 |
 |
- Search
| Neurospine > Volume 17(1); 2020 > Article |
|
|
Abstract
Objective
To determine the risk factors associated with radiographic changes and clinical outcomes following 3-level anterior cervical discectomy and fusion (ACDF) using rigidplate constructs and cortico-cancellous allograft. ACDF has demonstrated efficacy for treatment of multilevel degenerative cervical conditions, but current data exists in small heterogeneous forms.
Methods
A retrospective review included 98 patients with primary 3-level ACDF surgery at one institution from 2008 to 2013 with minimum 1-year follow-up. Cervical sagittal vertical axis (SVA), segmental height, fusion, and lordosis radiographs were measured preoperatively and at 2 postoperative periods.
Results
Rates of asymptomatic pseudarthroses and total reoperations were 18% and 4%, respectively. Results demonstrated immediate improvements in cervical lordosis (5.5┬░, p < 0.01) and segmental height (5.0-mm increase, p < 0.01) with little changes in the cervical SVA (3.2-mm increase, p < 0.01). The segmental height decreased from immediate postoperative period to final follow-up (1.7-mm decrease, p < 0.01). Older age was protective against radiolucent lines (p < 0.05). Patient-reported outcomes significantly improved following surgery (p < 0.01). Current smoking status and diagnosis of diabetes mellitus had no impact on radiographic or clinical outcomes. Risk factors were not identified for the 5 reoperations (4%).
Conclusion
Three-level ACDF with rigid-plating and cortico-cancellous allograft is an effective procedure for degenerative diseases of the cervical spine without the application of additional adjuncts or combined anteriorposterior cervical surgeries. Significant improvements in cervical lordosis, segmental height, and segmental alignment can be achieved with little change in cervical SVA and a low rate of reoperations over short-term follow-up. Similarly, patient-reported outcomes show significant improvements.
Anterior cervical discectomy and fusion (ACDF) is associated with successful outcomes for a variety of degenerative conditions of the cervical spine via neural decompression and stabilization [1-5]. ACDF for 1 or 2 levels using allograft and rigid plating gives a high union rate and beneficial clinical outcomes. However, concerns regarding the potential stress-shielding associated with rigid plating, the lack of healing potential with allograft, pseudarthrosis, subsidence, loss of lordosis, and reoperation have previously been discussed particularly for multilevel cases [6-8].
Historically outcomes following multilevel ACDF have been varied. Rigid anterior plate fixation demonstrated high fusion rates and satisfactory clinical outcomes [1,9-16]. Yet other studies resulted in concerning failure rates (up to 23%) and pseudarthrosis (up to 53%) due to the increased number of interfaces requiring fusion [5,17,18]. Advocates of alternative procedures which include the use of bone morphogenetic protein (BMP), combined anterior and posterior procedure, use of autogenous iliac bone, anterior cervical corpectomy and fusion or hybrid procedures, have contended that there is difficulty obtaining complete decompression, greater postoperative micromotion, and a higher rate of suboptimal postoperative sagittal alignment following 3-level ACDF with allograft and plating alone [1,12-16,19-21].
As such, the purpose of the current study is to detail the radiographic and clinical outcomes of a series of 3-level primary ACDF procedures using a rigid construction with cortico-cancellous allograft at a single institution.
This study was approved by the Institutional Review Board (IRB) of Rush University Medical Center (IRB No. 14100606IRB01). A retrospective review was performed on a consecutive series of patients undergoing primary 3-level ACDF over a 5-year period (2008ŌĆō2013) by a single surgeon at a single institution. Exclusion criteria included revision surgeries, infections, anterior/posterior surgeries, and follow-up of less than 12 months. Demographics such as age, sex, body mass index, and Charleston Comorbidity Index were recorded. Perioperative data such as operative time, estimated blood loss, and length of stay were analyzed. Implant details, complications, and reoperations were noted. Patient-reported outcomes (PROs) were assessed using the Neck Disability Index (NDI), visual analogue scale (VAS) neck, and arm survey scores.
Surgery was performed utilizing a standard Smith-Robinson anterior approach via a left-sided transverse incision. Subperiosteal dissection both cephalad and caudal were performed, making sure not to extend beyond the midpoint of the supraand subjacent vertebral bodies. A complete discectomy was performed at each level of interest with adequate neural decompression of nerve roots and dura was achieved. Careful to not violate the subchondral bone, the cartilaginous endplates were removed. The posterior longitudinal ligament (PLL) was evaluated at the posterior annular attachments. Depending on the degree of myelopathy, the PLL was either maintained or resected. A 3-mm burr hole was created in the middle of the endplate to improve the vascularity and infuse bone marrow content from the vertebra to the graft. Distraction of the disc space was about 2 mm, which was performed by either a lamina spreader or Caspar pins. Fresh-frozen cortico-cancellous structural allograft was inserted into the disc space to achieve fusion and a ŌĆ£rigidŌĆØ anterior plate system (Eagle Plate, Depuy Synthesis, Raynham, MA, USA) was applied, ensuring that there was no violation of screws or plate into the target disc.
Anteroposterior and lateral cervical radiographs were obtained during the preoperative, immediate postoperative (2-week postoperative follow-up appointment), and final follow-up period (Fig. 1). All radiographs were analyzed to calculate and compare changes in cervical sagittal vertical axis (SVA), segmental height, and cervical lordosis between all time periods. The SVA was measured as the horizontal distance from the posterior-superior corner of C7 to a vertical line that bisected the C2 centroid. Segmental lordosis was measured using the Cobb angle between the upper endplate of the vertebral body above the fusion to the lower endplate of the endplate of the body below the fusion. Segmental height was measured from the midpoint of the superior endplate of the superior vertebral body to the midpoint of the inferior plate of the inferior body. Subsidence, the presence of the fusion device sinking into one or both of the adjacent vertebral bodies (loss of vertebral height > 2 mm), was also evaluated. Lastly, successful fusion was assessed on plain radiographs for the presence of anterior and posterior bone bridging with < 1 mm of interspinous motion on plain radiographs that were magnified 150% [22]. Additionally, fusion was determined to be present if there was < 4 mm of superjacent interspinous motion. Further analysis was performed on the patients that had loss of correction from immediate postoperative to final radiographic findings.
Radiographic measurements from preoperative, immediate postoperative, and final follow-up were compared using the Wilcoxon matched-pairs signed-rank test. Bivariate and multivariate analyses were used to identify risk factors for patient report and radiographic outcomes such as: reoperations, graft lucencies, loss of lordosis, loss of segmental height, and progressive cervical SVA. The level of significance was set at p < 0.05. The final multivariate model was selected through a backwards stepwise process in which all variables were initially included in the model and then the variables with the highest p-values were sequentially excluded from the model until only variables with p < 0.05 remained. All analyses were performed using Stata ver. 13.1 (StataCorp LP, College Station, TX, USA).
A total of 123 consecutive patients that underwent a 3-level ACDF were initially identified. Twenty-five patients were excluded because their procedures involved revisions, infections, or combined anterior/posterior approaches, or because they had follow-up of less than 1 year. In total, 98 patients met inclusion criteria for the study (Table 1). The mean age at time of surgery was 55.2┬▒6.1 years. Average follow-up was 24.3┬▒13.7 months. In total, 13 patients (13.3%) were active tobacco smokers and 12 patients had a diagnosis of diabetes mellitus (12.3%). Indication for surgery in the majority of patients included a diagnosis of either cervical spondylotic myelopathy (42%) or degenerative cervical disc disease (56%) with multilevel foraminal stenosis from disc herniations. The C4ŌĆō7 segments (72.5%) were the most common operative levels. Cortico-cancellous allograft and rigid locking plates were exclusively used (100.0%). Averages of estimated blood loss, operative time, and length of stay were 126 mL, 137 minutes, and 2.3 days, respectively. The mean American Society of Anesthesiologists physical status classification system grade was 2.3.
The mean radiographic follow-up was 23.7┬▒13.7 months. At the time of final follow-up, cervical lordosis improved by an average of 5.0┬░ (p < 0.001) and segmental height increased by an average of 3.2 mm (p < 0.001). On average, at final follow-up, segmental alignment improved by 7.1┬░ (p < 0.001) from preoperative measurements (Table 2). A diagnosis of diabetes mellitus and current tobacco smoking were not significant for differences in radiographic parameters. Of those measurements, only segmental height was found to have significantly decreased from immediate postoperative to final follow-up images (-1.7 mm, p < 0.001). The incidence of a postoperative loss of lordosis of at least 5┬░ was 23.5%. An overall gain in SVA of at least 5 mm was seen in 15.3% of patients. A loss of at least 2 mm in segmental height was seen in 40.8% of patients, while asymptomatic radiolucent lines were seen around at least one graft in 18% of patients. Of the 294 levels evaluated, 24 levels showed some level of radiolucency and lack of both anterior and posterior bone bridging on plain radiographs at final follow-up (8%). Subsidence was observed in 12 patients (12%).
ACDFs performed on C5ŌĆōT1 were more likely than ACDFs performed on other levels to show a loss of lordosis from the immediate postoperative to final follow-up images (RR, 3.5; 95% CI, 1.3ŌĆō9.1; p < 0.001) (Table 3). There were no significant incidences or risk factors associated with changes in either segmental alignment (Table 4) or segmental height loss (Table 5). Greater age was found to be protective against radiolucent lines, both at ages 50ŌĆō59 years (RR, 0.4; 95% CI, 0.2ŌĆō0.9) and greater than 60 years (RR, 0.1; 95% CI, 0.0ŌĆō0.6) (p < 0.001) (Table 6). No significant individual patient or surgical risk factors were identified in patients who gained at least 5 mm of SVA or loss segmental height of at least 2 mm.
A total of 5 patients required a reoperation (5.1%). Two patients underwent posterior laminoforaminotomy for delayed unilateral radiculopathy at the superior segments. Additionally, 2 patients underwent posterior spine fusion and instrumentation for symptomatic pseudarthrodesis at the inferior levels.
One patient underwent an uninstrumented ACDF at the caudal level for stenosis due to adjacent segment disease. Significant improvements were observed in NDI (preoperative: 52.3┬▒19.6 vs. postoperative: 22.5┬▒24.2; p < 0.001), VAS-neck (preoperative: 7.5┬▒3.3 vs. postoperative: 2.2┬▒2.3; p < 0.001), and VAS-arm (preoperative: 5.3┬▒4.2 vs. postoperative: 1.4┬▒2.5; p < 0.001) survey scores from preoperative to final follow-up values (Table 7).
ACDF with anterior plates is a common and successful means of addressing the degenerative process of the cervical spine. For multilevel disease, a variety of surgical options have been developed to address concerns regarding cervical alignment, risks of pseudarthrosis, and adjacent segment disease. Anterior cervical plating has been shown to maintain cervical lordosis and is associated with improved clinical outcomes when compared to non-plated constructs [23].
This study presents relevant clinical and radiographic data regarding a large population undergoing primary 3-level ACDF with a rigid-plating system and cortico-cancellous allograft. On average, there were significant improvements in cervical lordosis, segmental height, and segmental alignment with little change in cervical SVA and a low rate of reoperations. Only segmental height was shown to decrease from immediate postoperative to final follow-up images, suggesting that there is subsidence even with the use of the rigid plate. Zhou et al. [10] identified 15 consecutive patients that underwent a 3-level ACDF with polyetheretherketone (PEEK) cages. They found that 13 of the 15 patients achieved solid fusion at an average time of 5.7 months while also effectively correcting cervical lordosis. Similar findings were demonstrated by Song et al. [9] in 43 consecutive patients that underwent 3- or 4-level ACDFs with PEEK cage and semirigid plate construct. Significant improvements were shown in postoperative Cobb angle, lordotic angle, and disk height that were maintained through the final follow-up. The overall improvements in cervical alignment and low reoperation rates, using a rigid anterior plate and cortico-cancellous allograft, found in this study are consistent with that of previous studies [1,12-16].
The clinical implications of subsidence continue to be a topic of contemporary research. Previous risk factors for subsidence occurring in ACDFs include smaller interbody footprint size, possible endplate violation, and fusion of more than 2 levels [23-25]. In addition, Chen et al. [25] reported that cage subsidence was more common with titanium cages than PEEK cages in patients with 3-level CSM undergoing ACDF. Cage subsidence has been associated with increased cervical kyphosis, foraminal stenosis, instability, and ultimately pseudarthrosis [26,27]. However, others have suggested that subsidence does not significantly impact successful fusion or clinical outcome in patients undergoing ACDF, irrespective of the measurement technique or term definition [10,14,24]. The current study observed a moderate rate of subsidence, but could not identify any patient or surgical risk factors for subsidence. Subsidence may not per se be entirely detrimental if settling of the cage leads to containment of the bone and subsequent healing. The amount of subsidence may be clinically relevant, in that if there is excessive subsidence before healing of the graft, recurrent foraminal stenosis, kyphosis, and pseudarthrosis might result. Rigid plating does not appear to prevent subsidence but it may lessen the amount of subsidence. It is important to note, even in setting of these radiographic subsidence observations, clinical outcome may not be affected, as NDI, VAS-arm, VAS-neck scores all significantly improved from preoperative scores.
The clinical implications of cervical malalignment have become increasingly discussed. Correlations have been found between sagittal alignment (as measured by the SVA) and functional outcomes following ACDFs [28-31]. Our study showed that ACDFs performed on C5ŌĆōT1 were more likely than ACDFs performed on other levels to show a loss of lordosis from the immediate postoperative to final follow-up images. The decreased lordosis between C5ŌĆōT1 following surgery may be associated to the anatomy of the cervicothoracic junction, in which, there is more loading and stresses at the lower cervical and the cervicothoracic junction of a mobile, lordotic cervical spine and rigid, kyphotic thoracic region. [32] Roguski et al. [31] found that cervical sagittal balance was an independent predictor of health-related quality of life (HRQoL) in patients undergoing decompressive surgery for cervical spondylotic myelopathy; patients with postoperative C2ŌĆō7 SVA values greater than 40 mm did not show improvements in scores on the Short-Form 36 physical component summary, despite improvements in myelopathy after surgery. Similarly, Uchida et al. [28] reported significantly improved postoperative kyphotic angles following anterior cervical surgery that correlated with improved Japanese Orthopedic Association functional outcomes scores. The sagittal imbalance may independently manifest as neck pain, muscle fatigue, dysphagia, and alterations in visual horizon. [29]. We found cervical lordosis can improve following a 3-level ACDF with minimal effect on the cervical SVA. In the setting of the radiographic parameters described in this study, patients reported significant improvements in their clinical survey outcome scores.
Allograft choices are abundant, with options of cortical or cancellous, as well as fresh, frozen or freeze-dried options. Frozen cortico-cancellous allograft was used in all patients in order to optimize the biomechanics and healing of the graft. As previously mentioned, a puncture hole is created in the endplate with goals to enhance vascularity and allow incorporation of bone marrow cells and protein which support osteoinductive and osteogenic activity. Acceptable fusion rates and restoration/maintenance of sagittal balance can be achieved in a 3-level ACDF without the use of more adjuncts (i.e., bone morphogenic protein, demineralized bone matrix) or a combination anteriorposterior cervical fusion, which can significantly increase the cost and introduce additional complications to the surgery [33-35]. At the final follow-up, a high incidence of radiographic lucencies was seen around the interbodies within the younger patient population. One explanation for this may be that the lower activity levels of older patients may play a protective role against delayed healing from increased motion. There is a paucity of orthopaedic literature regarding radiographic lucencies following ACDF procedures. The implications of these radiolucencies remain unclear as none of the patients with radiolucencies required a reoperation within our follow-up period. Similarly, PROs were significantly improved as measured by NDI, VAS-neck, and VAS-arm scores, despite the radiolucencies observed.
There are several limitations to the present study. There was no control group to which outcomes could be compared. We considered including other surgical techniques for cervical spondylotic myelopathy as a control, but this cohort would have necessarily consisted of a heterogenous patient population with fewer numbers and would have further confused results. In a sense, the current study population functioned as its own control group during the subanalysis. Additional radiographic changes that may occur outside of our follow-up period would not have been captured in this study. Additionally, given the retrospective nature of this study, follow-up time was not standardized for the group and each patientŌĆÖs final radiographic outcome date was variable. This series represents the experiences of one institution and therefore the results may not be generalizable to other surgeons or hospitals. Although the follow-up period may be sufficient to assess for fusion, symptomatic nonunions and subsequent subsidence may present later on. We will continue to follow this patient population and assess for any future presentations of symptomatic nonunions/subsidence.
Three-level ACDF with rigid-plating and cortico-cancellous allograft is an effective procedure for degenerative diseases of the cervical spine without the application of additional adjuncts or combined anteriorposterior cervical surgeries. Significant improvements in cervical lordosis, segmental height, and segmental alignment can be achieved with little change in cervical SVA and a low rate of reoperations over short-term follow-up. Similarly, PROs show significant improvements following these 3-level ACDF procedures.
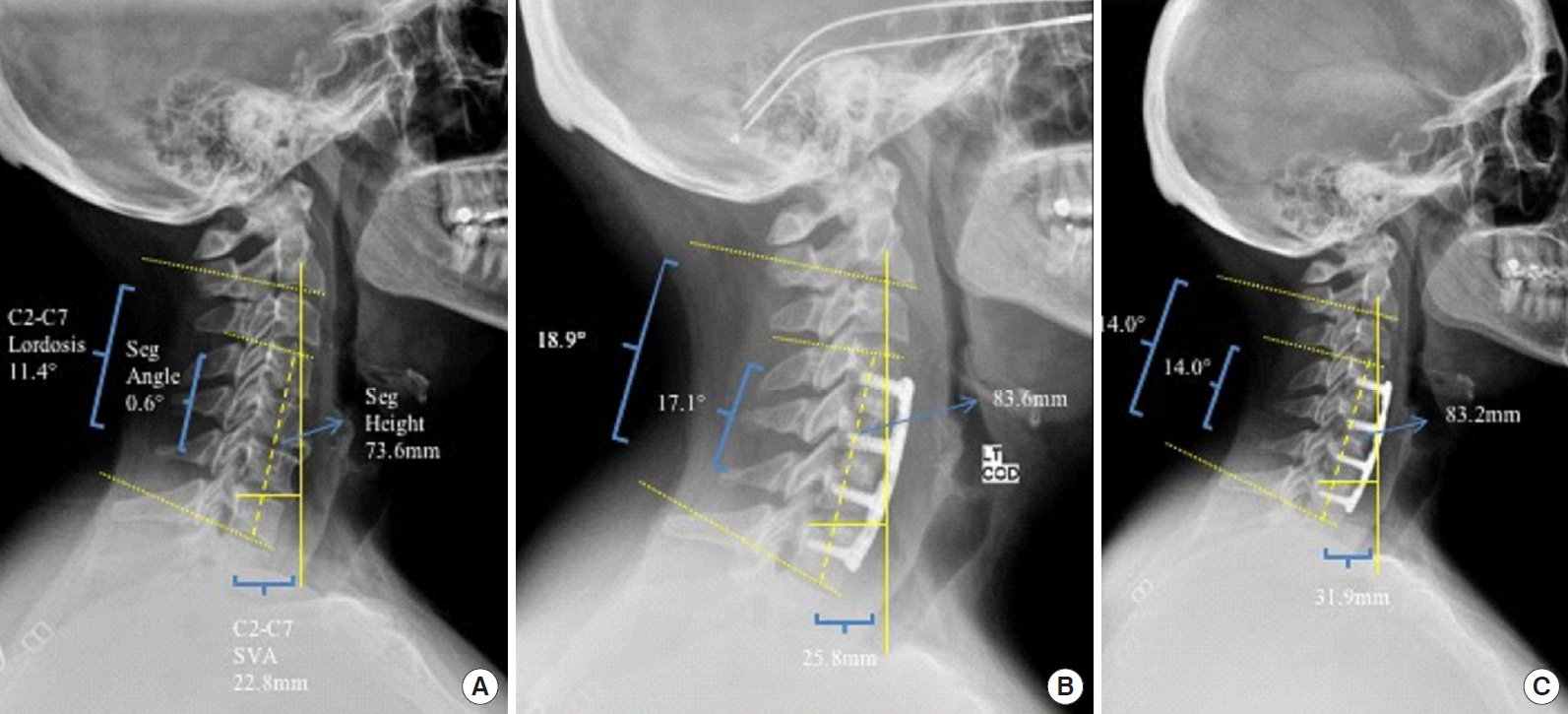
Fig.┬Ā1.
Lateral cervical spine radiographics obtained preoperatively (A), 2 weeks postoperatively (B), and the final follow-up (18 months) (C). Radiographic parameters measured include C2ŌĆō7 lordosis, segmental angle (seg angle), C2ŌĆō7 sagittal vertical axis (SVA), and segmental height (seg height).
Table┬Ā1.
Patient population (n=98)
| Variable | Value |
|---|---|
| Age (yr) | |
| ŌĆā18ŌĆō49 | 32 (32.7) |
| ŌĆā50ŌĆō59 | 35 (35.7) |
| ŌĆāŌēź 60 | 31 (31.6) |
| Current smoker | 13 (13.3) |
| Diabetes mellitus | 12 (12.2) |
| Levels | |
| ŌĆāC3ŌĆō6 | 23 (23.5) |
| ŌĆāC4ŌĆō7 | 71 (72.5) |
| ŌĆāC5ŌĆōT1 | 4 (4.1) |
| Allograft | |
| ŌĆāYes | 98 (100.0) |
| Graft type | |
| ŌĆāBiologic | 98 (100.0) |
| ŌĆāFusion | 95 (96.9) |
| Body mass index (kg/m2) | 29.3 ┬▒ 7.2 |
| Charleston Comorbidity Index | 2.29 ┬▒ 2.29 |
| Operative time (min) | 150 ┬▒ 32 |
| Estimated blood loss (mL) | 111 ┬▒ 130 |
| Length of stay* (day) | 1.84 ┬▒ 0.88 |
Table┬Ā2.
Radiographic measurements and differences between radiographic measurements over time
| Variable |
Radiographic measurements |
Differences between radiographic measurements over time |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Preop. | Immediate postop. | Latest follow-upŌĆĪ (mo) |
Preop. to immediate postop. |
Preop. to latest follow-up |
Immediate postop. to latest follow-up |
||||
| Diff. | p-valueŌĆĀ | Diff. | p-valueŌĆĀ | Diff. | p-valueŌĆĀ | ||||
| Lordosis | 4.3 ┬▒ 9.9 | 10 ┬▒ 9.3 | 9.2 ┬▒ 8.6 | +5.5 | < 0.001* | +5.0 | < 0.001* | -0.5 | 0.211 |
| Sagittal vertical axis | 28.1 ┬▒ 12.2 | 31.3 ┬▒ 12 | 30.2 ┬▒ 11.5 | +3.2 | < 0.001* | +1.7 | 0.042* | -1.4 | 0.060 |
| Segmental angle | 0.3 ┬▒ 9.2 | 8.8 ┬▒ 6.8 | 7.4 ┬▒ 6.7 | +8.4 | < 0.001* | +7.1 | < 0.001* | -1.4 | 0.010* |
| Segmental height | 70.7 ┬▒ 7.4 | 75.8 ┬▒ 7.4 | 74.1 ┬▒ 7.5 | +5.0 | < 0.001* | +3.2 | < 0.001* | -1.7 | < 0.001* |
Table┬Ā3.
Incidence and risk factors for loss of lordosis of 5┬░ or more from immediate postoperative to final follow-up
| Variable | Incidence (%) |
Bivariate analyses |
Multivariate analysisŌĆĀ |
||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | ||
| Overall | 23.5 | ||||||
| Age (yr) | 0.630 | > 0.05 | |||||
| ŌĆā18ŌĆō49 | 18.8 | Reference | NA | ||||
| ŌĆā50ŌĆō59 | 22.9 | 1.2 | 0.5ŌĆō3.1 | ||||
| ŌĆāŌēź 60 | 29.0 | 1.5 | 0.6ŌĆō3.9 | ||||
| Levels | < 0.001* | < 0.001* | |||||
| ŌĆāC3ŌĆō6 | 21.7 | Reference | NA | ||||
| ŌĆāC4ŌĆō7 | 21.1 | 1.0 | 0.4ŌĆō2.4 | 1.0 | 0.4ŌĆō2.4 | ||
| ŌĆāC5ŌĆōT1 | 75.0 | 3.5 | 1.3ŌĆō9.1 | 3.5 | 1.3ŌĆō9.1 | ||
| Diabetes | > 0.05 | ||||||
| ŌĆāNo | 19.7 | Reference | NA | ||||
| ŌĆāYes | 25.0 | 1.1 | 0.4ŌĆō2.7 | 0.491 | |||
| Current smoker | > 0.05 | ||||||
| ŌĆāNo | 20.0 | Reference | NA | ||||
| ŌĆāYes | 30.7 | 1.1 | 0.5ŌĆō2.5 | 0.301 | |||
Table┬Ā4.
Incidence and risk factors for gain of sagittal vertical axis of 5 mm or more from immediate postoperative to final follow-up
| Variable | Incidence (%) |
Bivariate analyses |
Multivariate analysisŌĆĀ |
||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | ||
| Overall | 15.3 | ||||||
| Age (yr) | 0.710 | > 0.05 | |||||
| ŌĆā18ŌĆō49 | 18.8 | Reference | NA | ||||
| ŌĆā50ŌĆō59 | 11.4 | 0.6 | 0.2ŌĆō2.0 | ||||
| ŌĆāŌēź 60 | 16.1 | 0.9 | 0.3ŌĆō2.5 | ||||
| Levels | 0.790 | > 0.05 | |||||
| ŌĆāC3ŌĆō6 | 17.4 | Reference | NA | ||||
| ŌĆāC4ŌĆō7 | 14.1 | 0.8 | 0.3ŌĆō2.4 | ||||
| ŌĆāC5ŌĆōT1 | 25.0 | 1.4 | 0.2ŌĆō9.9 | ||||
| Plate type | 0.707 | > 0.05 | |||||
| ŌĆāBiomet | 21.7 | Reference | NA | ||||
| ŌĆāDepuy eagle | 11.6 | 0.5 | 0.2ŌĆō1.7 | ||||
| ŌĆāDepuy sky | 12.5 | 0.6 | 0.1ŌĆō2.6 | ||||
| Diabetes | > 0.05 | ||||||
| ŌĆāNo | 13.9 | Reference | NA | ||||
| ŌĆāYes | 25.0 | 1.2 | 0.4ŌĆō3.1 | 0.301 | |||
| Current smoker | > 0.05 | ||||||
| ŌĆāNo | 14.5 | Reference | NA | ||||
| ŌĆāYes | 30.7 | 1.3 | 0.4ŌĆō3.3 | 0.214 | |||
Table┬Ā5.
Incidence and risk factors for loss of segmental height of 2 mm or more from immediate postoperative to final follow-up
| Variable | Incidence (%) |
Bivariate analysis |
Multivariate analysisŌĆĀ |
||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | ||
| Overall | 40.8 | ||||||
| Age (yr) | 0.672 | > 0.05 | |||||
| ŌĆā18ŌĆō49 | 34.4 | Reference | NA | ||||
| ŌĆā50ŌĆō59 | 42.9 | 1.2 | 0.7ŌĆō2.3 | ||||
| ŌĆāŌēź 60 | 45.2 | 1.3 | 0.7ŌĆō2.4 | ||||
| Levels | 0.087 | > 0.05 | |||||
| ŌĆāC3ŌĆō6 | 47.8 | Reference | NA | ||||
| ŌĆāC4ŌĆō7 | 36.6 | 0.8 | 0.5ŌĆō1.3 | ||||
| ŌĆāC5ŌĆōT1 | 75.0 | 1.6 | 0.8ŌĆō3.2 | ||||
| Plate type | 0.358 | > 0.05 | |||||
| ŌĆāBiomet | 39.1 | Reference | NA | ||||
| ŌĆāDepuy eagle | 46.5 | 1.2 | 0.6ŌĆō2.2 | ||||
| ŌĆāDepuy sky | 18.8 | 0.5 | 0.2ŌĆō1.5 | ||||
| Diabetes | > 0.05 | ||||||
| ŌĆāNo | 38.4 | Reference | NA | ||||
| ŌĆāYes | 41.7 | 1.1 | 0.6ŌĆō2.4 | 0.424 | |||
| Current smoker | > 0.05 | ||||||
| ŌĆāNo | 36.5 | Reference | NA | ||||
| ŌĆāYes | 46.2 | 1.2 | 0.6ŌĆō2.8 | 0.109 | |||
Table┬Ā6.
Incidence and risk factors for radiolucent lines around graft at final follow-up
| Variable | Incidence (%) |
Bivariate analysis |
Multivariate analysisŌĆĀ |
||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | ||
| Overall | 18.0 | ||||||
| Age | 0.023* | 0.023* | |||||
| ŌĆā18ŌĆō49 | 23.9 | Reference | Reference | ||||
| ŌĆā50ŌĆō59 | 9.8 | 0.5 | 0.3ŌĆō1.2 | 0.5 | 0.2ŌĆō1.0 | ||
| ŌĆāŌēź 60 | 6.5 | 0.3 | 0.1ŌĆō0.9 | 0.3 | 0.1ŌĆō0.9 | ||
| Levels | 0.946 | > 0.05 | |||||
| ŌĆāC3ŌĆō6 | 17.1 | Reference | NA | ||||
| ŌĆāC4ŌĆō7 | 18.6 | 1.0 | 0.4ŌĆō2.0 | ||||
| ŌĆāC5ŌĆōT1 | 19.2 | 1.1 | 0.3ŌĆō5.1 | ||||
| Plate type | 0.175 | > 0.05 | |||||
| ŌĆāBiomet | 19.6 | Reference | NA | ||||
| ŌĆāDepuy eagle | 17.9 | 0.8 | 0.3ŌĆō1.3 | ||||
| ŌĆāDepuy sky | 16.2 | 0.7 | 0.1ŌĆō1.5 | ||||
| Diabetes | > 0.05 | ||||||
| ŌĆāNo | 16.3 | Reference | NA | ||||
| ŌĆāYes | 25.0 | 1.3 | 0.4ŌĆō2.2 | 0.878 | |||
| Current Smoker | > 0.05 | ||||||
| ŌĆāNo | 14.1 | Reference | NA | ||||
| ŌĆāYes | 30.7 | 1.6 | 0.3ŌĆō3.6 | 0.091 | |||
REFERENCES
1. Han YC, Liu ZQ, Wang SJ, et al. Is anterior cervical discectomy and fusion superior to corpectomy and fusion for treatment of multilevel cervical spondylotic myelopathy? A systemic review and meta-analysis. PLoS One 2014 9:e87191.



2. Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg 1996 85:206-10.


3. Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 1993 75:1298-307.


4. Riley LH Jr, Robinson RA, Johnson KA, et al. The results of anterior interbody fusion of the cervical spine. Review of ninety-three consecutive cases. J Neurosurg 1969 30:127-33.


5. Bolesta MJ, Rechtine GR 2nd, Chrin AM. Three- and four-level anterior cervical discectomy and fusion with plate fixation: a prospective study. Spine (Phila Pa 1976) 2000 25:2040-4.


6. Vaccaro AR, Falatyn SP, Scuderi GJ, et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord 1998 11:410-5.


7. Rao RD, Gourab K, David KS. Operative treatment of cervical spondylotic myelopathy. J Bone Joint Surg Am 2006 88:1619-40.


8. Wu WJ, Jiang LS, Liang Y, et al. Cage subsidence does not, but cervical lordosis improvement does affect the long-term results of anterior cervical fusion with stand-alone cage for degenerative cervical disc disease: a retrospective study. Eur Spine J 2012 21:1374-82.



9. Song KJ, Yoon SJ, Lee KB. Three- and four-level anterior cervical discectomy and fusion with a PEEK cage and plate construct. Eur Spine J 2012 21:2492-7.




10. Zhou J, Li X, Dong J, et al. Three-level anterior cervical discectomy and fusion with self-locking stand-alone polyetheretherketone cages. J Clin Neurosci 2011 18:1505-9.


11. Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J 2003 3:451-9.


12. Guo Q, Bi X, Ni B, et al. Outcomes of three anterior decompression and fusion techniques in the treatment of three-level cervical spondylosis. Eur Spine J 2011 20:1539-44.




13. Shamji MF, Massicotte EM, Traynelis VC, et al. Comparison of anterior surgical options for the treatment of multilevel cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976) 2013 38(22 Suppl 1):S195-209.


14. Hwang SL, Lin CL, Lieu AS, et al. Three-level and four-level anterior cervical discectomies and titanium cage-augmented fusion with and without plate fixation. J Neurosurg Spine 2004 1:160-7.


15. Lin Q, Zhou X, Wang X, et al. A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J 2012 21:474-81.



16. Liu Y, Hou Y, Yang L, et al. Comparison of 3 reconstructive techniques in the surgical management of multilevel cervical spondylotic myelopathy. Spine 2012 37:E1450-8.


17. Lowery GL, McDonough RF. The significance of hardware failure in anterior cervical plate fixation. Patients with 2- to 7-year follow-up. Spine (Phila Pa 1976) 1998 23:181-6.


18. Paramore CG, Dickman CA, Sonntag VK. Radiographic and clinical follow-up review of Caspar plates in 49 patients. J Neurosurg 1996 84:957-61.


19. Shen HX, Buchowski JM, Yeom JS, et al. Pseudarthrosis in multilevel anterior cervical fusion with rhBMP-2 and allograft: analysis of one hundred twenty-seven cases with minimum two-year follow-up. Spine (Phila Pa 1976) 2010 35:747-53.


20. Fraser JF, H├żrtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine 2007 6:298-303.


21. Bayley JC, Yoo JU, Kruger DM, et al. The role of distraction in improving the space available for the cord in cervical spondylosis. Spine (Phila Pa 1976) 1995 20:771-5.


22. Song KS, Piyaskulkaew C, Chuntarapas T, et al. Dynamic radiographic criteria for detecting pseudarthrosis following anterior cervical arthrodesis. J Bone Joint Surg Am 2014 96:557-63.


23. Kao TH, Wu CH, Chou YC, et al. Risk factors for subsidence in anterior cervical fusion with stand-alone polyetheretherketone (PEEK) cages: a review of 82 cases and 182 levels. Arch Orthop Trauma Surg 2014 134:1343-51.




24. Karikari IO, Jain D, Owens TR, et al. Impact of subsidence on clinical outcomes and radiographic fusion rates in anterior cervical discectomy and fusion: a systematic review. J Spinal Disord Tech 2014 27:1-10.


25. Chen Y, Wang X, Lu X, et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7-year follow-up. Eur Spine J 2013 22:1539-46.




26. Siddiqui AA, Jackowski A. Cage versus tricortical graft for cervical interbody fusion. A prospective randomised study. J Bone Joint Surg Br 2003 85:1019-25.


27. Fujibayashi S, Neo M, Nakamura T. Stand-alone interbody cage versus anterior cervical plate for treatment of cervical disc herniation: sequential changes in cage subsidence. J Clin Neurosci 2008 15:1017-22.


28. Uchida K, Nakajima H, Sato R, et al. Cervical spondylotic myelopathy associated with kyphosis or sagittal sigmoid alignment: outcome after anterior or posterior decompression. J Neurosurg Spine 2009 11:521-8.


29. Fehlings MG, Gray R. Importance of sagittal balance in determining the outcome of anterior versus posterior surgery for cervical spondylotic myelopathy. J Neurosurg Spine 2009 11:518-9.


30. Villavicencio AT, Babuska JM, Ashton A, et al. Prospective, randomized, double-blind clinical study evaluating the correlation of clinical outcomes and cervical sagittal alignment. Neurosurgery 2011 68:1309-16.



31. Roguski M, Benzel EC, Curran JN, et al. Postoperative cervical sagittal imbalance negatively affects outcomes after surgery for cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2014 39:2070-7.



32. Louie PK, Presciutti SM, Iantorno SE, et al. There is no increased risk of adjacent segment disease at the cervicothoracic junction following an anterior cervical discectomy and fusion to C7. Spine J 2017 17:1264-71.


33. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011 11:471-91.






























