater was opened, and a major compression of the spinal cord could be appreciated; at this point, a biopsy of the anterior and posterior roots was obtained at level C5 under microscopy. Analysis was performed on the fresh biopsy sample after inclusion. The dura mater was then closed after a duroplasty and the laminoplasty stabilised by osteosynthesis using a plate at each decompressed level. An autologous posterolateral graft was placed in the contralateral side [9] (Fig. 3).
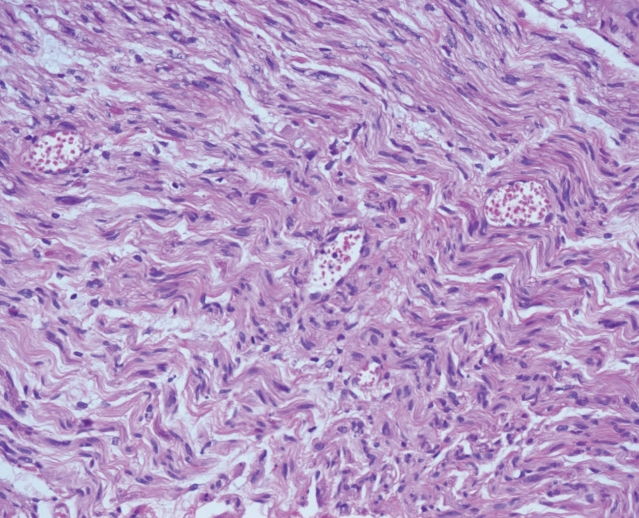
The postsurgical period was uncomplicated, with moderate analgesic intake and on postoperative day 15, the patient was transferred to a physiotherapy center. The biopsy was performed on an accessory radicular nerve to avoid major iatrogenic lesions and the result showed oedematous changes and inflammation in the perineural tissue with limited deposit of amorphous substance which has been described as a characteristic of the CIDP [3] (Fig. 4). The first postoperative clinical and radiological controls performed at 3 and 6 months after the surgery showed a progressive neurological improvement with primary recovery of the upper limbs functions followed by a progressive recuperation of the lower limbs. After 6 months, the patient was able to get out of his seat unassisted and walk a few paces using either a stick or a walking frame. The patient continued to improve and recovered autonomy with a walking frame; he was able to walk a few paces without technical assistance and was practically autonomous in self-care in a specially adapted environment (fine prehension remained deficient with distal sensory deficit).
During the last 10 years, the patients underwent periodical hospitalizations in a recovery center due to relapsing phases of neurological impairment. A therapy with Tegeline (Human Immunoglobulin, Vidal) was carried on with iv perfusions every 6 to 8 weeks. Unfortunately, the patients had a coronavirus disease 2019 infection in November 2020 that strongly affected his general performance status. During his last period at the recovery center in September 2021, he presented severe amyotrophy of his 4 limbs without signs of cervical myelopathy and was still able to walk and be autonomous in self-care daily activities. A last MRI performed at the end of 2020 showed a stability of the radicular thickening without radiological signs of cervical myelopathy (Fig. 5).
Although polyradiculoneuropathy has been described as a progressive chronic disease presenting as bilateral distal motor deficit, loss of sensitivity and hyporeflexia, the absence of clear consensual criteria for diagnosis of CIDP rendered the patient treatment more complex [10-12]. Today the diagnosis of CIDP is based on several points and a good correlation between clinical, laboratory, and radiological findings is essential [13]. The diagnosis should relay on the following elements: (1) complete blood count, electrolyte analysis, screening for underlying diseases such as human immunodeficiency virus, protein electrophoresis (to rule out other coexisting pathologies); (2) electromyogram with conduction blocks, increased motor and sensory latency, absence of F waves; (3) analysis of CSF with screening for albumin-cytological dissociation and monoclonal peak; (4) imaging is also necessary, particularly if there is central nervous system involvement: MRI remains the examination of choice [7,10,13]; (5) nerve or roots biopsy to differentiate CIDP from NF-1 (presence of peripheral nerve sheet tumour) and CMT (both pathologies have the “onion bulb” formation but only CIDP have inflammatory changes such as oedematous stroma with endoneural T-lymphocytes and macrophages) [3].
Radicular hypertrophy in CIDP is rare and was noted in 11% of patients in certain series [4,7,9,14-19], and mostly responsible for lumbar compressions [11,14-24]. The cervical localisation remains exceptional [8].
Even though various studies have reported favourable response of such hypertrophy to corticosteroids [7,17,22], our patient remained refractory to such treatment for several years. Although the cause of this hypertrophy is uncertain, the numerous inflammatory episodes appear to result in demyelination, leading to cell deposition because of regeneration, which then creates an onion-shaped mass [19,25]. Some authors have suggested a relation of CIDP with diabetes, but in our case, we cannot confirm this hypothesis since our patient isn’t diabetic [5,6].
Neural biopsies may be of diagnostic assistance [26] if they reveal deposits of amorphous and inflammatory substances. However, there is some discussion in the current literature concerning the utility of biopsy [19,25].
The currently recommended treatment for CIDP is medical [27,28], with first-line therapy consisting of immunoglobulins and intravenous corticosteroids [27,29-31]. Good results have also been achieved with plasmapheresis [27,31-33]. While monoclonal antibodies have also been beneficial to some degree, they have not been given as first-line therapy. Other forms of treatment are used more reluctantly because of the occasionally major associated adverse effects.
The patient described herein presented a very rare complication of CIDP, namely medullary compression due to radicular cervical hypertrophy. This type of compression is described in the literature, but usually not associated to CIDP cases [34-36]. To our knowledge this is the first report in the literature of opendoor technique laminoplasty used to treat radicular hypertrophy with spinal canal compression. Our long follow-up shows the good outcome of this conservative technique which didn’t destabilizes the spine [37]. Following surgery our patient could gain back his autonomy, returning to his daily activities. Because of the relapsing nature of this pathology, he necessitated of continuous medical intravenous treatment and periods of rehabilitation in a dedicated center. Nevertheless, at 10 years of FU, he keeps his autonomy and did not represent signs of cervical myelopathy which testifies the efficacy of the cervical decompression procedure [3].
The present case illustrates the value of an open-door laminoplasty in the event of medullary compression due to CIDP; the clinical outcome was satisfactory with major recovery of autonomy that was kept at 10-year follow-up without radiological signs of new cervical spine compression. The main treatment of CIDP is medical but in some rare cases a surgical treatment of neural structures compression might be necessary, and this pathology should be kept in mind in the differential diagnosis of spinal roots hypertrophy.
NOTES
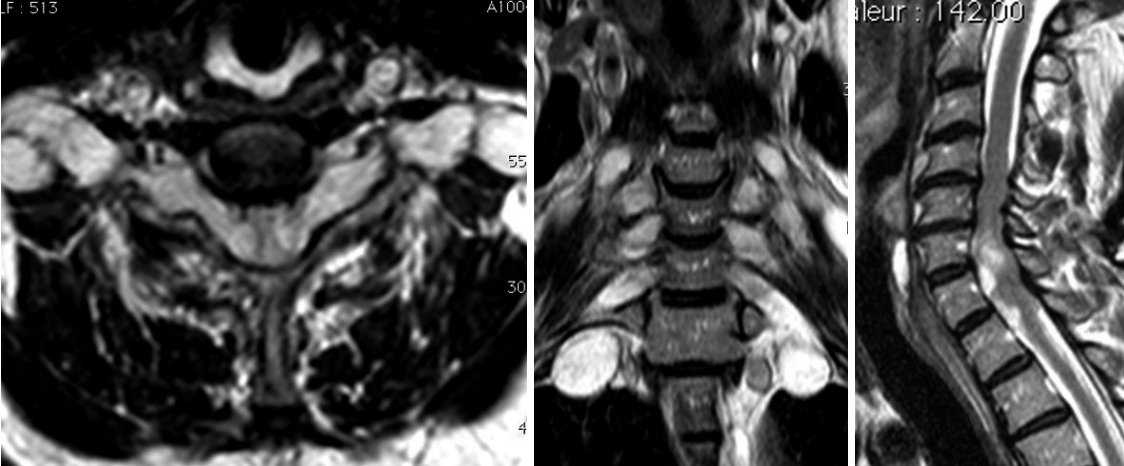
Fig. 1.
Preoperative magnetic resonance imaging: multiple level radicular hypertrophy with C5–7 myelopathy signs.
REFERENCES
1. Sander HW, Latov N. Research criteria for defining patients with CIDP. Neurology 2003;60(8 Suppl 3):S8-15.


2. Tanaka K, Mori N, Yokota Y, et al. MRI of the cervical nerve roots in the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy: a single-institution, retrospective case-control study. BMJ Open 2013;3:e003443.



3. Hasan MT, Patil S, Chauhan V, et al. Spinal cord compression from hypertrophic nerve roots in chronic inflammatory demyelinating polyradiculoneuropathy-a case report. Surg Neurol Int 2021;12:114.



4. Tazawa K, Matsuda M, Yoshida T, et al. Spinal nerve root hypertrophy on MRI: clinical significance in the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy. Intern Med 2008;47:2019-24.


5. Bril V, Blanchette CM, Noone JM, et al. The dilemma of diabetes in chronic inflammatory demyelinating polyneuropathy. J Diabetes Complications 2016;30:1401-7.



6. Rajabally YA, Stettner M, Kieseier BC, et al. CIDP and other inflammatory neuropathies in diabetes-diagnosis and management. Nat Rev Neurol 2017;13:599-611.



7. Schady W, Goulding PJ, Lecky BR, et al. Massive nerve root enlargement in chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 1996;61:636-40.



8. Freitas MR, Nascimento OJ, Soares CN, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: two cases with cervical spinal cord compression. Arq Neuropsiquiatr 2005;63:666-9.


9. Duggins AJ, McLeod JG, Pollard JD, et al. Spinal root and plexus hypertrophy in chronic inflammatory demyelinating polyneuropathy. Brain 1999;122:1383-90.


10. Koski CL, Baumgarten M, Magder LS, et al. Derivation and validation of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy. J Neurol Sci 2009;277:1-8.


11. Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Report from an Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Neurology 1991;41:617-8.


12. Barohn RJ, Kissel JT, Warmolts JR, et al. Chronic inflammatory demyelinating polyradiculoneuropathy. Clinical characteristics, course, and recommendations for diagnostic criteria. Arch Neurol 1989;46:878-84.


13. Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society--first revision. J Peripher Nerv Syst 2010;15:295-301.


14. Pytel P, Rezania K, Soliven B, et al. Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) with hypertrophic spinal radiculopathy mimicking neurofibromatosis. Acta Neuropathol 2003;105:185-8.



15. Staff NP, Figueroa JJ, Parisi JE, et al. Hypertrophic nerves producing myelopathy in fulminant CIDP. Neurology 2010;75:750.



16. Aïdi S, El Alaoui Faris M, Amarti A, et al. Chronic inflammatory demyelinating polyradiculoneuropathy with hypertrophy of spinal roots, brachial plexus and cranial nerves. Rev Neurol (Paris) 2002;158:819-23.

17. Mizuno K, Nagamatsu M, Hattori N, et al. Chronic inflammatory demyelinating polyradiculoneuropathy with diffuse and massive peripheral nerve hypertrophy: distinctive clinical and magnetic resonance imaging features. Muscle Nerve 1998;21:805-8.


18. Midroni G, Dyck PJ. Chronic inflammatory demyelinating polyradiculoneuropathy: unusual clinical features and therapeutic responses. Neurology 1996;46:1206-12.


19. Matsuda M, Ikeda S, Sakurai S, et al. Hypertrophic neuritis due to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): a postmortem pathological study. Muscle Nerve 1996;19:163-9.


20. Kretzer RM, Burger PC, Tamargo RJ. Hypertrophic neuropathy of the cauda equina: case report. Neurosurgery 2004;54:515-8. discussion 518-9.



22. Fukae J, Okuma Y, Noda K, et al. A quadriplegic patient with chronic inflammatory demyelinating polyneuropathy (CIDP) who responded well to corticosteroids and intravenous immunoglobulin therapy. No To Shinkei 2001;53:1115-8.

23. Goldstein JM, Parks BJ, Mayer PL, et al. Nerve root hypertrophy as the cause of lumbar stenosis in chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve 1996;19:892-6.


24. Ginsberg L, Platts AD, Thomas PK. Chronic inflammatory demyelinating polyneuropathy mimicking a lumbar spinal stenosis syndrome. J Neurol Neurosurg Psychiatry 1995;59:189-91.



25. Dyck PJ, Lais AC, Ohta M, et al. Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc 1975;50:621-37.

26. Vallat JM, Tabaraud F, Magy L, et al. Importance of the nerve biopsy for the diagnosis of atypical forms of chronic inflammatory demyelinating polyradiculoneuritis: 8 cases. Bull Acad Natl Med 2003;187:387-99. discussion 399-403.

27. Tracy JA, Dyck PJ. Investigations and treatment of chronic inflammatory demyelinating polyradiculoneuropathy and other inflammatory demyelinating polyneuropathies. Curr Opin Neurol 2010;23:242-8.


28. Villa AM, Garcea O, Di Egidio M, et al. Interferon beta-1a in chronic inflammatory demyelinating polyneuropathy: case report. Arq Neuropsiquiatr 2004;62:892-4.


29. Dyck PJ, O'Brien PC, Oviatt KF, et al. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann Neurol 1982;11:136-41.


30. Van Schaik IN, Winer JB, De Haan R, et al. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev 2002;(2):CD001797.
31. Mehndiratta MM, Hughes RA. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev 2001;(3):CD002062.

32. Dyck PJ, Daube J, O'Brien P, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med 1986;314:461-5.


33. Dyck PJ, Pineda A, Swanson C, et al. The Mayo Clinic experience with plasma exchange in chronic inflammatory-demyelinating polyneuropathy (CIDP). Prog Clin Biol Res 1982;106:197-204.

34. Wehling P, Cleveland S, Reinecke J, et al. Magnetic stimulation as a diagnostic tool in cervical nerve root compression and compression-induced neuropathy. J Spinal Disord 1995;8:304-7.


35. Murata K, Morishita S, Nakamuro T, et al. A case report of the compression syndrome due to hypertrophic neuropathy. Rinsho Shinkeigaku 1991;31:213-5.


- TOOLS
-
METRICS

-
- 1 Crossref
- Scopus
- 3,543 View
- 206 Download
- Related articles in NS
-
Journal Impact Factor 3.2