Rabbit Annulus Fibrosus Cells Express Neuropeptide Y, Which Is Influenced by Mechanical and Inflammatory Stress
Article information
Abstract
Objective
Rabbit annulus fibrosus (AF) cells were exposed to isolated or combined mechanical and inflammatory stress to examine the expression of neuropeptide Y (NPY). This study aims to explore the ability of AF cells to produce NPY in response to mechanical and inflammatory stress.
Methods
Lumbar AF cells of 6- to 8-month-old female New Zealand white rabbits were harvested and exposed to combinations of inflammatory (interleukin-1β) and mechanical (6% or 18%) tensile stress using the Flexcell System. NPY concentrations were measured in the media via enzyme-linked immunosorbent assay. The presence of NPY receptor-type 1 (NPY-1R) in AF cells of rabbit intervertebral discs was also analyzed via immunohistochemistry and immunofluorescence.
Results
Exposure to inflammatory stimuli showed a significant increase in the amount of NPY expression compared to control AF cells. Mechanical strain alone did not result in a significant difference in NPY expression. While combined inflammatory and mechanical stress did not demonstrate an increase in NPY expression at low (6%) levels of strain, at 18% strain, there was a large—though not statistically significant—increase in NPY expression under conditions of inflammatory stress. Lastly, immunofluorescence and immunohistochemistry of AF cells and tissue, respectively, demonstrated the presence of NPY-1R.
Conclusion
These findings demonstrate that rabbit AF cells are capable of expressing NPY, and expression is enhanced in response to inflammatory and mechanical stress. Because both inflammatory and mechanical stress contribute to intervertebral disc degeneration (IDD), this observation raises the potential of a mechanistic link between low back pain and IDD.
INTRODUCTION
Chronic back pain is one of the most ubiquitous and enigmatic clinical entities causing patients to present to the healthcare system [1], affecting upward of 80% of individuals worldwide at some point in their lives [2]. Furthermore, back pain has both a devastating functional and financial impact on society, costing the United States alone more than $100 billion in direct and indirect costs annually [3]. Unfortunately, this number is likely to increase as the world’s population continues to age [4]. Discogenic back pain, back pain arising from the intervertebral disc (IVD), is a widely known contributor to back pain and is particularly difficult to treat clinically. Despite promising research into the pathogenesis, diagnosis, and potential treatment of discogenic back pain, it continues to be a pervasive problem with devastating socioeconomic effects.
It is clear that chronic and excessive loading of the spine with and without inflammatory mediators have a role in the progression of intervertebral disc degeneration (IDD) [5–7]. Interleukin-1 (IL-1), an inflammatory mediator, has been linked to IDD with increased expression demonstrated in degenerated disc tissue [8]. Both beneficial and deleterious responses have been seen in response to mechanical loading, including matrix production, matrix degradation, proteoglycan synthesis, and inflammatory modulation. These cellular pathways are highly dependent on age, frequency, and magnitude of cell loading [9,10]. However, determining how these cellular responses to inflammatory and mechanical stress relates to the experience of pain is more complex and currently poorly understood. This is supported by the fact that despite the widespread nature of IDD on imaging studies, not all patients experience pain-related symptoms [11,12]. Consequently, understanding how nociception is modulated in symptomatic disc degeneration is vital for the appropriate treatment of IDD clinically.
Pain producing cytokines or neuromodulator proteins represent a potential mechanistic link between the degenerative cascade and the experience of pain-related symptoms. Neuropeptide Y (NPY) is a 36 amino acid peptide, first isolated and sequenced in 1982, with known nociceptive function in both the central and peripheral nervous systems [13]. NPY has been shown to play an important role in stressful and painful conditions including posttraumatic stress disorder (PTSD) [14], fibromyalgia [15], osteoarthritis [16], neck, and back pain [17]. Additionally, NPY has been previously shown to be present in the sympathetic nerve fibers in the annulus fibrosus (AF) of rat lumbar spines [18] and human degenerated discs, making it of particular interest in IDD [19]. Most recently, it was demonstrated that variations in systemic serum NPY concentrations were correlated with back pain and pain-related function in individuals with axial low back pain [20], and thought to potentially serve as a biomarker for active discogenic pain. However, further investigation is needed to determine if IVD cells are capable of producing or responding to neuromodulating proteins, and if and how they interact with inflammatory and mechanical signaling cascades that coexist in vivo.
Several different receptors of NPY are expressed in mammalian tissue [21,22]. Specifically, the NPY receptor-type 1 (NPY-1R) has shown to be expressed in neurons located in the rat dorsal root ganglion [23] as well as tenocytes of the Achilles tendon [24]. Increases in NPY-1R expression and signaling have been demonstrated after peripheral nerve injury and inflammatory stress [25], suggesting an autocrine or paracrine effect of NPY on pain modulation [26]. However, the presence and role of NPY-1R in IVD tissue has yet to be identified.
It has been shown that detrimental mechanical stress and inflammation have a negative synergistic effect on the catabolic cascade, causing altered disc cell function, increased catabolic enzymes, and decreased matrix synthesis [7,27]. However, determining how these same conditions affect nociceptive pathways is unclear. This study aimed first to determine if AF cells can express NPY, and subsequently to determine the expression profile of NPY in response to varying degrees of isolated or combined mechanical and inflammatory stress. Additional objectives were to investigate the presence and location of NPY-1R in rabbit AF cells. We hypothesized that NPY increases in response to mechanical loading and inflammatory stimulation, which are similar conditions known to create a catabolic metabolic environment within the disc [9,27], and that NPY-1R expression would be present.
MATERIALS AND METHODS
1. NPY Expression by AF Cells in Response to Mechanical and Inflammatory Stress
This study was approved by the Institutional Animal Care and Use Committee at the institution of the principal investigator. We examined the response of rabbit AF cells to inflammatory stress alone as well as different magnitudes of applied tensile strain. The tensile strain was applied in both a noninflammatory and inflammatory environment. NPY expression was measured as the ratio of NPY expression to total protein expression. Data were then compared to unloaded control AF cells.
Lumbar IVD tissue was extracted from female New Zealand white rabbits (age 6 to 8 months) immediately after sacrifice to yield independent cell culture preparations. Whole AF tissue (including both inner and outer AF) was carefully dissected from lumbar IVDs and digested with 0.2% pronase, followed by 0.02% collagenase to yield isolated AF fibrochondrocytes. Fibrochondrocytes were cultured in F-12, 10% fetal bovine serum (FBS), 1% penicillin-streptomycin at 37°C, and 5% CO2 until 90% confluence, as previously described [28]. Primary cells were transferred to flexible culture plates coated with collagen I (BF-3001C, Bioflex, Flexcell International Corp., Burlington, NC, USA) at a concentration of 300,000 cells per well and allowed to increase for 3 days. Sixteen hours before experiments, media were changed to F-12, 1% FBS, and 1% penicillin-streptomycin to facilitate biological assays. The tensile strain was then applied at 0.5 Hz for 24 hours, at 2 different levels of strain: 6% and 18% using the Flexcell Tension Plus System (FX-3000, Flexcell International Corp.), previously shown to be anti-inflammatory and pro-catabolic, respectively (Fig. 1) [9]. Cyclic tensile strain at this level has been shown previously not to change AF cell adhesion or morphology [28]. For those conditions with inflammatory stress, cells were preincubated for 30 minutes with 1-ng/mL recombinant IL-1β (201-LB/CF, R&D Systems, Minneapolis, MN, USA).
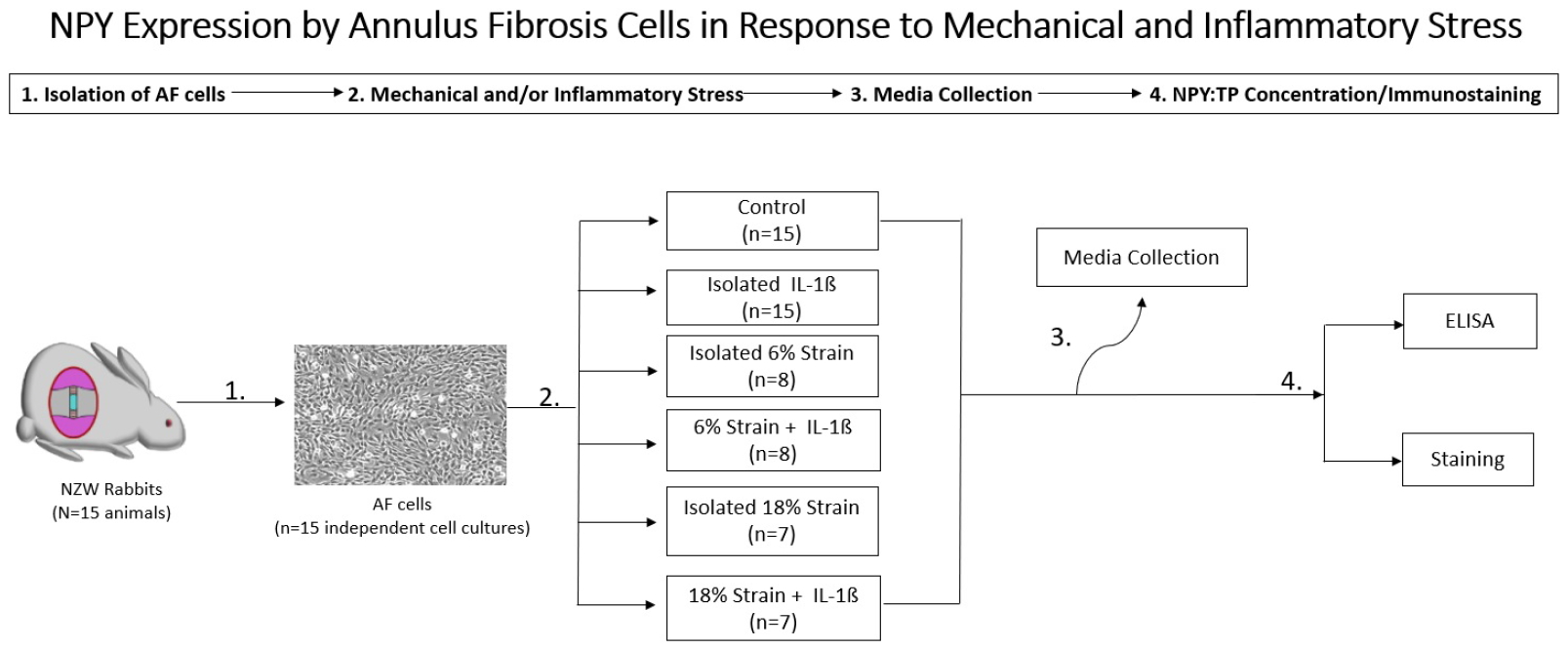
Experimental design to measure neuropeptide Y (NPY) expression by annulus fibrosus cells in response to mechanical and inflammatory stress. AF, annulus fibrosus; TP, total protein; NZW, New Zealand white; ELISA, enzyme-linked immunosorbent assay.
Unstretched controls were maintained under identical conditions on unstretched flexible plates. Media was collected from all samples after 24 hours of strain application, analyzed for NPY using a commercially available enzyme-linked immunosorbent assay kit (Ray Biotech, Inc., Norcross, GA, USA), and normalized by total protein concentration using bicinchoninic acid assay as per the manufacturer’s instructions (Pierce BCA Protein Assay Kit, 23225, ThermoFisher Scientific, Rockford, IL, USA). Values represent the average of trials from independent cell isolations consisting of pooled lumbar discs from independent animals. There were 8 independent cell isolations tested under conditions of 6% strain and 7 independent cell isolations tested under conditions of 18% strain. Each cell culture subjected to inflammation and/or strain was compared to the unstretched, unstimulated control cells isolated from the same rabbit. Data was reported as a ratio of NPY to total protein, and groups were compared using paired, 2-tailed Student t-tests with statistical significance set at the p < 0.05 level.
2. Whole Disc Immunohistochemistry for NPY-1R
Whole lumbar discs were harvested from a single New Zealand white rabbit then decalcified in Decalcifier I solution at 4°C for 2 weeks. The disc tissues were dehydrated through a graded alcohol series and then embedded in paraffin (Tissue Tek processor and Leica embedder, Buffalo Grove, IL, USA). They were cut in the sagittal plane at 6-μm thickness with a microtome and mounted on Superfrost Plus microscope slides (ThermoFisher Scientific). For immunohistochemistry, detection of NPY-1R was performed on the deparaffinized disc tissue section. The sections were pretreated with chondroitinase ABC (0.25 U/mL; Sigma, St. Louis, MO, USA) for 1 hour at 37°C to disrupt tissue surface glycoconjugates. The pretreatment eliminated endogenous peroxidase activity with 3% hydrogen peroxide. Permeabilization and blocking were done with 10% goat serum, 1% BSA, and 0.25% triton X-100 in PBS for 30 minutes. The primary antibody against NPY-1R (1:50, ab35336, Abcam plc, Cambridge, UK) was applied overnight at 4°C. The sections were thoroughly washed with 0.05% Tween20 in PBS. Biotinylated goat anti-rabbit secondary antibody (1:200, BA-1000, Vector Laboratories, Burlingame, CA, USA) was applied for 30 minutes, and the sections were rewashed with 0.05% Tween20 in PBS. This was followed by avidin-biotin amplification (ABC Elite, Vector Laboratories) for 30 minutes. AEC substrate/chromogen Kit (ScyTek Laboratoties, Inc., Logan, UT, USA) was used for 10 minutes and rinsed in running water for 5 minutes. Hematoxylin was used as a counterstain. All pictures were collected on a Nikon E800 microscope (Nikon, Tokyo, Japan) at 40x to 100X magnification. Rabbit AF without primary antibody was used as negative controls. Stained cells were counted and divided by the total number of cells noted in the outer AF of the stained discs to determine a rate of positivity.
3. AF Cell Immunofluorescence for NPY-1R
NPY-1R was visualized on AF cells by immunofluorescent labeling using anti-NPY-1R antibodies. The rabbit AF cells were cultured in 8-well chamber slides (Nunc lab-Tek II, Chamber Slide System, ThermoFisher Scientific) with F-12, 10% FBS, 1% penicillin-streptomycin at 37°C, and 5% CO2 until 50% confluence. The AF cells were fixed in 4% paraformaldehyde for 15 minutes at room temperature. After washing in PBS, they were incubated with 10% goat serum, 1% BSA, and 0.1% Triton X-100 in PBS at room temperature for 30 minutes. Then AF cells were incubated with sheep anti-NPY-1R antibody (1:200, ab35336, Abcam plc) applied overnight at 4°C. Next, the cells were washed twice with 1x PBS and incubated with the donkey polyclonal secondary antibody to sheep IgG (1:500, Alexa Fluor 594, ab150180, Abcam plc) at a dilution of 1:500 for 60 minutes at room temperature. At last, they were counterstained with DAPI (4’,6-diamidino-2-phenylindole) at a dilution of 1:500. Fluorescence analysis was performed with a microscope (Eclipse TE2000-U Inverted Microscope, Nikon).
AF cells incubated with secondary, but no primary antibody was used as a negative control. Jurkat cells, an immortalized line of human T lymphocytes, were used as a control and incubated with the primary and secondary antibody as described above to establish antibody specificity.
RESULTS
1. NPY Expression Response to Inflammatory or Mechanical Stress
Under inflammatory stress alone, there was a significant increase in the amount of NPY expression compared to control cells (p = 0.031) (Fig. 2). In contrast, under mechanical stress alone (Fig. 2), neither 6% strain (p = 0.265) nor 18% strain (p = 0.543) resulted in a significant increase in NPY expression compared to unloaded control.

Neuropeptide Y (NPY) expression by annulus fibrosus (AF) cells in response to inflammatory, mechanical, or combined stress. Values represented as a ratio of NPY concentration to total protein concentration (NPY: total protein). Bars indicate mean values with error bars representing one standard error. NPY expression for each condition was compared to control cells and between stressful conditions. *p <0.05. C, control; S, strain; IL, interleukin-1β.
2. Expression of NPY in AF Cells in Response to Combined Mechanical and Inflammatory Stress
While combined inflammatory and mechanical stress did not demonstrate an increase in NPY expression at low (6%) levels of strain, at 18% strain there was a large—though not statistically significant—increase in NPY expression under conditions of inflammatory stress (p = 0.154).
3. AF and Whole Disc Immunostaining for NPY-1R
Immunohistochemistry showed positive staining for NPY1R on rabbit whole disc, with specificity confirmed by negative controls without primary antibody for NPY-1R. Abundant expression of NPY-1R was most pronounced in the most peripheral layers of the outer AF (Fig. 3, right). Moderate expression was noted in the cartilaginous endplate. Minimal to no expression of NPY-1R was detected in the nucleus pulposus or inner AF. The overall rate of positivity of cells in the outer AF was found to be 53%. Again, most positive cells were located in the most peripheral layers of the outer AF. NPY-1R expression in rabbit AF cells in cell culture was further confirmed by immunofluorescence (Fig. 4). NPY-1R immunofluorescence was not detected in Jurkat cells, a negative control. These results indicate the presence of the receptors on AF cells.
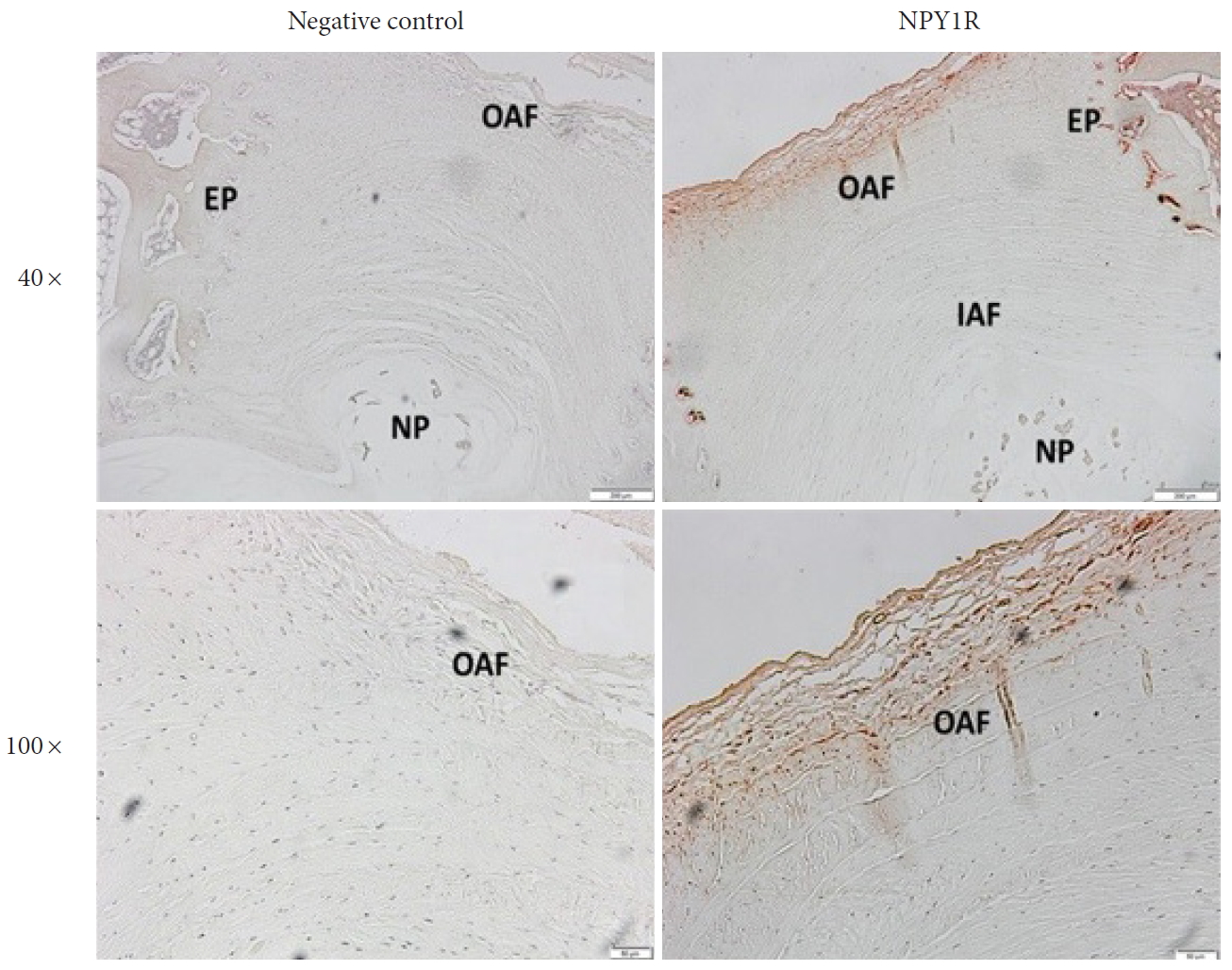
Examination of neuropeptide Y receptor type 1 (NPY-1R) expression by immunohistochemistry on rabbit disc tissues. Representative images of immunohistochemistry revealed the NPY-1R (red) expressed in the outer annulus fibrosus (OAF). Upper, original magnification: ×40, scale bar=200 μm; low, original magnification: ×100, scale bar=50 μm. The left panel showed negative control with no primary antibody. The right panel showed positively stained (red) annulus fibrosus cells for NPY-1R in the OAF and cartilaginous endplate (EP) regions. IAF, Inner annulus fibrosus; NP, nucleus pulposus.
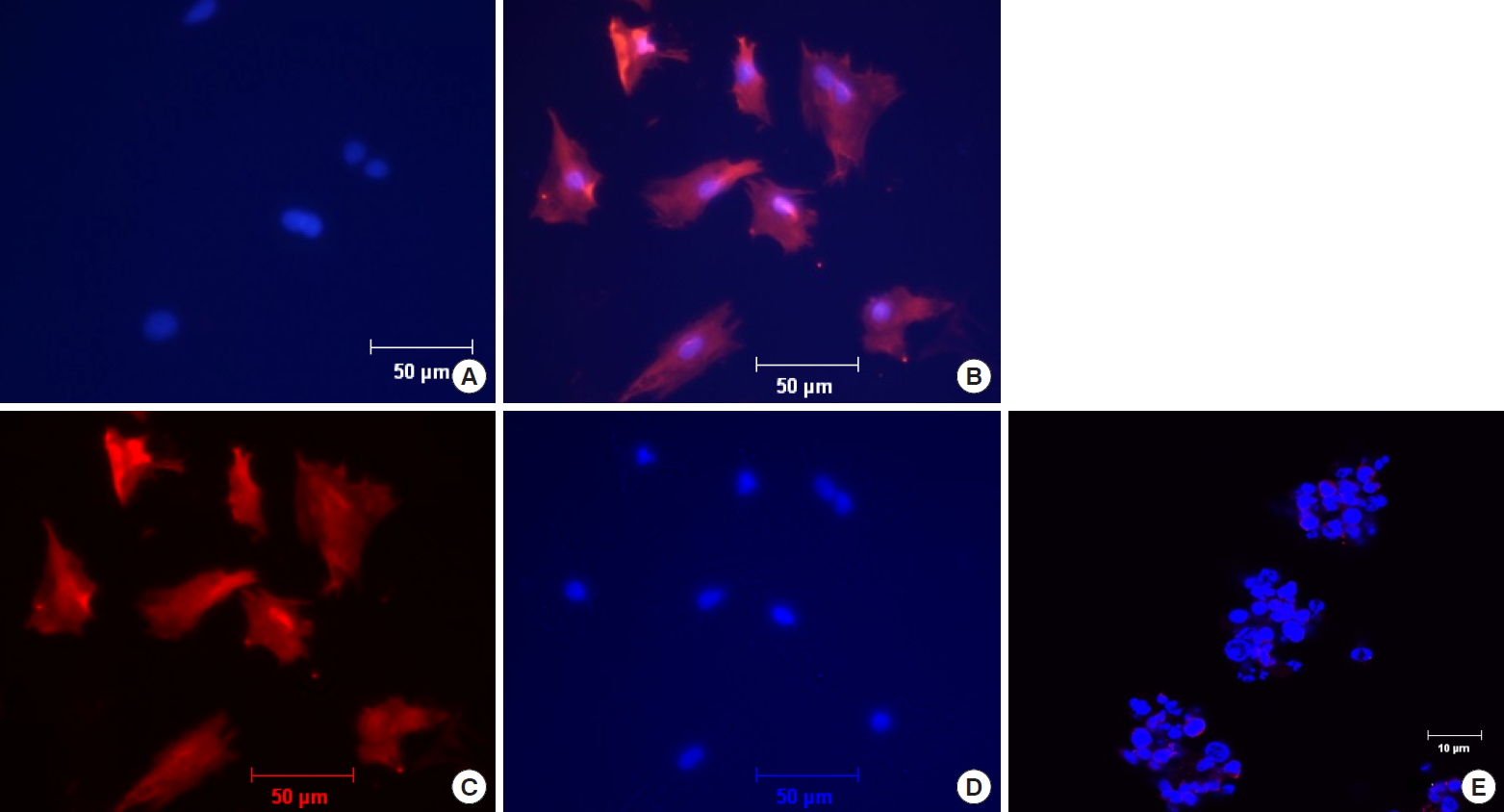
Examination of neuropeptide Y receptor type 1 (NPY-1R) expression by Immunofluorescence on rabbit annulus fibrosus (AF) cells. The AF cells were immunostained for NPY-1R (red), and nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) (blue). (A) Negative control of AF cells incubated with secondary but no primary antibody at ×100 original magnification. (B) NPY-1R immunofluorescence reactivity for AF cells at ×100 original magnification. (C, D) Unmerged photographs of panel B showing positive immunofluorescence reactivity for AF cells to NPY-1R (C) and DAP1 (D). (E) Jurkat cells incubated with primary and secondary antibody, demonstrating no immunostaining at ×400 original magnification as a control to establish antibody specificity.
DISCUSSION
Although prior literatures have shown the presence of the wellknown nociceptive modulatory, NPY, in AF tissue [18,19], little has been investigated regarding changes in NPY levels following inflammatory and mechanical stress in the IVD. The current study demonstrates that AF NPY expression is modulated by inflammatory stress and varying magnitudes of mechanical stress. Specifically, this study demonstrates that when compared to controls, rabbit AF cells produce a significantly greater amount of NPY in response to inflammatory conditions, and there is a trend towards further amplified expression when mechanical and inflammatory stress are combined in the same environment. The increased expression of NPY demonstrated in this study is seen under the same conditions previously reported to cause the inflammatory and catabolic cascade seen in IVD degeneration in vitro [9,27]. Furthermore, NPY-1R was demonstrated to be present in the rabbit AF via immunofluorescence (IF) and immunohistochemistry. (IHC) The presence of NPY-1R was almost exclusively noted in the cells of the outer AF on immunohistochemistry (IHC), with very little NPY-1R positive cells seen in the inner AF. However, immunofluorescence (IF) staining seemed to suggest that a vast majority of AF cells were positive for NPY-1R. The discrepancy between our IHC and IF results may be explained by the possibility that there was a disproportionate amount of cell growth from cells originating in the outer AF in the cell culture used for IF analysis. Unfortunately, there are currently no in vitro assays available to discriminate between outer and inner AF cells in culture. The presence of NPY-1R in cells of the AF raises the possibility for potential paracrine modulation of NPY pathways. These results suggest a potential nociceptive link between alteration of IVD cell-matrix homeostasis and the systemic perception of pain and pain-related behaviors.
NPY has been shown to have a complex biologic function, and its role in nociceptive signaling is diverse. NPY has been associated with both pro- and antinociceptive responses. For example, if delivered centrally (i.e., spinal cord) it has potential to act as an analgesic [25,29], however, in the peripheral nervous system and soft tissues it has been shown to have the opposite effect, causing increased pain and inflammation in several conditions including knee osteoarthritis [30], temporomandibular joints [31], fibromyalgia [15], and peripheral nerve injury [32]. Furthermore, NPY has also been shown to have regulatory roles in both PTSD [14] and appetite [33], linking the neuropeptide with stress-related conditions [34]. Beyond nociception, NPY is a potent regulator of bone metabolism and synthesis via indirect and direct action on osteoblasts [35,36], suggesting that NPY may have more than an isolated nociceptive role in musculoskeletal tissues.
Serum NPY concentration is increased in patients with low back pain and pain-related behaviors [20], and now is produced by the disc cells as well as have receptors present in the cells and tissue. Although it is not definitively known how NPY may enter the systemic circulation from the discs, NPY has been shown to cross through the blood-brain barrier in a nonsaturable manner, indicating that it is highly diffusible across vessel walls and likely can move freely between the extracellular disc matrix and the bloodstream [37]. This potential link between IDD and low back pain perception is one of utmost importance for several reasons. It is widely accepted that despite the high prevalence of imaging findings of degenerative disc disease, imaging often does not correlate with symptoms [38]. Thus, NPY has the potential to be a novel biomarker to assess symptomatic IDD in vivo.
There are several limitations to this study that are worth noting. First, changes in expression were noted in vitro, which may not accurately reflect the in vivo response to inflammatory and mechanical stress. Cell culture systems used in this study do not take into account the complex cell-matrix interactions that play pivotal roles in governing mechanical and inflammatory signaling pathways in vivo [39]. Furthermore, mammalian cells undergo more complex loading patterns than isolated tensile stress, and IL-1 in isolation is an oversimplification of in vivo inflammation biology, which includes IL-6 and tumor-necrosis factor-α, as well as nitric oxide and prostaglandins, among others [40]. Additionally, experiments were performed using a female rabbit model. Changes in NPY concentrations in human AF cells in response to stress may differ from the rabbit cells employed in our experiments, and that sex differences may exist. Finally, many mediators not measured here were likely altered in addition to NPY. However, reporting all measures of NPY to a ratio of total protein produced allowed us to conclude that the elevated expression of NPY was indeed due to the conditions being tested and not from overall nonspecific protein increase. Larger, more complex in vivo analysis with local NPY-1R inhibition and assessment for the presence or absence of pain-related behaviors are warranted for future investigation.
Overall, the current study establishes the production of the neuropeptide NPY by rabbit AF cells and expression of the associated NPY-1R. Additionally, we show that NPY expression in rabbit AF cells increase in response to inflammation and combined inflammation and mechanical loading, laying the foundation for a potential mechanistic link between pain-related behavior and IDD. We hope that this research provides the groundwork for future in vivo studies assessing NPY’s potential role in disc degeneration and discogenic pain.
CONCLUSION
The findings demonstrate that rabbit AF cells are capable of expressing NPY, and expression is enhanced in response to inflammatory stress. The findings further suggest a trend towards the amplified expression of NPY in response to combined inflammatory and mechanical stress. Because both inflammatory and mechanical stress contribute to IDD, this observation raises NPY signaling as a potential of a mechanistic link between low back pain and IDD.
Notes
The authors have nothing to disclose.
Acknowledgements
Internal funds from the University of Pittsburgh/UPMC Departments of Physical Medicine and Rehabilitation and Orthopaedic Surgery were used to support this research.
The authors thank Dr. Kevin Gno for his assistance with protein expression analysis and Jessa Darwin for editorial assistance on the manuscript.
