Clinical Characteristics, Surgical Outcomes, and Risk Factors for Emergency Surgery in Patients With Spinal Metastases: A Prospective Cohort Study
Article information
Abstract
Objective
To elucidate the patient characteristics and outcomes of emergency surgery for spinal metastases and identify risk factors for emergency surgery.
Methods
We prospectively analyzed 216 patients with spinal metastases who underwent palliative surgery from 2015 to 2020. The Eastern Cooperative Oncology Group performance status, Barthel index, EuroQol-5 dimension (EQ5D), and neurological function were assessed at surgery and at 1, 3, and 6 months postoperatively. Multivariate analysis was performed to identify risk factors for emergency surgery.
Results
In total, 146 patients underwent nonemergency surgery and 70 patients underwent emergency surgery within 48 hours of diagnosis of a surgical indication. After propensity score matching, we compared 61 patients each who underwent nonemergency and emergency surgery. Regardless of matching, the median performance status and the mean Barthel index and EQ5D score showed a tendency toward worse outcomes in the emergency than nonemergency group both preoperatively and 1 month postoperatively, although the surgery greatly improved these values in both groups. The median survival time tended to be shorter in the emergency than nonemergency group. The multivariate analysis showed that lesions located at T3–10 (p = 0.002; odds ratio [OR], 2.92; 95% confidence interval [CI], 1.48–5.75) and Frankel grades A–C (p < 0.001; OR, 4.91; 95% CI, 2.45–9.86) were independent risk factors for emergency surgery.
Conclusion
Among patients with spinal metastases, preoperative and postoperative subjective health values and postoperative survival are poorer in emergency than nonemergency surgery. Close attention to patients with T3–10 metastases is required to avoid poor outcomes after emergency surgery.
INTRODUCTION
Recent progress in cancer diagnosis and treatment has resulted in increasing numbers of cancer survivors with bone metastases. Because the spine is the most common site of bone metastases, the prevalence of spinal metastases has also been increasing. Approximately 10% to 20% of patients with spinal metastases develop spinal element destruction and epidural compression, leading to serious symptoms including neurological dysfunction and intractable pain [1,2]. These symptomatic spinal metastases can severely deteriorate patients’ performance status (PS), activities of daily living (ADL), and quality of life (QoL), which are critical indicators for cancer therapy until the terminal phase.
Current evidence shows that spine surgery for symptomatic spinal metastases can improve patients’ ambulatory status [3,4]; health state parameters, including PS, ADL, and QoL [5-8]; and survival [3,8]. Because spinal metastases mostly progress asymptomatically, prophylactic surgical indications have not been established, and some patients with symptomatic spinal metastases require emergency surgery because of progressive neurological dysfunction. Emergency spine surgery for other disorders [9,10] as well as other surgeries [11] generally result in poor outcomes. However, few studies have investigated the clinical outcomes of emergency surgery for spinal metastases. Therefore, it is essential to clarify the clinical outcomes of emergency surgery for spinal metastases. In addition, identifying the characteristics of patients with spinal metastases who are likely to require emergency surgery is important. This will provide valuable insights to both patients and clinicians, including spine surgeons, oncologists, and radiologists, enabling them to provide timely treatment before severe symptoms develop. However, the risk factors for emergency surgery in patients with spinal metastases remain unclear. We thus designed the present prospective cohort study to elucidate the characteristics and clinical outcomes of patients with spinal metastases who underwent emergency surgery and to identify risk factors for emergency surgery for spinal metastases.
MATERIALS AND METHODS
1. Ethics Statement
This study was approved by the Institutional Review Board (IRB) of Kobe University Hospital (IRB No. 1733). Written informed consent was obtained from each patient in accordance with the principles of the Declaration of Helsinki and the laws and regulations of our country.
2. Patients
In total, 264 consecutive patients who had symptomatic spinal metastases with surgical indications in our hospital from 2015 to 2020 were prospectively enrolled. Metastasis was diagnosed by plain radiography, computed tomography, magnetic resonance imaging, bone scintigraphy, positron emission tomography, and histological evaluation of needle biopsy samples. Tumors that did not originate from a biopsy site and had no identifiable primary site were diagnosed as spinal metastases from an unknown primary tumor [12]. The patients were divided into 2 groups: those who underwent emergency surgery and those who underwent nonemergency surgery. Patients with at least one of the following were considered to have a surgical indication: (1) progressive neurological deficits, (2) a potentially unstable spine (Spinal Instability Neoplastic Score [SINS] [13] of 7–12) or unstable spine (SINS of ≥ 13), or (3) intractable pain resistant to conservative care, including opioids. Emergency surgery was performed on patients who underwent surgery within 48 hours of diagnosis of a surgical indication because of rapidly progressive neuropathy or advanced neuropathy except for complete paraplegia for > 48 hours. Patients whose incomplete paralysis was ameliorated with bed rest underwent semiurgent surgery within 2 weeks of diagnosis; these patients were included in the nonemergency surgery group. Patients with impaired consciousness were excluded because of the inability to accurately describe their subjective health state. To maintain case homogeneity, patients with oligometastasis who underwent total en bloc spondylectomy for curative treatment were also excluded. The surgeon comprehensively chose the surgical procedure based on the patient’s neurological function, degree of spinal cord compression, and SINS. Generally, patients with a potentially unstable spine (SINS of 7–12) or unstable spine (SINS of ≥ 13) and tumor-induced spinal canal stenosis with neurological dysfunction underwent posterior instrumentation with decompression. Patients with a SINS of ≥ 7 and intact neurological function underwent posterior instrumentation without decompression. Patients with a SINS of ≥ 7 and moderate to severe spinal cord compression underwent posterior instrumentation with decompression even if they had no neurological dysfunction at the time of surgery. A few patients without apparent instability (SINS of ≤ 6) underwent posterior instrumentation with decompression to prevent future instability due to progression of the residual tumor postlaminectomy. All surgeries involved single-stage posterior stabilization with fixation using lateral mass screws for the cervical spine and pedicle screws for the thoracic and lumbar spine and/or posterior decompression. Posterior decompression was generally performed with partial removal of the tumor to the extent feasible from only the posterolateral aspect. Tumor reduction from the anterior aspect of the spinal cord was not performed except in separation surgery, creating a 2- to 3-mm tumor-free interval between the spinal cord and the tumor for safe application of stereotactic body radiation therapy or intensity-modulated radiation therapy for the target adjacent to the spinal cord. Neither piecemeal spondylectomy, corpectomy, nor an anterior approach was performed. No preoperative embolization was performed. All immobilization devices, including corsets and collars, were removed postoperatively. The treatment plan was determined by a multidisciplinary team focused on bone metastases. Postoperative radiotherapy and/or chemotherapy was performed after 2 weeks postoperatively.
3. Evaluation
The postoperative survival duration was defined as the time from the date of surgery to the latest follow-up examination or death. At the start of the study (baseline), we registered the patients’ age, sex, and primary tumor type as preoperative demographic factors. The new Katagiri score [14] and revised Tokuhashi score [15] were used to estimate the patients’ prognosis, and that part of the new Katagiri score [14] pertaining to the primary lesion was used to evaluate the malignancy grade of the primary tumor (slow/moderate/rapid growth). Moreover, the primary tumor type was classified by sex specificity and sex predominance. Prostate and testicular cancers were considered male-specific cancers, whereas breast, ovarian, and uterine cancers were considered female-specific cancers. Bladder, laryngeal, and esophageal cancers were considered tumors with male predominance, and thyroid and gallbladder cancers were considered tumors with female predominance based on a prior report [16]. The remaining tumors were categorized as tumors without sex predominance. The SINS [13] was used to evaluate the degree of spinal instability, and the tumor location was categorized based on the spinal section of the lesion in the SINS as follows: semirigid spine (T3–10), mobile spine (C3–6, L2–4), or junctional spine (occiput–C2, C7–T2, T11–L1, L5–S1). Additionally, the metastatic epidural spinal cord compression (MESCC) scale was used to evaluate the severity of epidural spinal cord compression by the tumor [17]. We comprehensively determined the lesion level responsible for symptoms, including pain and neurological dysfunction, based on computed tomography, magnetic resonance imaging, neurological findings, and the SINS. As surgery-related factors, we investigated the operative time, blood loss, number of fused vertebrae, surgical procedure (posterior instrumentation with or without decompression), screw technique (open or percutaneous), and Clavien-Dindo classification grade ≥ II postoperative complications [18]. Given the nature of surgery for spinal metastases, grade 2 blood transfusion was not included as a complication. The Eastern Cooperative Oncology Group PS (ECOG PS) [19], Barthel index (BI) [20], and EuroQol-5 dimension (EQ5D) score [21] were used to evaluate the PS, ADL, and QoL, respectively. Neurological function was assessed with the Frankel grade [22]. Clinical follow-up was routinely performed at 1, 3, and 6 months postoperatively and then every 3 months. Patients who were alive and could not consult our department were contacted by telephone to obtain the latest follow-up information. For patients who died, we obtained information from the patient’s family or institution of transfer.
4. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics ver. 28.0 (IBM Co., Armonk, NY, USA) with significance set at a p-value of < 0.05. Student t-test or the Mann-Whitney U-test was used to compare continuous variables and Pearson chi-square test or Fisher exact test was used to compare categorical variables between the emergency and nonemergency groups. The overall survival rate was calculated by the Kaplan-Meier method, and the 2 groups were compared by the log-rank method. For chronological evaluation of the endpoints, the Kruskal-Wallis test and Scheffe post hoc test were used to assess the significance of differences between the 2 groups. The propensity scores were calculated based on the patients’ age, sex, SINS, new Katagiri score, primary tumor malignancy, primary tumor type according to sex predominance, and MESCC grade. The C-statistic, indicating goodness of fit, was 0.710. Patients who underwent emergency surgery or nonemergency surgery were matched based on their propensity scores with the caliper set at < 0.2.
All baseline variables with a p-value of < 0.10 in the comparison between the nonemergency and emergency groups were candidates for inclusion in the multivariable logistic regression analysis to identify risk factors for emergency surgery.
RESULTS
1. Patient Demographics and Clinical Characteristics at Surgery
In total, 216 patients with symptomatic spinal metastases were enrolled (mean age, 66.4 years; range, 24–92 years). After all patients had provided informed consent, 38 patients who were unwilling to undergo surgery and 4 patients with impaired consciousness were excluded. Three patients who had experienced complete paraplegia for > 48 hours and were unlikely to recover did not undergo spinal surgery. Additionally, 3 patients who underwent total en bloc spondylectomy were excluded. The nonemergency group comprised 146 patients (mean age, 70 years; range, 24–92 years), and the emergency group comprised 70 patients (mean age, 65 years; range, 38–84 years). No patients were lost to follow-up before death. There were no significant differences between the 2 groups in preoperative demographics and clinical characteristics at surgery except for sex (p = 0.012), SINS (p = 0.020), primary tumor type according to sex predominance (p = 0.030), and location of spinal metastases (p < 0.001). With regard to surgery-related factors, blood loss in the emergency group (median, 313 mL; interquartile range [IQR], 100–600 mL) was significantly higher than that in the nonemergency group (median, 180 mL; IQR, 89–346 mL) (p = 0.005). All patients in the emergency group underwent posterior instrumentation and decompression, whereas 102 of 146 patients (69.9%) in the nonemergency group underwent posterior instrumentation and decompression (p < 0.001). The percutaneous pedicle screw technique was more likely to be chosen in the nonemergency than emergency group (37.0% vs. 5.7%, respectively; p < 0.001) because percutaneous pedicle screws were not regularly stocked in our institution. All the subjective health values at baseline were significantly poorer in the emergency than nonemergency group (ECOG PS, p = 0.023; BI, p < 0.001; EQ5D score, p < 0.001). The ratio of patients with preoperative radiotherapy tended to be higher in the nonemergency than emergency group (32.2% vs. 20.0%, respectively), although the difference did not reach statistical significance (p = 0.062) (Table 1).
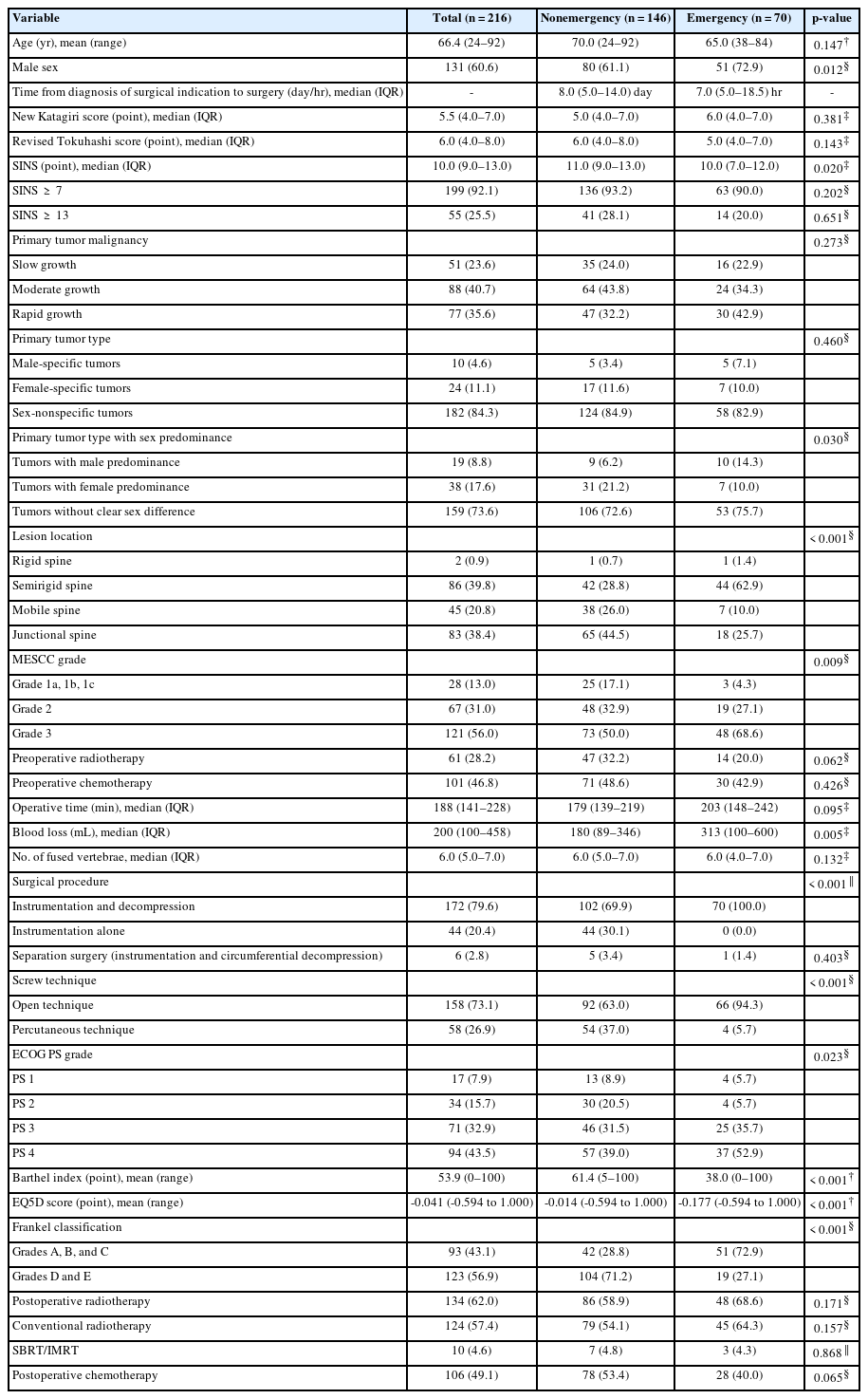
Demographics and clinical characteristics of patients in nonemergency and emergency groups at baseline before propensity score matching (crude analysis)
Table 2 shows the comparison of 1:1 matched patients in the nonemergency (n = 61) and emergency (n = 61) groups. There were no significant differences in the patients’ demographics or tumor characteristics between the 2 groups. However, because of the significant difference in neurological function between the 2 groups (p < 0.001), there was also a significant difference in the surgical procedure between the 2 groups. Additionally, blood loss in the emergency group (median, 317 mL; IQR, 100–600 mL) was significantly higher than that in the nonemergency group (median, 175 mL; IQR, 80–368 mL) (p = 0.039). Similar to the crude analysis, the subjective health values at baseline tended to be poorer in the emergency than nonemergency group (ECOG PS, p = 0.061; BI, p < 0.001; EQ5D score, p = 0.218).
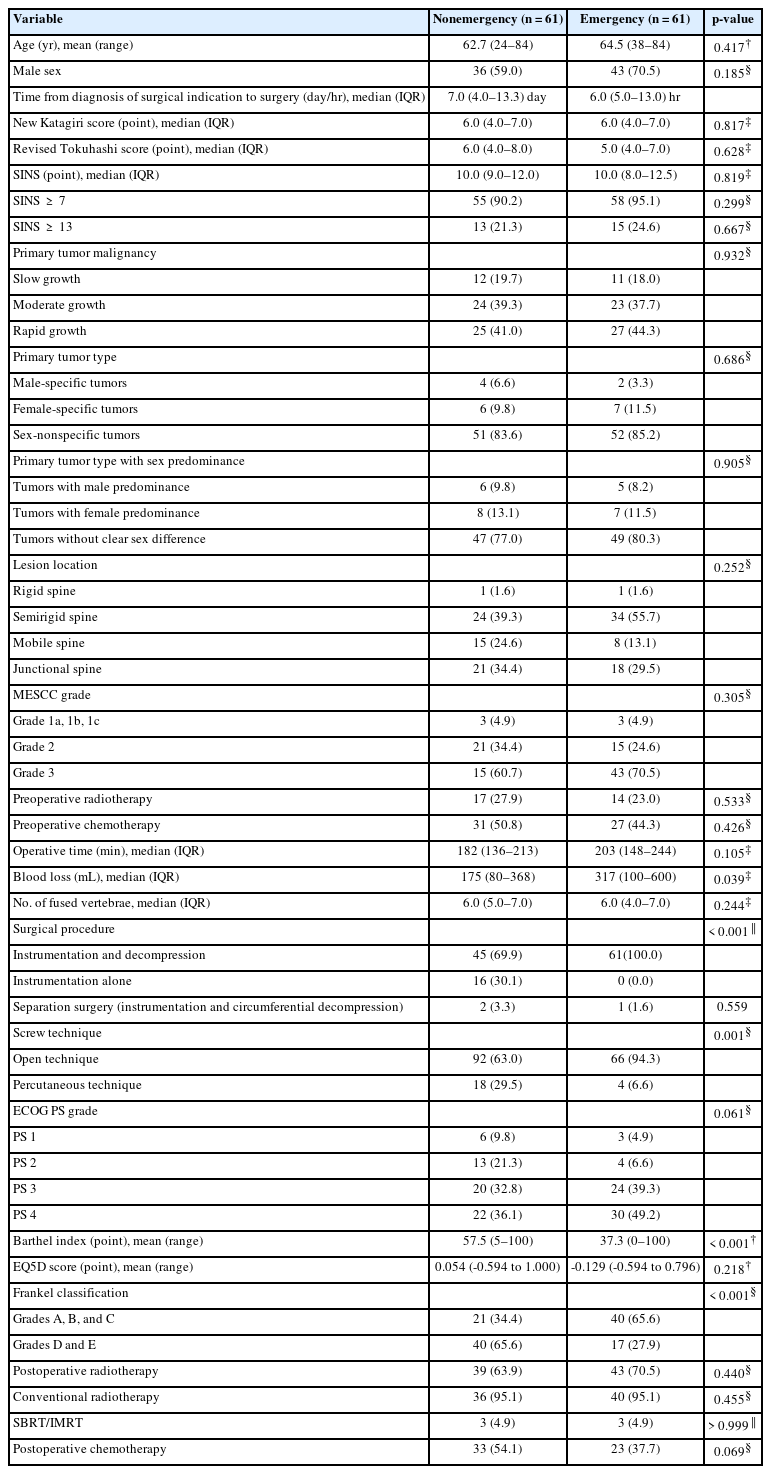
Demographics and clinical characteristics of patients in nonemergency and emergency groups at baseline after propensity score matching
The primary tumors are listed in Supplementary Table 1. Lung cancer was the most common type of primary cancer in both groups (approximately 20%), followed by renal cell cancer in the nonemergency group (11.0%) and hepatocellular carcinoma in the emergency group (11.4%). Although there was no significant difference in the degree of malignancy of the primary tumor between the 2 groups, 13 (8.9%) patients with thyroid cancer underwent nonemergency surgery, whereas no patients with thyroid cancer underwent emergency surgery. Prostate and liver cancer were more common in the emergency group. The primary tumors in matched patients are also listed in Supplementary Table 1.
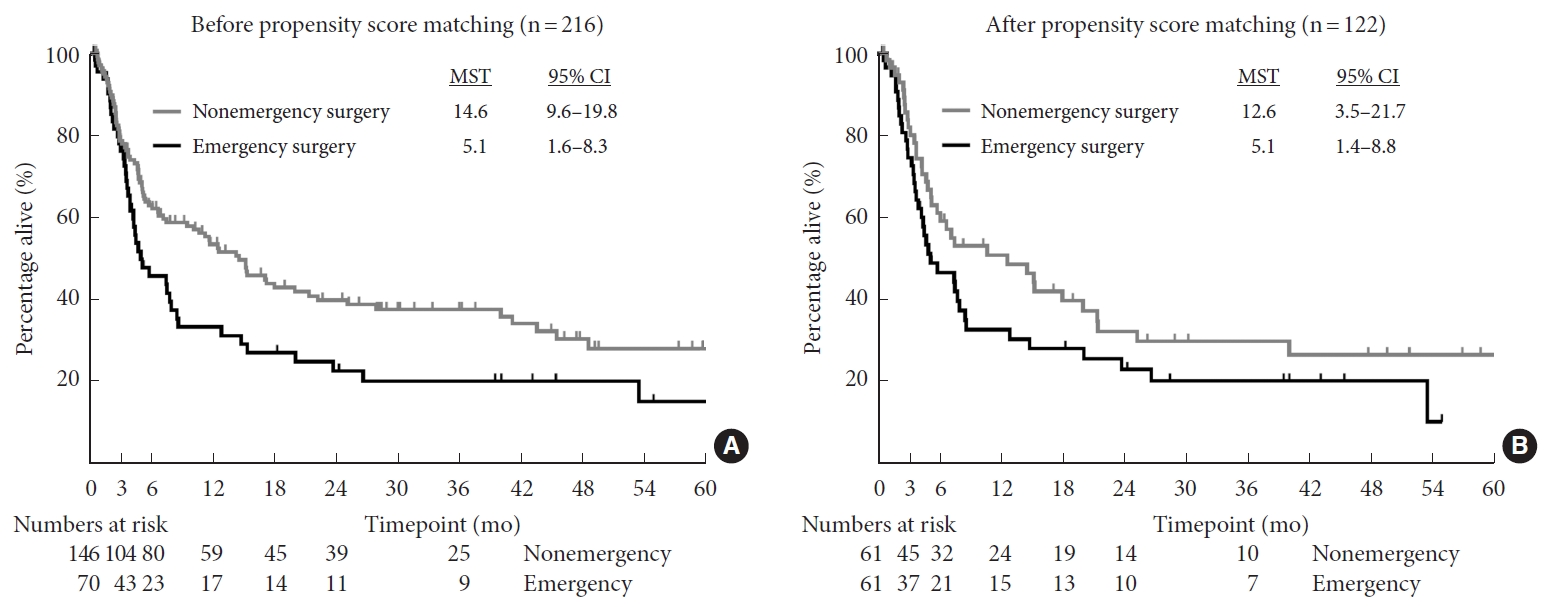
2. Survival Rate
The 1-month survival rate was 97.1% and 95.5% in the nonemergency and emergency groups, respectively. The 3-month survival rate was also similar between the 2 groups (78.7% and 76.1%, respectively). However, the 6-month survival rate was lower at 62.9% and 45.6% in the nonemergency and emergency groups, respectively, and the 1-year survival rate further decreased to 53.4% and 33.2%, respectively. The median survival time after surgery was 14.6 months (95% confidence interval [CI], 9.6– 19.8) in the nonemergency group and 5.1 months (95% CI, 1.6– 8.3) in the emergency group (p = 0.008) (Fig. 1A). After propensity score matching, the median survival time also tended to be longer in the nonemergency group (12.6 months; 95% CI, 3.5–21.7) than in the emergency group (5.1 months; 95% CI, 1.4–8.7). However, the difference did not reach statistical significance (p = 0.115) (Fig. 1B).
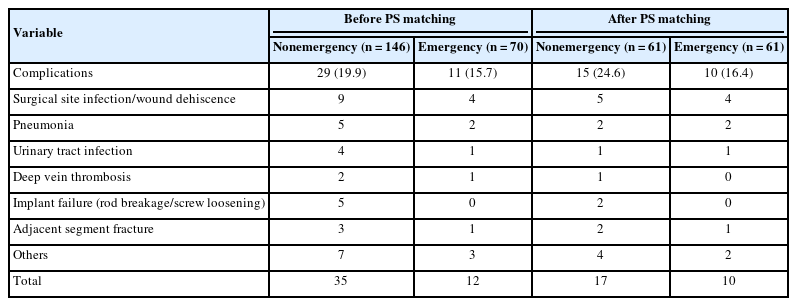
3. Complications
Postoperative complications occurred in 29 patients (19.9%) in the nonemergency group and 11 patients (15.7%) in the emergency group (Table 3). Among the matched patients, 15 of 61 patients (24.6%) in the nonemergency group and 10 of 61 patients (16.4%) in the emergency group developed postoperative complications. The complication rate was not significantly different between the 2 groups in either the crude analysis (p = 0.463) or propensity score-matched analysis (p = 0.099). The total number of postoperative complications in the nonemergency and emergency groups was 35 and 12, respectively (Table 3). The most common complication was surgical site infection/wound dehiscence, which was observed in 9 of 146 patients (6.2%) in the nonemergency group and 4 of 70 patients (5.7%) in the emergency group. The second most common complication was pneumonia, which was observed in 5 of 146 patients (3.4%) in the nonemergency group and 4 of 70 patients (2.9%) in the emergency group. This trend was consistent with that after propensity score matching. The other complications are listed in Table 3.
4. ECOG PS (PS)
The median PS at surgery was 3 in the nonemergency group and 4 in the emergency group (p = 0.023) (Table 1). At 1 month postoperatively, the PS had improved to 2 in the nonemergency group and 2.5 in the emergency group. In the nonemergency group, it further improved and reached 1 at 3 months postoperatively, and this was maintained until 6 months. In the emergency group, it further improved to 2 at 3 months postoperatively and reached 1 at 6 months. The PS in the nonemergency group was significantly better than that in the emergency group at 1 and 3 months postoperatively (p = 0.002 and p = 0.047, respectively). There was no significant difference at 6 months postoperatively (Fig. 2). Among the matched patients, the median PS at surgery was 3 in both groups. At 1 month postoperatively, the median PS had improved to 2 in the nonemergency group but had not changed in the emergency group (p = 0.042). In the nonemergency group, it further improved to 1 at 3 months postoperatively, and this was maintained until 6 months. In the emergency group, it improved to 2 at 3 months postoperatively and 1 at 6 months postoperatively. There was no significant difference between the 2 groups at 3 and 6 months postoperatively (Fig. 2).
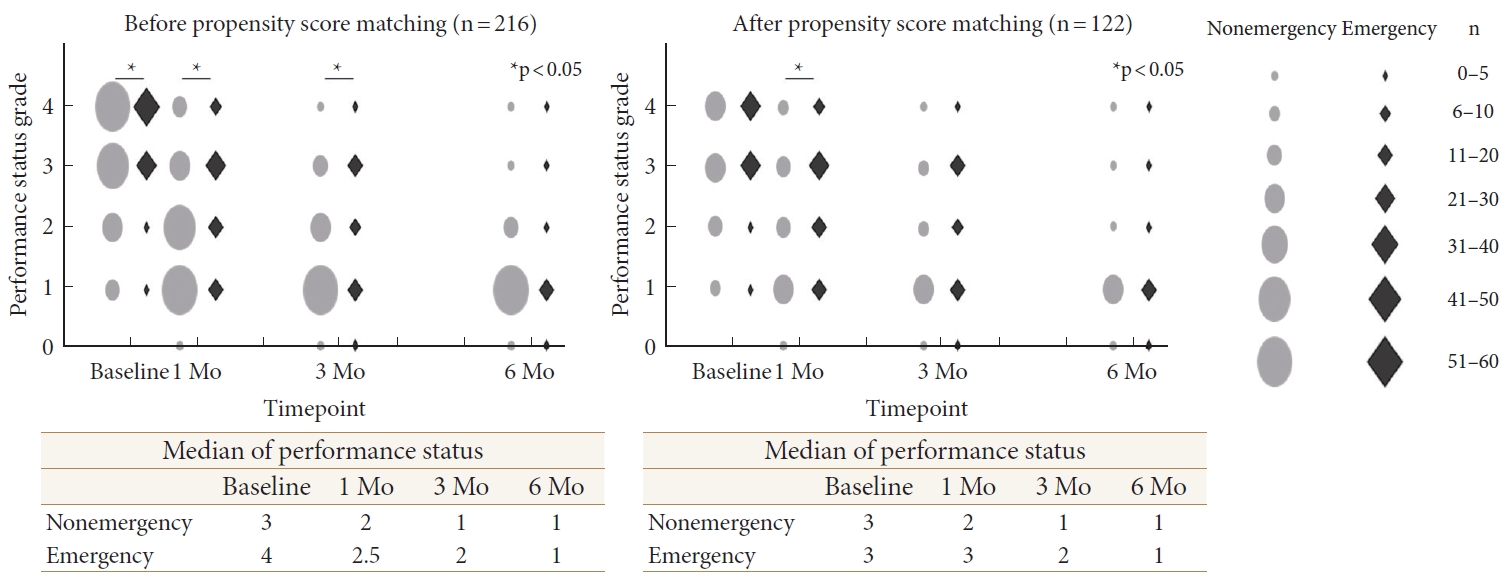
Performance status in nonemergency and emergency groups preoperatively and at 1, 3, and 6 months postoperatively. Crude and propensity score matching analysis were shown at the left side and right side, respectively. Gray circles and black diamonds indicate nonemergency and emergency group, respectively. *p < 0.05 between nonemergency and emergency group using Pearson chi-square test.
5. BI (ADL)
The mean BI at surgery was 61.4 (range, 5–100) in the nonemergency group and 38.0 (range, 0–100) in the emergency group (p < 0.001) (Table 1). At 1 month postoperatively, the mean BI had greatly improved to 80.7 (range, 0–100) in the nonemergency group and 62.9 (range, 0–100) in the emergency group. At 3 and 6 months postoperatively, the BI was maintained and gradually improved in both groups. The chronological course of the BI showed significant differences between the 2 groups at all time points (1 month, p < 0.001; 3 months, p = 0.001; 6 months, p = 0.020) (Fig. 3A). Among matched patients, the chronological course of the BI in the emergency group was also lower than that in the nonemergency group at all time points, although the difference at 6 months postoperatively did not reach statistical significance (1 month, p = 0.010; 3 months, p = 0.015; 6 months, p = 0.061) (Fig. 3B).
6. EQ5D Score (QoL)
The mean EQ5D score at surgery was -0.014 (range, -0.594 to 1.000) in the nonemergency group and -0.170 (range, -0.594 to 0.516) in the emergency group (p < 0.001) (Table 1). At 1 month postoperatively, the mean score had improved to 0.655 (range, -0.594 to 1.000) in the nonemergency group and to 0.457 (range, -0.594 to 1.000) in the emergency group. At 3 months postoperatively, the mean score had further improved to 0.747 (range, -0.166 to 1.000) in the nonemergency group and markedly improved to 0.694 (range, -0.043 to 1.000) in the emergency group. At 6 months postoperatively, both groups showed a modest deterioration of the EQ5D score, but the score was generally maintained. The chronological course of the EQ5D score showed a significant difference between the 2 groups at 1 month postoperatively (p = 0.010) but no difference at 3 and 6 months postoperatively (p = 0.985 and p = 0.544, respectively) (Fig. 4A). The EQ5D score in both groups similarly improved with time, and there was a significant difference between the 2 groups at 1 month postoperatively (p = 0.011) but no difference at 3 and 6 months postoperatively (p = 0.985 and p = 0.544, respectively) (Fig. 4B).

EQ5D score in nonemergency and emergency groups preoperatively and at 1, 3, and 6 months postoperatively. (A) Crude analysis. (B) Propensity score matching analysis. EQ5D, EuroQol-5 dimension. *p < 0.05 between nonemergency and emergency groups using the Kruskal-Wallis test and Scheffe post hoc test.
7. Frankel Classification (Neurological Function)
We evaluated changes in the Frankel classification from surgery to the last follow-up within 6 months. In the nonemergency group, 49 of 51 patients with Frankel grade E at surgery retained their neurological status. No patients in the emergency group had Frankel grade E at surgery. Excluding patients with a neurologically normal status at baseline, 61 of 95 patients (64.2%) in the nonemergency group showed improvement of more than one grade, whereas 51 of 70 patients (72.9%) in the emergency group showed improvement of more than one grade. There was no significant difference between the 2 groups (p = 0.240). After matching and excluding patients with a neurologically normal status at baseline, 30 of 47 patients (63.8%) in the nonemergency group showed improvement of more than one grade, whereas 45 of 61 patients (73.7%) in the emergency group showed improvement of more than one grade.
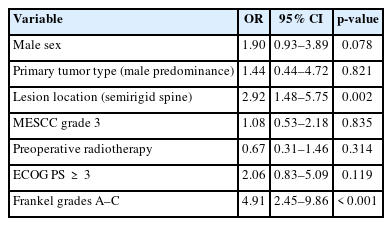
8. Multivariate Analysis of Risk Factors for Emergency Surgery
Based on the comparison of demographics and characteristics at baseline between the 2 groups, male sex (p = 0.012), primary tumor type with male predominance (p = 0.030), lesion location in the semirigid spine (T3–10) (p < 0.001), MESCC grade 3 (p = 0.009), and preoperative radiotherapy (p = 0.062) were considered potential risk factors for emergency surgery (Table 1). Additionally, emergency surgery was associated with poor ECOG PS (p = 0.023), BI (p < 0.001), EQ5D score (p < 0.001), and Frankel grade (p < 0.001) (Table 1). Because the inclusion of many closely correlated covariates in the multivariate analysis caused the model to become technically difficult and unstable, we chose male sex, SINS, primary tumor type with male predominance, lesion location in the semirigid spine (T3–10), MESCC grade 3, preoperative radiotherapy, preoperative ECOG PS of ≥ 3, and preoperative Frankel grades A–C as eligible predictors for the multivariate analysis. The multivariate analysis showed that lesion location in the semirigid spine (T3–10) (p = 0.002; odds ratio [OR], 2.92; 95% CI, 1.48–5.75) and preoperative Frankel grades A–C (p < 0.001; OR, 4.91; 95% CI, 2.45–9.86) were independent risk factors for emergency surgery (Table 4). In particular, lesion location at T3 and T4 tended to be associated with a higher risk for emergency surgery. Among 17 patients with lesion location at T3, 6 patients underwent nonemergency surgery and 11 patients underwent emergency surgery. Among 8 patients with lesion location at T4, 2 patients underwent nonemergency surgery and 6 patients underwent emergency surgery (Table 5).
DISCUSSION
In the current study, we demonstrated that patients with spinal metastases who underwent emergency surgery faced more critical problems in terms of PS, ADL, and QoL (especially preoperatively and in the early stage after surgery) and tended to have shorter survival times after surgery than patients with spinal metastases who underwent nonemergency surgery. Additionally, spinal metastases in the semirigid spine (T3–10) were identified as an independent significant risk factor for emergency surgery in the multivariate analysis.
In general, emergency surgery might be associated with a higher risk of poor outcomes and complications than elective surgery. One study showed that in patients undergoing total hip arthroplasty, nonelective surgery was associated with a longer operative time, higher readmission rate, and higher complication rate [11]. Prior reports regarding spine surgery showed higher surgical site infection/wound dehiscence rates in nonelective surgery for spinal injury [9] and higher costs for anterior cervical discectomy and fusion [23]. However, few studies have investigated the clinical outcomes of emergency surgery for spinal metastases, and these studies were limited by their retrospective design [24-26]. We thus designed the present prospective cohort study to reveal the clinical characteristics and outcomes of patients with spinal metastases who underwent emergency surgery versus nonemergency surgery.
Generally, patients with symptomatic spinal metastases have neurological dysfunction and intractable pain at baseline, leading to serious deterioration of PS, ADL, and QoL [5-8]; these problems are particularly severe for patients who require emergency surgery. Retrospective studies by van Tol et al. [25,26] demonstrated that patients with neurological deficits and/or gross mechanical instability who underwent emergency surgery within 3 days of symptom onset had worse neurological function (Frankel classification), PS (Karnofsky PS), and QoL (EQ5D score) at baseline. Although their definition of “emergency” slightly differs from ours, these findings support our results. One review demonstrated that preoperative QoL, baseline functional status, and neurological status were predictors of postoperative QoL [2], which is also in line with our findings. We additionally evaluated the BI, which reflects patients’ ADL, and found that the mean BI at surgery was > 60 in the nonemergency group and < 40 in the emergency group. This finding indicates that patients who undergo nonemergency surgery are minimally dependent in daily life and that patients who undergo emergency surgery need some assistance in most of their daily life. Taken together, these data indicate that the differences between these 2 groups of patients can be highly relevant from the perspective of patient-related factors as well as objective factors such as neurological function.
Among the surgery-related factors, blood loss was significantly higher in the emergency than nonemergency group in this study. In patients undergoing total hip arthroplasty, emergency surgery is also associated with much higher transfusion and complication rates [11]. Similarly, in patients undergoing metastatic spine surgery, more blood loss and complications also occur in emergency than nonemergency surgery [25]. However, in the current study, there was no significant difference in the complication rate between the 2 groups. The higher rate of implant failure due to relatively longer survival in the nonemergency group may have affected this result. This finding may have also been partly attributed to the higher rate of preoperative radiotherapy in the nonemergency group, possibly leading to wound problems.
With regard to the postoperative course, spinal surgery greatly improved these health state values in both groups. Even emergency surgery provided favorable outcomes regardless of the short preparation time that limits the availability of instruments and qualified medical staff. However, in the emergency surgery group, the PS and QoL were worse preoperatively and postoperatively and the survival rate tended to be shorter in both the crude analysis and propensity score-matching analysis. These findings may have been due to the poorer status at baseline in the emergency surgery group and are in line with a previous retrospective study [24]. Regardless of matching, the ADL in patients who underwent emergency surgery was worse at 1 and 3 months postoperatively, although it remained > 60. These findings reflect the indispensability of timely treatment. At 6 months after surgery, no significant difference was observed in either the PS or EQ5D score between the 2 groups. This may be attributed to having reached a ceiling in most patients and the difference in the survival rate between the 2 groups at 6 months postoperatively. Notably, more than 25% of patients who underwent emergency surgery died between 3 and 6 months postoperatively. Patients with better function are less likely to die within 6 months.
Therefore, timely surgery for patients with spinal metastases can dictate patients’ subjective health and even survival. However, the indication for prophylactic surgery in patients with spinal metastases has not been established. We thus sought to identify the independent risk factors for emergency surgery, providing an opportunity for treatment before the development of severe symptomatic spinal metastases. In our analysis, lesion location in the semirigid spine (T3–10) and Frankel grades A–C were identified as independent risk factors for emergency surgery in patients with spinal metastases. Because Frankel grades A–C were strongly associated with an indication for emergency surgery, it should be taken for granted. Importantly, this is the first study to identify lesion location in the semirigid spine (T3–10) as a risk factor for emergency surgery. Interestingly, lesion location at T3 and T4 tended to be associated with a higher risk for emergency surgery. T3 and T4 are the 2 most cranial levels from T3 to T10 and are in kyphotic alignment. Therefore, patients with metastatic tumors in the semirigid spine (particularly T3 and T4) may develop severe neurological deficits without severe mechanical pain because of the anatomical characteristics, high stability, and narrow spinal canal in this region. One study showed that lesion location at C7–L1 is associated with a higher risk of neurological deficit [27], which may support our findings. Although lesions in the semirigid spine are assigned a score of only 1 point in the SINS system, clinicians might underestimate the risks of severe symptoms in patients with spinal metastases located at T3–10. We should consider early intervention to avoid poor outcomes and short survival before severe symptoms develop in patients with spinal metastases at T3–10.
Our study has a few limitations. First, this was a single-institution study, and the patients consisted of a single race because of our national characteristics. A national database in the United States showed that patients who were Black, had lower income, and had public insurance had a greater risk of emergency surgery [24]. Second, we compared the emergency and nonemergency groups without adjusting for subjective health values including PS, BI, and EQ5D score in the propensity score-matching analysis. However, we focused on clarifying actual clinical settings rather than the difference between the effect of emergency surgery and nonemergency surgery.
CONCLUSION
Among patients with spinal metastases, the subjective health values at baseline and in the early phase after surgery and the overall survival rate are poorer in patients who undergo emergency than nonemergency surgery. Lesion location at T3–10 was identified as an independent risk factor for emergency surgery in patients with spinal metastases. Therefore, clinicians should pay attention to patients with T3–10 metastases to provide timely treatment before severe symptoms develop.
Supplementary Material
Supplementary Table 1 can be found via https://doi.org/10.14245/ns.2347012.506.
Primary tumor types in nonemergency and emergency groups at surgery before and after propensity score matching
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: YK, KKakutani, YS; Data curation: YK, KKakutani, YS, TY, YT, KM, HO, TM, MR, NK, KKuroshima, YH, RK; Formal analysis: YK, KKakutani, TY, YT, RK; Methodology: YK, KKakutani, YS; Project administration: YK, KKakutani, YS, TY, YT, KM, HO, TM, MR, NK, KKuroshima, YH, RK; Visualization: YK, KKakutani, YT, HO; Writing - original draft: YK, KKakutani; Writing - review & editing: KKakutani, YS, TY, YT, KM, HO, TM, MR, NK, KKuroshima, YH, RK.