 |
 |
- Search
|
|
||
Abstract
Objective
Although endoscopic drill has the advantages in manipulation and hemostasis, whose low efficiency and blurred vision reduce the efficacy of lumbar endoscopic unilateral laminotomy with bilateral decompression (LE-ULBD). The present study was designed to evaluate the safety and efficacy of full-visualized trephine/osteotome in the LE-ULBD surgery for severe lumbar stenosis.
Methods
Fifty-seven severe lumbar stenosis patients who underwent LE-ULBD between January 2020 to January 2023 were enrolled, who were divided into drill and visualized trephine groups. The medical records including demographics, operative duration, intraoperative electrophysiological findings, postoperative hospital stay or hospital stay, postoperative outcomes and complications were retrospectively reviewed and analyzed.
Results
A total of 57 patients included 15 in drill and 42 in trephine group were enrolled in the study. There was significant difference in the pre- and postoperative visual analogue scale and Oswestry Disability Index scores in both groups (p < 0.05). The mean operative duration in the trephine group (101.05±12.18 minutes) was shorter than that in the drill group (134.67±9.68 minutes) (p < 0.05). There was no statistical difference between the 2 groups in electrophysiological monitoring, posthospital stays, postoperative outcomes and complications. Abnormal free-electromyography (EMG) were recorded in 2 (13.3%) and 5 patients (11.9%) in the drill and trephine group. Intraoperative somatosensory evoked potential changes occurred in 3 (20%) and 3 patients (7.1%) in the drill and trephine group and all patients recovered immediately when surgery ended. No serious complications and recurrence occurred in all the patients.
Degenerative lumbar stenosis is a common spinal disease in elderly patients [1,2], which is attributed to facet osteoarthritis, ligamentum flavum (LF) hypertrophy, disc pathology, and spondylolisthesis, either alone or in any combination leading to symptoms such as leg/back pain, numbness and lower extremity weakness. Although with confirmed efficacy, the traditional open surgery is always accompanied with many complications such as wound infection, spinal nonfusion and failed back surgery syndrome especially in elderly patients because of excessive destruction of muscle and bone structures [3-5]. To reduce iatrogenic injury to normal anatomic structures during surgery, more minimally invasive techniques are introduced. Unilateral laminotomy with bilateral decompression (ULBD) in combination with the microscope has been regarded as standard minimally invasive treatment for the lumbar stenosis since reported by Spetzger et al. [6] in 1994. Endoscopic spine surgery in lumbar disease has continuously evolved over the past 3 decades since several pioneers introduced [7-11]. However, lumbar endoscopic ULBD (LE-ULBD) surgery have not be widely popularized due to the steep learning curve [9], high complication rates [12], low efficiency and inadequate decompression [13]. The widely used endoscopic bone removal tool is high-speed drill, although which has the advantages in manipulation and hemostasis, its low efficiency, vibration and blurred vision influence the widespread of LE-ULBD [14]. We performed full-visualized LE-ULBD surgery by a new designed full-visualized trephine/osteotome under general anesthesia and electrophysiological monitoring, which is rarely seen in previous reports. In this article, we shared our surgical experience focusing on the visualized trephine/osteotome and inside-out technique.
This is a retrospective study. We retrospectively reviewed our patients from January 2020 to January 2023, who underwent full-visualized LE-ULBD procedures under electrophysiological monitoring and general anesthesia. All patients have undergone conservative treatment preoperatively and failed, who were divided into 2 groups according to bone removal by endoscopic drill or trephine/osteotome. All the patients were operated by Dr. Zhong. The research complies with the guidelines for human studies. Informed consent for surgery was obtained from the all participants in the study. This study was approved by Institutional Review Board (IRB) of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (IRB No. XHEC-D-2023-106).
All patients underwent pre- and postoperative clinical evaluation including lumbar/computed tomography (CT)/magnetic resonance imaging (MRI)/dynamic x-ray. The inclusion criteria are described as follows: (1) neurogenic intermittent claudication with or without radiculopathy; (2) preevaluation including lumbar CT/MRI/dynamic x-ray shows sever central lumbar spinal stenosis (grade C with or without lateral recess stenosis and grade D [15]); (3) failed conservative treatments for at least 6 weeks. Patients are excluded with segmental instability, cauda equina syndrome or the patient with multiple responsible levels.
The medical records including demographics, intraoperative findings, operation time, length of hospital stay, postoperative outcomes and complications were reviewed. All patients were followed up by telephone or outpatient clinic interview. Both back and sciatic pain were assessed by visual analogue scale (VAS 0–10) and Oswestry Disability Index (ODI). All patients underwent a lumbosacral CT within 24 hours postoperatively to evaluate the change of cross-sectional area pre- and postoperatively.
The sensory and motor function were monitored by somatosensory evoked potential (SSEP) and free-electromyography (EMG) respectively. Motor activity of the operative level and below was monitored by free-EMG bilaterally, meanwhile, sensory activity was monitored by SSEP. The monopolar needle electrodes (20 mm/28G; 25 mm/27G or 37 mm/27G) were placed 5–10 mm apart into characteristic muscle groups that were physiologically innervated by the targeted nerve root. For recording, filters were set at 10 Hz to 10 kHz and sensitivity at 100 µV to 1 mV. The time base was 20 to 500 msec. A Nicolet Endeavor (Viasys Healthcare/Nicolet Biomedical, Madison, WI, USA) was used for all examinations. EMG signals were continuously made audible by loudspeakers and displayed on a monitor screen. The free-EMG in the form of isolated spikes or phasic bursts recorded intraoperatively and the SSEP amplitude reduction of more than 50% were considered abnormal.
All procedures were performed via a translaminar rather interlaminar approach under general anesthesia with prone position on a radiolucent table. The upper laminar-medial facet junction was located by intraoperative fluoroscopy. After the skin incision was confirmed by the fluoroscopy and a 10-mm vertical skin incision was made, a stepwise-dilating cannulas and working cannula were introduced along the guiding rod. X-ray fluoroscopy was performed again to confirm the location.
Endoscopic radiofrequency electrodes and forceps were used to clean all the soft tissue as much as possible to expose the facet joints and superior and inferior lamina, otherwise, it will take more time to hemostasis and identify the anatomic landmark. Fully exposure of the upper 1/2 lamina, medial border of facet, 1/3 lower lamina and spinolamianr junction before removal of bony structure is critical in the process. After identification of the anatomical landmarks, the laminectomy starts at the inferior border of the cranial hemilamina in the spinolaminar junction going cranially until the origin of the LF by visualized trephine with semiring technique. Identification of the uppermost attachment of the LF provides an important landmark to confirm the cranial limit of the decompression. Medial laminotomy and early piecemeal removal of the detached LF border should reveal the spinal canal and the spinal dura which can be followed laterally to guide the surgeon in preforming medial facetectomy. Early exposure of the lateral margin of the traversing nerve root may reduce nerve damage and may prevent excessive facetectomy [16] (Fig. 1).
Removal of spinolaminar basement is critical for contralateral decompression in PE-ULBD, which focused on undercutting the base of the spinous process and the contralateral lamina by visualized trephine (Fig. 2), osteotome and Kerrison punches until the small detachment of the cranial contralateral LF. The trephine was used to undercut the bone and the osteotome was adopted to remove smaller obstacle. Once the water pressure created a window to make the epidural fat and neural structures visible, then rotate and tilt the endoscope to decompress the LF and view the contralateral side (Figs. 3, 4).
Once the contralateral superior articular process (SAP) was visualized, blunt dissectors were used to carefully detach adhesions between the dura and LF, then remove it piecemeal by Kerrison. As the decompression progresses, the field of vision widen by manipulating the endoscope to maximize the angle of sight to fully decompress the contralateral dura and traversing nerve root (Figs. 3, 4).
Unlike the transforaminal approach, the cannulas insertion in the translaminar approach tends not to cause abnormal EMG changes because the cannulas aim at the upper lamina rather than the interlaminar space. It is easy to irritate the nerve root during intracanal especially lateral recess decompression process. Burst potentials always mean direct or indirect nerve root irritation. When abnormal free-EMG occurs, we should stop to react and deal with the abnormality immediately based on the abnormal EMG and intraoperative findings. During intracanal decompression process, the traversing nerve root may be compressed by the cannula or be irritated by the forceps, bipolar or Kerrison. If the abnormal free-EMG was caused by canula, it should be adjusted to be more dorsal. If the stimulation was caused by decompression manipulation, the free-EMG would stop when manipulation stops.
The SSEP is highly sensitive, but also has the highest false positive incidence, which could be affected by many factors. However, the amplitudes of SSEP in patients with severe spinal stenosis are always low or even cannot be detected. Although the determinization in significance of SSEP abnormality was always controversial, the manipulation should be stopped immediately especially during contralateral decompression and the judgment should be made based on free-EMG and SSEP findings when abnormal SSEP occurred intraoperatively.
A total of 57 patients were enrolled in the study, which included 15 and 42 patients in drill and trephine/osteotome group with an average age of 75.5±5.4 and 73.1±6.9 years old respectively (p > 0.05). The mean symptoms duration in drill group was 15.1±8.06 months, compared with 12.9±9.7 months in the trephine group (p>0.05) (Table 1).
The preoperative and postoperative VAS and ODI scores in both the 2 groups were significantly different (p<0.05), as was the operation duration, which was 134.67±9.68 and 101.05±12.18 minutes respectively in the drill and trephine/osteotome group (p<0.05). There was no difference in the posthospital stay in the 2 groups, which was 2.3±0.7 and 2.2±0.6 days respectively in the drill and trephine/osteotome group (p>0.05). All except 5 patients were followed up by telephone or interview. No recurrence occurred in the 2 groups during the follow-up (Tables 2, 3). During the follow-up, 3 patients including 1 in the drill and 2 in the trephine/osteotome group complained of postoperative back pain and all but 1 relieved 6 months later. No other complications happened in all the patients during the follow-up.
There was no statistical difference between the 2 groups in electrophysiological monitoring. Abnormal free-EMG in the form of isolated spikes or phasic bursts were recorded in 2 (13.3%) and 5 patients (11.9%) in the drill and trephine/osteotome group during intracanal and foraminal decompression. Intraoperative SSEP changes occurred in 3 (20%) and 3 patients (7.1%) in the drill and trephine/osteotome group and all patients recovered to less than 50% reduction of SSEP amplitude immediately when surgery ended. No complications occurred in all the patients with electrophysiological abnormalities, which was attributed to the timely adjustment of manipulation intraoperatively.
Endoscopic spine surgery has been evolving rapidly in the past decades, starting from contraindications and eventually becomes feasible for lumbar stenosis. Due to the wide range of treatable lumbar stenosis and the reported favorable clinical outcomes, the endoscopic posterior approach has attracted more and more attention [17-22].
As we all know, the outside-in technique was most accepted in the LE-ULBD surgery [23], whose procedure involves removal of bony structure including the lamina, articular facet joint first, then the entire LF en bloc or piecemeal to expose the dura and nerve root. However, the outside-in technique has disadvantages including uncertain removal border, dura tear and nerve injury. Lee reported 5% of excessive facet resection in his cases [13], which would lead to iatrogenic destabilization. Meanwhile, ligament flavum and dura are closely adhesive because of loss of epidural fat in severe lumbar spinal stenosis.
The inside-out technique we described here is completely different from what is meant by the foraminal approach. The translaminar inside-out technique involves exposure of the dura and nerve root priorly or concurrently with bony and LF removal, which could avoid excessive bony resection because of visualized dura and nerve structure. Full endoscopic surgery also can precisely decompress the nerve, during which bone and LF were resected piecemeal and no effort is made to remove it en bloc. In our cases, there was no dura and nerve root injury observed during decompression.
Although the drill has the advantages in manipulation and hemostasis, its low efficiency, vibration and blurred vision influence the widespread of LE-ULBD. Ahn reported the rate of postoperative dysesthesia was 6.1% [14], which might be due to the thermal or vibration injury by the drill postoperatively. Compared with endoscopic drill, fully endoscopic trephine is a timesaving and safe equipment to undercut the osteophyte, which has been approved in our research. Endoscopic osteotome can also be used to accurately remove small osteophytes especially the medial border of facet.
The newly designed full-visualized trephine applied in this study advanced with rotation under full vision instead of x-ray (Fig. 2). Unlike endoscopic drill, whose vision would be blurred because of abrasive powder and drill’s direction that influences the progress of the operation, the full-visualized trephine could always be visualized clearly with semiring technique (Fig. 4). What we refer to as the semiring technique is that when the ring is applied, only half or less of the ring is filled with bone rather than the entire ring. The semiring technique could ensure the dura and nerve root visible during the bone removal, which could decrease the incidence of nerve injury. It can also speed up the bone removal process because the semiring’s bone is much easier to remove than full-ring’s.
Besides the full-visualized in-outside technique, the intraoperative EMG monitoring was also performed to provide double protection of the nerve.
The combination of free-EMG and SSEP intraoperatively appears to have a high enough sensitivity to identify nerve structures irritation, which could help to remind the surgeon to avoid the nerve injury by adjusting the cannulas and manipulation timely based on intraoperative electrophysiological monitoring and findings. SSEP contains many inherent problems including high unreliability and false positive rate to moderate spinal cord dysfunction [24], which makes SSEP rarely useful in the lumbar surgery intraoperatively. However, SSEP could monitor the overall sensory conduction of the spinal cord, which is important in patients with severe spinal canal stenosis.
Since motor evoked potential techniques could only monitor conduction of approximately 1/25 motor axons in the motor nerve root [24]. Therefore, free- and triggered-EMG techniques have been the best way to monitor the electrophysiological activity of the muscle fibers, which could provide the surgeon with real-time feedback information on the function of the motor nerve. The purpose of motor nerve root monitoring is to check their function and integrity during intraoperatively in order to avoid irreversible injury. Burst potentials always means direct nerve root contact. As long as the irritation is not heavy, there will be no serious sequelae with removal of harmful factors timely.
Full-visualized trephine/osteotome has been approved to be convenient, safe and efficient in our study, which combined with translaminar inside-out technique and EMG monitoring especially free-EMG may offer a new choice in LE-ULBD surgery for lumbar stenosis patients. However, this is an observational retrospective study within a single center, which may lead to the bias in the final results. A multicenter, prospective study with a large sample size should be performed.
NOTES
Fig. 1.
The LE-ULBD surgical procedure by inside-out technique. The bilateral decompression process follows the sequence (1 to 6). Fully exposure of the upper 1/2 lamina, medial border of facet, 1/3 lower lamina and spinolamianr junction before removal of bony structure is critical in the process. The laminectomy starts at the inferior border of the cranial hemilamina in the spinolaminar junction going cranially until the origin of the ligamentum flavum by visualized trephine with semiring technique. Medial laminotomy and early piecemeal removal of the detached ligamentum flavum border could guide the surgeon to reduce nerve and dural damage incidence and may prevent undue facetectomy.

Fig. 2.
The full-visualized endoscopic trephine and working cannula (Jianbo Medical Device Co., Hangzhou, China).
Fig. 3.
(A, B) The magnetic resonance imaging in the upper row shows severe central and lateral spinal canal stenosis of the L4/5. (C, D) The lower row shows preoperative and postoperative computed tomography scans.
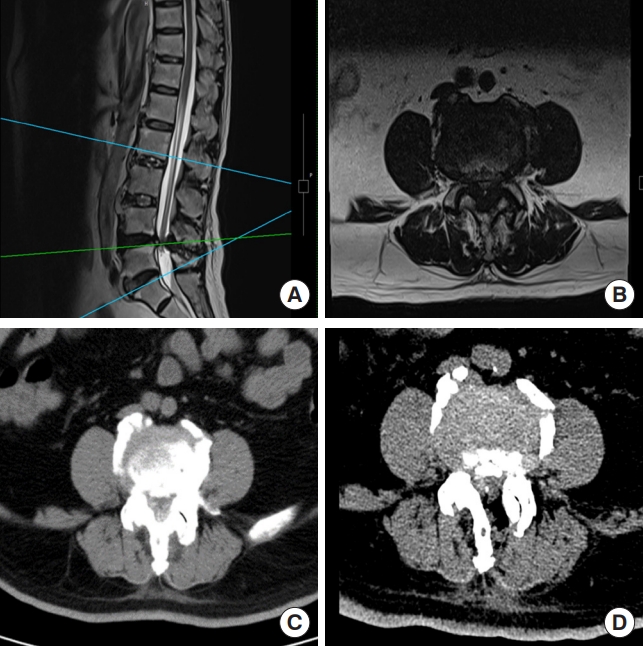
Fig. 4.
Intraoperative view of an illustrated case. An 80-year-old male with bilateral lower extremity pain for more than 1 week (lumbago visual analogue scale [VAS] 5, leg pain VAS 10, unable to walk). Panels A and B show the removal of the lamina with the full-visualized semiring technique and osteotomy. Panels C and D show the ipsilateral and contralateral nerve decompression with the inside-out technique. Early exposure of the lateral margin of the traversing nerve root may reduce nerve damage and may prevent undue facetectomy.

Table 1.
Patient demographics and characteristics of clinical manifestation
Table 2.
Pre- and postoperative assessment of clinical manifestation in the trephine/osteotome group
| Variable | Preoperative | Postimmediate | Post 1 month | Post 6 months | Post 12 months | Post last-FU |
|---|---|---|---|---|---|---|
| VAS | 5.6 ± 2.6 | 0.3 ± 0.6† | 0.4 ± 0.7 | 0.3 ± 0.7 | 0.2 ± 0.4 | 0.2 ± 0.2 |
| ODI | 51 ± 26.8 | 33.8 ± 6.6† | 5.5 ± 3.3‡ | 4.3 ± 2.6 | 3.7 ± 2.4 | 4.0 ± 2.4 |
Table 3.
Pre- and postoperative assessment of clinical manifestation in the drill group
| Variable | Preoperative | Postimmediate | Post 1 month | Post 6 months | Post 12 months | Post last-FU |
|---|---|---|---|---|---|---|
| VAS | 5.6 ± 2.0 | 0.7 ± 1.3† | 0.6 ± 1.2 | 0.5 ± 0.7 | 0.5 ± 0.7 | 0.5 ± 1.2 |
| ODI | 48.9 ± 19.9 | 37.3 ± 8.3† | 5.7 ± 3.1‡ | 2.6 ± 2.3 | 2.7 ± 2.4 | 2.8 ± 2.4 |
REFERENCES
1. Kim JH, Kim HS, Kapoor A, et al. Feasibility of full endoscopic spine surgery in patients over the age of 70 years with degenerative lumbar spine disease. Neurospine 2018;15:131-7.




2. Shin EK, Kim CH, Chung CK, et al. Sagittal imbalance in patients with lumbar spinal stenosis and outcomes after simple decompression surgery. Spine J 2017;17:175-82.


3. Tonomura H, Hatta Y, Mikami Y, et al. Magnetic resonance imaging evaluation of the effects of surgical invasiveness on paravertebral muscles after muscle-preserving interlaminar decompression (MILD). Clin Spine Surg 2017;30:E76-82.


4. Sihvonen T, Herno A, Paljärvi L, et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine (Phila Pa 1976) 1993;18:575-81.


5. Zoidl G, Grifka J, Boluki D, et al. Molecular evidence for local denervation of paraspinal muscles in failed-back surgery/postdiscotomy syndrome. Clin Neuropathol 2003;22:71-7.

6. Spetzger U, Bertalanffy H, Naujokat C, et al. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part I: anatomical and surgical considerations. Acta Neurochir (Wien) 1997;139:392-6.

7. Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician 2015;18:61-70.


8. Franco D, Mouchtouris N, Gonzalez GA, et al. A review of endoscopic spine surgery: decompression for radiculopathy. Curr Pain Headache Rep 2022;26:183-91.



9. Ru N, Su C, Li J, et al. Varied low back pain induced by different spinal tissues in percutaneous endoscopic lumbar discectomy: a retrospective study. Pain Physician 2022;25:E331-9.

10. Ju CI, Lee SM. Complications and management of endoscopic spinal surgery. Neurospine 2023;20:56-77.




11. Hara T, Ohara Y. Perioperative management for full-endoscopic lumbar discectomy: consideration from the perspective of preventing complication. Neurospine 2023;20:28-32.




12. Lewandrowski KU. Incidence, management, and cost of complications after transforaminal endoscopic decompression surgery for lumbar foraminal and lateral recess stenosis: a value proposition for outpatient ambulatory surgery. Int J Spine Surg 2019;13:53-67.



13. Lee CW, Yoon KJ, Kim SW. Percutaneous endoscopic decompression in lumbar canal and lateral recess stenosis - the surgical learning curve. Neurospine 2019;16:63-71.




14. Li ZZ, Hou SX, Shang WL, et al. Percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar decompression for lateral recess stenosis through transforaminal approach: technique notes and 2 years follow-up. Clin Neurol Neurosurg 2016;143:90-4.


15. Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 2010;35:1919-24.


16. Lim KT, Meceda EJA, Park CK. Inside-out approach of lumbar endoscopic unilateral laminotomy for bilateral decompression: a detailed technical description, rationale and outcomes. Neurospine 2020;17(Suppl 1):S88-98.




17. Lee CH, Choi M, Ryu DS, et al. Efficacy and safety of fullendoscopic decompression via interlaminar approach for central or lateral recess spinal stenosis of the lumbar spine: a meta-analysis. Spine (Phila Pa 1976) 2018;43:1756-64.

18. Jiang Q, Ding Y, Lu Z, et al. Comparative analysis of nonfull and full endoscopic spine technique via interlaminar approach for the treatment of degenerative lumbar spinal stenosis: a retrospective, single institute, propensity scorematched study. Global Spine J 2023;13:1509-21.



19. Hashimoto A, Tezuka F, Yamashita K, et al. Planned four-stage transforaminal full-endoscopic lumbar decompression under local anesthesia in a patient with severe comorbidity. NMC Case Rep J 2021;8:221-7.



20. Perez-Roman RJ, Gaztanaga W, Lu VM, et al. Endoscopic decompression for the treatment of lumbar spinal stenosis: an updated systematic review and meta-analysis. J Neurosurg Spine 2022;36:549-57.


21. Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices 2014;11:605-16.


22. Salim AA, Yusof AH, Johari J, et al. Feasibility of unilateral approach for bilateral decompressive endoscopic spinal surgery for lumbar stenosis to improve back and leg pain: a consecutive single-center series of 60 patients. Front Surg 2020;7:507954.




- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























