 |
 |
- Search
| Neurospine > Volume 17(Suppl 1); 2020 > Article |
|
|

Abstract
Lumbar disc herniation (LDH) comprises one of the most common causes of low back pain. 35%ŌĆō72% of LDH is associated with disc fragment migration. The migration of the disc fragments can be high-grade up, low-grade up, high-grade down, and low-grade down. Spine surgeons deal with unique challenges during surgical management of migrated discs. Operational challenges with open surgery include extensive lamina excision, pars excision, and potential for iatrogenic instability without fixation. In contrast, rigid instruments and poor visualization are the challenges with transforaminal endoscopic spine surgery (ESS). Hence interlaminar approach with ESS is an excellent choice with these migrated LDH. The creation of a translaminar crater in the cranial lamina without dealing with the interlaminar window or ligamentum flavum could be an excellent option to deal with these herniations face front. The lamina is the only anatomical barrier between the endoscope and the migrated disc fragment. Hence with a translaminar approach, unnecessary flavectomy can be avoided. In this technical report and video, we demonstrate the surgical technique of performing the translaminar ESS for highly upmigrated LDH with the preservation of optimal natural anatomy.
Almost 80% of the population is reported to have an episode of low back pain in their lifetime, and the most frequent cause is intervertebral disc degeneration leading to lumbar disc herniation (LDH) and degenerative disc disease [1-3]. Nearly all cases of LDH are managed conservatively with surgery reserved for the cases failing conservative management [4]. Surgery for LDH has evolved from open discectomy to minimally invasive surgery (MIS) procedures like microdiscectomy, tubular discectomy, and recently endoscopic discectomy [5-7]. Endoscopic spine surgery (ESS) has significant benefits with smaller skin incision, continuous saline irrigation, and absence of retractor systems [8]. However, there is a steep learning curve to master these surgical skills [9,10].
ESS has gradually evolved to be the flag bearer of standard MIS procedures like discectomy with precise indications, proper diagnosis, and apprenticeship [11]. ESS for discectomy is as effective as traditional open surgery with an effective rate of 90% [12-14]. Initially described through transforaminal access, ESS has now evolved to the interlaminar corridor [5,6].
In LDH, the disc fragment migration occurs in 35%ŌĆō72% of cases [15,16]. ESS is technically difficult in migrated fragments, and the results are decided by the surgeons learning curve [16,17]. Migrated disc fragments in LDH are one of the common causes of endoscopic failure through the transforaminal route, and hence open surgery was recommended [16,18-20]. With open surgery, the removal of migrated disc fragments requires removal of the extensive lamina, pars interarticularis, facet joints leading to a potential for iatrogenic fracture and instability [16].
To overcome the obstacle of migrated disc fragments, various modifications of ESS have been introduced like: foraminoplasty, interlaminar corridor, and transiliac approach [6,21]. The interlaminar corridor makes the application of ESS to different types of migrated LDH like high-grade up, low-grade up, high-grade down, and low-grade down a possibility [16]. The upmigrated LDH are challenging cases with difficulty in finding the migrated fragment in the axilla of the exiting nerve root of the cranial level. Interlaminar ESS can tide over this technical difficulty in expert hands using the translaminar access (through the cranial lamina). This technical report describes the procedure of performing an interlaminar ESS through the translaminar access to deal with an L4ŌĆō5 highly upmigrated LDH.
A 68-year-man presented with acute onset left lower limb radiating pain. The pain was radiating along the posterior aspect of the thigh and calf. The pain was continuous with partial relief on sleeping in lateral decubitus position, and the hips flexed. The patient had a numeric rating scale score of 10 and neurology of left toe dorsiflexion weakness (Medical Research Council 3/5).
Magnetic resonance imaging (MRI) revealed the presence of an L4ŌĆō5 upmigrated LDH with significant compression of the L4 nerve root (Fig. 1). The facet joints had a coronal orientation with the computed tomography (CT) scan demonstrating a vacuum sign in the left L4ŌĆō5 facet joint. The CT scan ruled out any calcification of the herniated fragment (Fig. 2). There was no relevant back pain even though the x-ray images show a stable grade 1 anterolisthesis (Fig. 3).
The patient was given conservative management in the form of medications, physiotherapy, and selective nerve root blocks for 6 weeks, following which he had partial pain relief. A decision to perform an interlaminar ESS with a translaminar approach was made with the recurrence of severe pain. The patient underwent ESS and had complete pain relief in the postoperative period.
The video clip of the procedure is attached along with this paper. (Supplementary video clip 1). The steps are illustrated as follows.
The patient was administered general anesthesia and positioned prone on a Wilsons frame. Following draping, intraoperative fluoroscopy was used to locate the L4ŌĆō5 disc space, and a trocar was used to dock on the left lamina of the L4 vertebra with an 8-mm skin incision. The ideal trajectory for docking is centered over the main migrated fragment lying underneath the lamina and is determined by the preoperative axial imaging of CT scan and MRI. The docking is confirmed by intraoperative fluoroscopy with the help of a needle. A K-wire replaced the needle and sequential dilators were inserted to the surface of lamina. The docking point was well cranial to the interlaminar space of L4ŌĆō5 to tackle the upmigrated LDH face front (Fig. 4AŌĆōC). A working cannula was inserted over the dilators. This was followed by the insertion of stenosis endoscope (Vertebris Stenosis; RIWOSpine, Knittlingen, Germany). A radiofrequency probe was used to coagulate the paraspinal musculature and create a working space. This endoscope is composed of the outer diameter with 9.5-mm and 5.6-mm working channels. Space created exposed the bony surface of the left L4 lamina. The working cannula is then advanced over the lamina to create a crater over the lamina. A dissector was used to palpate the L4ŌĆō5 interlaminar space and the left pars interarticularis. This step helps to mentally map the important surgical landmarks. These surgical landmarks serve to avoid an unnecessary breach of the pars or the interlaminar space.
A thin-tipped high-speed endoscopic diamond burr is used to create a crater in the L4 lamina as shown in Fig. 1. This crater is progressively widened and deepened with the burr until the second cortex of the lamina is reached and becomes paper-thin (Fig. 5A, B). Adequate care should be taken at this step as the ligamentum flavum is very thin at this step and the burr tip can accidentally enter the epidural space. A crater of approximately 10-mm diameter is created over the lamina. Utmost care is taken to avoid violation of the isthmus and interlaminar space during burring. The paper-thin second cortex is then gradually removed with 3.5-mm/4-mm punch. As the base of this crater was excised, the thecal sac, nerve root, and the highly upmigrated herniated disc fragment were identified (Fig. 6). The 10.5-mm working cannula cannot pass the crater and hence the endoscope was changed (Fig. 7) to a smaller diameter interlaminar model (Vertebris Interlaminar; RIWOSpine). This endoscope is composed of the working cannula with an 8-mm outer diameter and endoscope with a 7.9-mm outer diameter and 4.1-mm working channel. Relatively smaller 8-mm working cannula can enter the translaminar crater and its beveled edge is used as a nerve root retractor in the following steps, as demonstrated in the video and intraoperative pictures (Fig. 8). The maneuverability of this working cannula is mostly in the axial rotatory fashion and 15┬░ŌĆō30┬░ in mediolateral and craniocaudal plane
1. A dissector was used to retract the thecal sac medially (Fig. 8A).
2. The beveled edge of the interlaminar endoscope was gradually rotated in an anticlockwise fashion (left-sided, this will be a clockwise rotation for right-sided pathology) (Fig. 8B).
3. Gradually the dissector is taken out of the field, and the beveled edge of the endoscope takes over the position of the dissector to retract the thecal sac medially (Fig. 8C).
4. Lastly, the working cannula is slightly pushed into the migrated herniated disc fragment so that it herniates into the cannula (Fig. 8D).
The annular defect that was visualized was sealed with radiofrequency ablation (Trigger-Flex, Elliquence, LLC, Baldwin, NY, USA). The endoscope was removed, and the skin closed with skin glue. The patient was extubated and shifted. He was mobilized within 4 hours of surgery and was completely painfree. The postoperative MRI shows complete removal of the disc fragment (Fig. 9). The postoperative x-ray radiograph depicts the radiolucency of the crater as compared to the preoperative x-ray (Fig. 10).
The indications for ESS have largely increased due to newer corridors like the interlaminar access [6,7,11]. With open surgery and other MIS procedures, the excision of highly upmigrated LDH involves opening of the interlaminar space and flavectomy [16]. With open surgery, the surgeonŌĆÖs view is located outside the spinal canal, and hence an excessive laminectomy may be required to remove the highly migrated disc fragments. With ESS, the surgeonŌĆÖs view is located inside the spinal canal, and hence excessive removal of lamina, pars, or facets may be avoided [16]. With translaminar access, the natural anatomy of the interlaminar window is hardly disturbed. The translaminar crater is strictly within the lamina of the cranial vertebra, and flavectomy is not necessary. The endoscope with the camera at the tip gives direct 360┬░ visualization of the translaminar crater from all angles. This technical report shows the feasibility of performing ESS in this challenging scenario with minimal patient morbidity and preservation of optimal natural anatomy through the translaminar crater.
Di Lorenzo et al. [22] reported a translaminar technique through the pars interarticularis for foraminal disc herniations in 1998. The pars interarticularis forms the roof of the neural foramen. A fenestration done at the lateral aspect of the pars interarticularis gives direct access to the posterior aspect of the foraminal LDH. This technique was described using a posterior midline incision and microscope but is one of the first descriptions of the translaminar approach. The authors illustrated the difficulty of approaching the L5ŌĆōS1 foramen due to extensive drilling required. This drilling may contribute to iatrogenic pars fractures and instability.
Dezawa et al. [23] in 2012 reported the translaminar technique with a full-endoscopic approach and named it PETA (percutaneous endoscopic translaminar approach). This technique uses a fluoroscopically made 8-mm incision directly over the cranial lamina on the side of the disc herniation. It is essential to rule out any calcification of the herniated disc fragment with CT. The lamina forms an anatomical barrier between the disc fragment located ventral to the lamina and medial to the pedicle. A 4- to 8-mm diameter bony crater is created over the lamina with an endoscopic burr and 4-mm punch.
Du et al. [24] reported a series of 7 patients with highly downmigrated soft LDH operated with percutaneous endoscopy through the translaminar route. Five of the 7 cases were revision cases with previous endoscopic surgery done either by transforaminal or interlaminar endoscopy. The patients had a mean follow-up of 13.8 months, and all had excellent or good outcomes without any recurrences. Their technique involved using preoperative axial MRI or CT scan images to measure the distance from the midline to the migration (usually 8ŌĆō10 mm) for planning the skin entry site of the needle and cannula of the endoscope. The authors prepared an 8-mm bony tunnel over the migrated disc herniation with a wide bottom for manipulation of the instruments. The advantages of this approach as mentioned by the authors are a potential to avoid an injury to the adjacent soft tissues, reduced risk of neural injury due to short distance between the working cannula and the target site and the advantage to explore the cranial and caudal extent of the herniation to remove the fragments completely. The facet joint is also sparred by the translaminar access. The limitations of this approach are the cases of central disc herniations where the fragments may be covered by the dura mater giving rise to a potential for recurrence due to retained fragments and the potential of dural tears during widening of the base of the crater. Also, cases of highly migrated disc herniation at upper lumbar level with a narrow lamina, cases with severe canal stenosis are relative contraindications for the translaminar technique. In cases with severe stenosis or hypertrophy of the ligamentum flavum the risk of iatrogenic injury to the duramater and nerve root is multiplied. In such a scenario it is better to do either laminectomy or laminotomy.
We have recently reported a series of 13 patients with highly upmigrated LDH operated via a translaminar key-hole approach [25]. Five patients of the 13 were cases of very high up migration. We reported a satisfaction rate of 92.3% on MacNab criteria at a mean follow-up of 20 months with no complications or recurrences. The same surgical technique as described in this paper was used in the case series. The advantages of a translaminar approach are avoidance of iatrogenic instability, shorter procedural time, and the shorter distance of manipulation in the canal, leading to a decreased risk of complications, reduced risk of epidural scar formation and the ability of patients to resume work soon. The disadvantages are that some parts of the disc in the hidden zone may still exist, but fragmentectomy is acceptable because the residual disc could be reabsorbed over time. Also, the disc fragments in continuity with the disc of origin may be difficult to remove with this approach. The cases with the fragments in the shoulder of the upper nerve root are better managed with an endoscopic transpedicular approach [26].
Xin et al. [27] reported a series of 11 highly migrated LDH operated by a modified translaminar osseous channel assisted percutaneous endoscopic technique. All the LDH were sequestered and highly migrated but the direction of migration was not mentioned and they had 9 cases with excellent, 1 case each with good and fair outcome on the Macnabs criteria. The approach described by the authors was not strictly translaminar and involved removing the lateral bone of the lamina with a trephine and using a beveled working sheath. They reported no recurrence and benefits like adequate exposure of the LDH and nerve root and reduced formation of epidural scarring.
Recently this approach has been reported for different indications like the series of 5 patients of ligamentum flavum hematoma removed using the translaminar route [28]. The authors argued that the full-endoscopic translaminar route is not only safe and effective in cases of ligamentum flavum hematoma but also better to confirm the correct diagnosis as the continuity of the hematoma capsule and facet joint capsule is visible under high definition endoscopic view. The same author also reported a case on decompression of the L5ŌĆōS1 foramen for a case of degenerative foraminal stenosis through the translaminar route [29]. The full-endoscopic translaminar route was also recently reported for the excision of an intradural extramedullary tumor of the spinal cord with very good results [30]. The same group utilized the translaminar endoscopic approach to excise extravasated cement leak causing radiculitis in a case of percutaneous vertebroplasty [31]. But all the authors warn that the full-endoscopic translaminar approach is technically challenging and requires a significant hand-eye coordination. Hence this surgery should be attempted only after developing good skills in routine endoscopic procedures.
The advantages of this translaminar technique are minimal disruption of the paraspinal musculature, almost no damage to the facet joint and most importantly an extensive hemi laminectomy and flavectomy are avoided as ligamentum flavum is not the anatomic barrier to the upmigrated disc fragment. As the distance between the base of the translaminar crater and neural elements is less in this approach, there is a risk of nerve root injury, dural tear or an epidural hematoma [23]. The endoscopic translaminar approach is dependent on oneŌĆÖs surgical skill and has specific indications like highly migrated disc herniations. We recommend surgeons with a good experience in interlaminar endoscopy to attempt this approach.
CONFLICT OF INTEREST
Dr. Jin-Sung Kim is presently a consultant for RIWOspine (Knittlingen, Germany) and a consultant for Elliquence, LLC (Baldwin, NY, USA). Except for that, the authors have nothing to disclose.
SUPPLEMENTARY MATERIALS
Supplementary video clip 1 can be found via https://doi.org/10.14245/ns.2040264.132.v.1.
Fig.┬Ā1.
(A) T2-weighted images of magnetic resonance imaging (MRI) lumbar spine in sagittal section show the highly upmigrated lumbar disc herniation. (B) On the axial MRI on the right, the herniated disc fragment can be seen just medial to the left pedicle of L4 vertebra.

Fig.┬Ā2.
Computed tomography images in sagittal (A) and axial (B) sections to rule out any calcification around the herniated disc fragment.
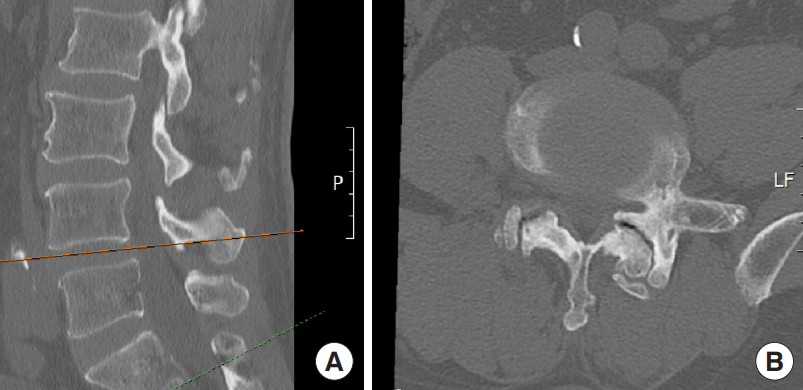
Fig.┬Ā3.
Dynamic x-ray radiograph images in standing position in neutral (A), flexion (B), and extension (C). The images depict a stable grade 1 anterolisthesis at the L4ŌĆō5 vertebral bodies.

Fig.┬Ā4.
(A) Intraoperative fluoroscopic image in the lateral projection depicting the trocar at the L4 lamina. (B) Fluoroscopic image in lateral position with working cannula that has replaced the trocar. (C) Fluoroscopic anteroposterior image of the working cannula seen to be docked at the left L4 lamina.
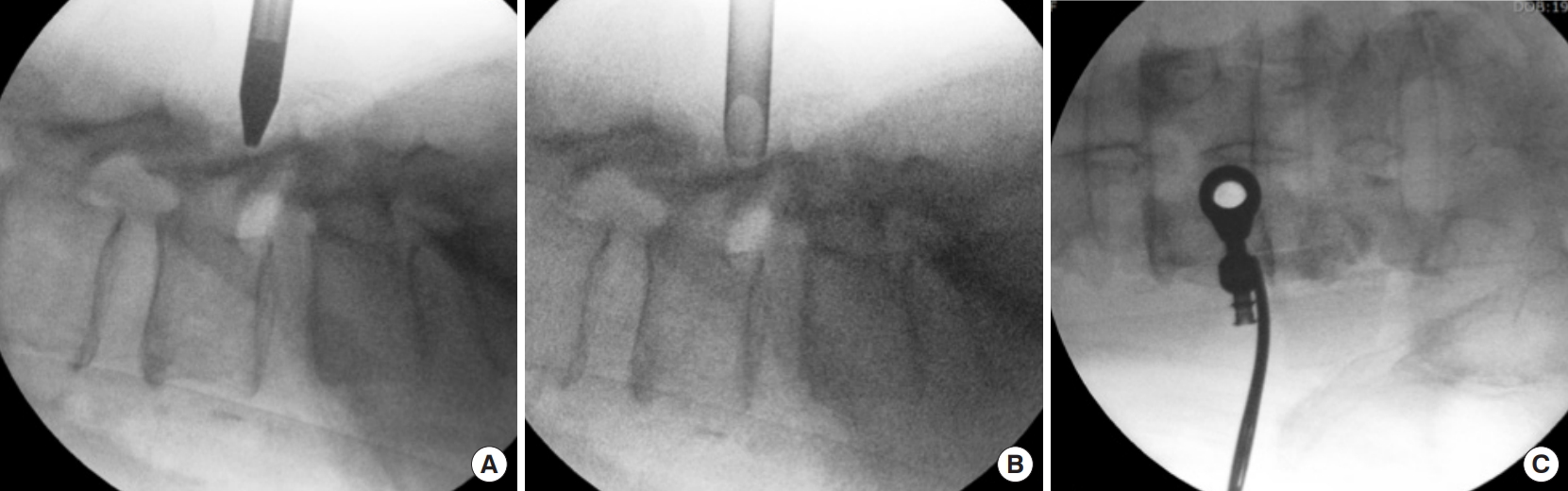
Fig.┬Ā5.
(A) X-ray radiograph of the lumbar spine in anteroposterior position (image mirrored for comparison with the 3-dimensional [3D] computed tomography image). The shaded yellow circle depicts the location of the translaminar crater. (B) 3D computed tomography image of the lumbar spine. The shaded yellow circle represents the area of the left L4 lamina that will be drilled to create the translaminar crater.

Fig.┬Ā6.
(A) Intraoperative endoscopic view of creating of the translaminar burr using a diamond-tipper high-speed burr. (B) Birds eye view of the crater. (C) First bites of the base of the crater with kerrisons punch. (D) Probing the lateral edge of the dural sac with a dissector.

Fig.┬Ā7.
Change of endoscopes from the larger diameter stenosis endoscope to smaller diameter interlamina endoscope.

Fig.┬Ā8.
(A) Step 1: The dural sac is retracted medially with a dissector. (B) Step 2: The beveled edge of the smaller working cannula rotated in anticlockwise direction. (C) Step 3: The beveled edge of the working cannula replaces the dissector and becomes the nerve root retractor. (D) Step 4: The migrated disc fragment herniates into the working channel.

Fig.┬Ā9.
T2-weighted magnetic resonance imaging in postoperative period depicting complete removal of the upmigrated disc fragment in both sagittal (A) and axial images (B).

Fig.┬Ā10.
X-ray radiographs in anteroposterior position (mirrored for comparison with the 3-dimensional CT scan images) depicting the preoperative (A) and postoperative images (B). The image on the right shows the postoperative radiolucency in the form of a circular crater on the left L4 lamina as pointed out by the red arrow.

REFERENCES
2. Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008 299:656-64.


3. Amin RM, Andrade NS, Neuman BJ. Lumbar disc herniation. Curr Rev Musculoskelet Med 2017 10:507-16.




4. Kanno H, Aizawa T, Hahimoto K, et al. Minimally invasive discectomy for lumbar disc herniation: current concepts, surgical techniques, and outcomes. Int Orthop 2019 43:917-22.



5. Yeung AT. Minimally invasive disc surgery with the yeung endoscopic spine system (YESS). Surg Technol Int 1999 8:267-77.

6. Choi G, Lee SH, Raiturker PP, et al. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery 2006 58(1 Suppl):ONS59-68.



7. Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008 33:931-9.


8. Siepe CJ, Sauer D, Michael Mayer H. Full endoscopic, bilateral over-the-top decompression for lumbar spinal stenosis. Eur Spine J 2018 27(Suppl 4):563-5.



9. Wang B, L├╝ G, Patel AA, et al. An evaluation of the learning curve for a complex surgical technique: the full endoscopic interlaminar approach for lumbar disc herniations. Spine J 2011 11:122-30.


10. Hsu HT, Chang SJ, Yang SS, et al. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J 2013 22:727-33.



11. Choi G, Pophale CS, Patel B, et al. Endoscopic spine surgery. J Korean Neurosurg Soc 2017 60:485-97.




12. Hermantin FU, Peters T, Quartararo L, et al. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am 1999 81:958-65.


13. Ahn Y, Lee SH, Lee JH, et al. Transforaminal percutaneous endoscopic lumbar discectomy for upper lumbar disc herniation: clinical outcome, prognostic factors, and technical consideration [published correction appears in Acta Neurochir (Wien). 2009 Nov;151(11):1561]. Acta Neurochir (Wien) 2009 151:199-206.



14. Liu C, Chu L, Yong HC, et al. Percutaneous endoscopic lumbar discectomy for highly migrated lumbar disc herniation. Pain Physician 2017 20:E75-84.

15. Ebeling U, Reulen HJ. Are there typical localisations of lumbar disc herniations? A prospective study. Acta Neurochir (Wien) 1992 117:143-8.



16. Choi KC, Lee DC, Shim HK, et al. A strategy of percutaneous endoscopic lumbar discectomy for migrated disc herniation. World Neurosurg 2017 99:259-66.


17. Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery 2015 76:372-80.



18. Lee S, Kim SK, Lee SH, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J 2007 16:431-7.



19. Kim CH, Chung CK, Woo JW. Surgical outcome of percutaneous endoscopic interlaminar lumbar discectomy for highly migrated disk herniation. Clin Spine Surg 2016 29:E259-66.


20. Lee SH, Kang BU, Ahn Y, et al. Operative failure of percutaneous endoscopic lumbar discectomy: a radiologic analysis of 55 cases. Spine (Phila Pa 1976) 2006 31:E285-90.


21. Osman SG, Sherlekar S, Malik A, et al. Endoscopic trans-iliac approach to L5-S1 disc and foramen - a report on clinical experience. Int J Spine Surg 2014 8:20.



22. Di Lorenzo N, Porta F, Onnis G, et al. Pars interarticularis fenestration in the treatment of foraminal lumbar disc herniation: a further surgical approach. Neurosurgery 1998 42:87-9.



23. Dezawa A, Mikami H, Sairyo K. Percutaneous endoscopic translaminar approach for herniated nucleus pulposus in the hidden zone of the lumbar spine. Asian J Endosc Surg 2012 5:200-3.


24. Du J, Tang X, Jing X, et al. Outcomes of percutaneous endoscopic lumbar discectomy via a translaminar approach, especially for soft, highly down-migrated lumbar disc herniation. Int Orthop 2016 40:1247-52.



25. Lin GX, Park CW, Suen TK, et al. Full endoscopic technique for high-grade up-migrated lumbar disk herniation via a translaminar keyhole approach: preliminary series and technical note. J Neurol Surg A Cent Eur Neurosurg 2020 Feb 11 [Epub]. https://doi.org/10.1055/s-0039-1700574.


26. Quillo-Olvera J, Akbary K, Kim JS. Percutaneous endoscopic transpedicular approach for high-grade down-migrated lumbar disc herniations. Acta Neurochir (Wien) 2018 160:1603-7.



27. Xin Z, Liao W, Ao J, et al. A modified translaminar osseous channel-assisted percutaneous endoscopic lumbar discectomy for highly migrated and sequestrated disc herniations of the upper lumbar: clinical outcomes, surgical indications, and technical considerations. Biomed Res Int 2017 2017:3069575.




28. Kaneko T, Oshima Y, Inoue H, et al. Successful treatment of lumbar ligamentum flavum hematoma using a spinal full-endoscopic system. J Spine Surg 2018 4:744-9.



29. Koga H. Improved percutaneous endoscopic translaminar approach for lumbar foraminal stenosis at L5/S1. Mini-invasive Surg 2017 1:3. -5. https://doi.org/10.20517/2574-1225.2016.07.


- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























