Getting Down to the Bare Bones: Does laminoplasty or laminectomy With Fusion Provide Better Outcomes for Patients With Multilevel Cervical Spondylotic Myelopathy?
Article information
Abstract
Objective
Cervical spondylotic myelopathy (CSM) is a degenerative disorder leading to progressive decline in spinal cord function. Cervical laminoplasty (CLP) and cervical laminectomy with fusion (CLF) are standard treatments for multilevel CSM. However, it is still unclear whether one procedure over the other provides better outcomes. Here, we performed a comprehensive review of published articles that compare the clinical outcomes and costs between CLP and CLF for CSM.
Methods
A literature search was performed using PubMed, Web of Science, and Cochrane databases. Strict exclusion criteria were applied, and included articles were then assessed for publication year, study design, and significant differences in outcome variables.
Results
From 519 studies identified with search terms, 38 studies were included for the qualitative analysis. Statistically significant differences in the clinical outcomes and costs were found in 18 studies. Eleven studies were prospective or retrospective, and 8 studies were meta-analyses. For the outcome variables of interest, results were reported by classifying into prospective studies, retrospective studies, and meta-analyses.
Conclusion
CLP and CLF are 2 of the most commonly performed surgical procedures for the treatment of CSM. Although CLP and CLF each provide satisfactory clinical outcomes for patients with CMS, CLP may result in better cervical range of motion and less cost, length of stay, operation time, blood loss, paraspinal muscular atrophy, and rate of nerve palsies as compared to CLF. The major limitation of CLP versus CLF comparison studies includes the heterogeneity in techniques and preoperative criteria. Thus, further validation and investigations in larger cohorts will be required.
INTRODUCTION
Cervical spondylotic myelopathy (CSM) is a degenerative condition that often results in gradual decline in spinal cord function over time, and as with most spinal cord conditions, early intervention results in superior prognosis for patients. Onset of cervical spondylosis is usually middle age, and the resulting degeneration induces motion abnormalities, disc compression, and uncovertebral/facet joint arthrosis [1]. In terms of etiology, this disorder, which involves progressive narrowing of the spinal canal, is the most common spinal disorder among the elderly population [2]. In CSM, effects are generally first seen in the lower extremities, as damage to the corticospinal and spinocerebellar tracts causes gait abnormalities. In later stages, impaired movement occurs in the upper extremities and sphincter activity can become abnormal [1]. Generally, CSM can be treated using both an anterior and posterior approach, and the anterior approach is most commonly used for 1- or 2-level CSM. However, for multilevel CSM, any anterior approach is more complex and can increase rates of complications including hoarseness, trigeminal nerve palsy, and dysphagia [2]. As such, among treatments for multilevel CSM, cervical laminoplasty (CLP) and cervical laminectomy with fusion (CLF) (posterior approaches) are 2 of the most commonly used. However, debate remains as to which actually provides better outcomes. CLF is favored over cervical laminectomy alone in cases of multilevel CSM, as fusion can reduce rates of postoperative segmental instability following CLF. CLP is an alternative approach in CSM that has been found to yield greater cervical range of motion postoperatively. The purpose of this study is to determine whether CLP or CLF provide better outcomes for patients with multilevel cervical compressive myelopathy. As previously stated, cervical laminectomy has historically been the method of choice in CSM although cervical laminectomy alone has been associated with reduced flexibility and stability of the spinal column, which can ultimately lead to repetitive microtrauma to the spinal cord and worsened neurological outcomes [1,3]. In a study comparing laminectomy and CLP in an in vivo animal model, it was found that laminectomy reduced cervical curvature index (CCI) by 59% at 16 weeks and 70% at 24 weeks postoperatively. As compared to intact animal specimens, there was also a significant increase in sagittalplane slack motion and general decrease in sagittal-plane stability owing to forward sagittal angulation following laminectomy [1,4]. Another drawback to laminectomy, when performed alone, is that recovery can be impaired in patients with congenitally short pedicles. Although laminectomy may initially provide these patients with decompression, recompression of the spinal cord can occur as a result of the scar that matures and contracts as it forms over the dura mater [5,6].
In the past 2 decades, cervical laminectomy—when performed with fusion—has demonstrated improved postoperative stability over cervical laminectomy alone. CLF addresses the potential instability resulting from removal of laminae, and it has been demonstrated to minimize complications (lower levels of kyphosis and neurological deterioration) and possibly improve long-term outcomes. Although CLF confers greater stability than laminectomy alone, CLF can interfere with natural spine movements. As such, complications specific to CLF—including rotational movement imbalance, screw loosening and avulsion, and broken rods/plates—have been reported [7-9].
Due to the risks associated with cervical laminectomy (with or without fusion), including intraoperative spinal cord injury, worsening cervical kyphosis, and complications related to the “laminectomy membrane” scar tissue overlying the dura following this procedure, many spine surgeons have worked extensively since the 1960’s to improve upon this approach [10]. In 1981, Hirabayashi and colleagues reported a new unilateral open-door CLP method that would allow for decompression of multiple segments while preserving muscle structures that would prevent postoperative worsening of cervical kyphosis and resulting segmental instability [10,11]. Since that time, various modifications of CLP have been developed to treat CSM. The benefits conferred by CLP are that the laminae remain for load bearing and attachment of the posterior muscles, and also that they prevent “laminectomy membrane” formation. Natural spinal biomechanics are thus preserved following surgery [1,2]. However, CLP is not without complications of its own: these include C5 nerve palsy, neck spasms, and shoulder pain [2,12].
MATERIALS AND METHODS
1. Search Strategy
In order to identify studies reporting on CLF and/or CLP in the treatment of CSM, a literature search was performed using PubMed, Web of Science, and Cochrane databases from database inception to August 6th, 2020. We adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) recommendations, and the following Boolean search terms were used: (‘cervical'’ AND ‘laminoplasty’ AND ‘cervical spondylotic myelopathy’) OR (‘cervical’ AND ‘laminectomy’ AND ‘cervical spondylotic myelopathy’). In total, our search yielded 0 studies from Web of Science, 490 from PubMed, and 0 from Cochrane for a total of 490 unique studies. Reference sections were scrutinized in order to ensure that relevant studies were not missed, yielding 29 additional studies, and duplicates were removed. Articles were then independently screened by 2 investigators (NJB and BVL) according to title and abstract. During this screening process, SS served as the final arbiter with respect to inclusion in the final analysis. Following this screen, 38 studies remained and were assessed in full text for final eligibility (Fig. 1).
2. Variables and Inclusion/Exclusion Criteria
Articles were eligible for inclusion if they met the following criteria: (1) manuscript availability in English or an English translation, (2) involving human subjects only, (3) primary studies or meta-analyses comparing outcomes between CLF and CLP for CSM. Additionally, studies were excluded if they: (1) involved thoracic or lumbar LF or LP or (2) did not compare CLF and CLP based on at least one of the following variables: pain, disability, motor function, range of motion, health and quality of life (QoL), complications, C5 palsy, blood loss, length of stay (LOS), and cost.
3. Study Analysis
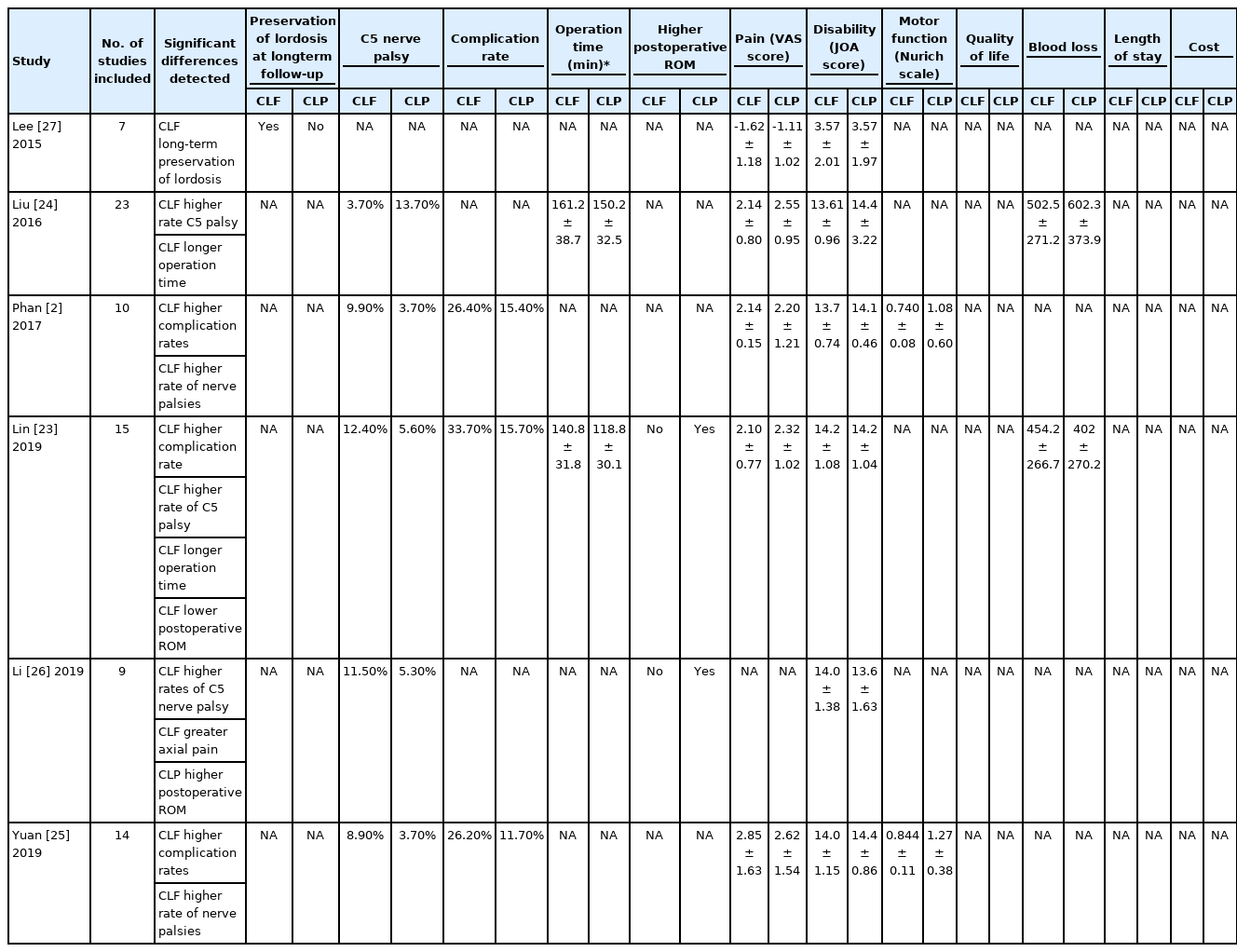
The 18 selected articles, which included meta-analyses, cohort studies, and systematic reviews, were then assessed for publication year and study design. The 6 meta-analyses were analyzed and the following outcomes were evaluated when reported, including pain, disability, motor function, range of motion, health, QoL, complication rate, C5 palsy, bleeding, lordosis, hospital LOS, operation time, and cost. These outcome variables were recorded from the 6 meta-analyses and any significant differences that detected were also noted in Table 1.
RESULTS
Our query yielded 16 studies, including 10 primary studies and 6 meta-analyses. For the outcome variables of interest, results were tabulated and are reported separately for prospective studies, retrospective studies, and meta-analyses (Tables 1, 2).
1. Primary Research Comparing CLF and CLP
1) Prospective studies
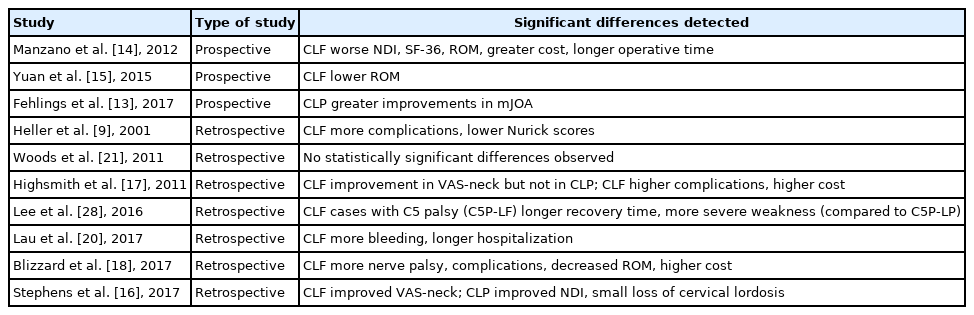
Although several prospective studies comparing CLF and CLP may suggest advantages of CLP over CLF, whether the differences are clinically significant is yet to be established. Fehlings and colleagues conducted a prospective cohort study of 266 patients using CLF (n = 166) and CLP (n = 100). In both groups, patients exhibited significantly improved modified Japanese Orthopaedic Association (mJOA), Nurick grade, Neck Disability Index (NDI), and Short Form 36 (SF-36) v2 physical and mental health scores at 24-month postoperation. Twenty-four-month mJOA scores showed greater improvements in the CLP group as compared to the CLF group [13].
A 2012 prospective, randomized controlled trial performed by Manzano et al. [14] found that CLP, in some regards, may be favorable to CLF. The study acknowledges small patient numbers (LF, n = 7; LP, n = 9), but reports that outcome measures—including NDI, SF-36, and range of motion (ROM)—improved significantly in patients who underwent CLP. CLF exhibited a greater increase in spinal canal area following operation. A unique aspect of this study—which is not commonly found among the literature of studies comparing CLP and CLF—is that it included a survey of spine surgeons. The authors found that the majority (70%) of North American spine surgeons prefer CLF to CLP for CSM. Furthermore, laminectomy alone was preferred by only 7% of polled spine surgeons. In addition, Manzano et al. [14] point to the potential influence that the strong preference for LF might have on future generations of spine surgeons. The authors also report increased operative time and greater costs associated with CLF at their home institution.
In a 2010–2012 prospective cohort study of 38 patients who underwent CLP (n = 20) or CLF (n = 18), ROM and neurological outcomes were assessed. Patients were assessed preoperatively and at 3-, 6-, 9-, and 12-month postoperation for 3-dimensional cervical ROM, JOA scores, visual analogue scale (VAS), and rate of overall complications. Both groups exhibited significant loss in ROM in 6 directions of motion, but at the 12-month follow-up, the CLP group demonstrated greater ROM in 5 of 6 directions (all except for bilateral rotations). In both groups, the most preserved ROM was in rotation, and the most severe reduction was in extension. There was no significant difference in JOA and VAS scores between the groups, and both surgical approaches resulted in improvements in these areas. Additionally, both techniques provided patients with significant improvement in neurological outcomes [15].
2) Retrospective studies
Two studies found that VAS-neck pain scores improved significantly in patients following CLF but not CLP [16,17]. However, 3 studies reported that postoperative VAS-total scores were similar in patients following CLP and CLF [16,18,19]. In terms of disability, only CLP was found to significantly improve NDI scores and minimize neck disability [16,19].
There was discordance among results for motor function, JOA, and Nurick myelopathy scores: while one study reported that Nurick scores improved similarly following both CLF and CLP; another reported that postoperative Nurick scores were significantly lower for CLF [17,20]. Several studies found that JOA scores were similar or that they improved similarly following operation [16-19]. In their matched cohort study, Heller et al. [9] reported that more patients exhibited improvements in strength, dexterity, and gait following CLP, however, Woods et al. [21] report similar gait improvements for both CLF and CLP. Two studies suggest that CLP leads to better postoperative ROM compared to CLF [18,19]. When postoperative health statuses and QoL were subjectively assessed (Short Form-12 and health-related QoL scores), scores were similar for patients who underwent CLF and CLP [18,19].
With respect to complication rates, results were inconsistent. Some reported lower long-term complication rates for CLP, and that complications were roughly twice as common following CLF [9,20]. Heller et al. [9] performed an independent matched cohort analysis consisting of 13 CLF patients and 13 CLP patients. In the CLP group, no complications were reported, while there were 14 complications among 9 of 13 patients from the CLF cohort: nonunion, progression of myelopathy, instrumentation failure, development of a significant kyphosis alignment, bone graft harvest site pain, deep infection, and degeneration requiring operation [9]. Finally, Woods et al. [21] found no significant difference in complication rate following CLP versus CLF. Blizzard et al. [18] reported higher rates of C5 palsy for patients who underwent CLF compared to CLP patients. From their retrospective study of 145 adult patients undergoing CLP or CLF for CSM it was found that CLP was associated with less bleeding. Although one study reported a significantly small loss of lordosis following CLP, it was otherwise found that CLP was associated with significantly lower rates of paraspinal muscular atrophy (PMA), which was associated with reports of better preoperative lordosis preservation for patients following CLP as compared to CLF [16,19,22]. Results for LOS were not unanimous, as 2 studies reported significantly shorter lengths of stay for patients following CLP, while another showed no significant difference [18,20]. CLF was associated with higher cost, and Highsmith et al. found that implant costs were almost 3 times higher for CLF than CLP (Table 2) [17,18].
2. Meta-Analyses or Systematic Reviews Comparing LF and LP
1) Meta-analyses
A comparison of outcomes between CLF and CLP has been performed multiple times in systematic reviews and meta-analyses published within the last decade (Table 1). The majority show no significant differences in postoperative JOA [2,23-25], postoperative VAS [2,23,25], postoperative CCI [2,23,25,26], Nurich grade [2,25], operative time [2], or blood loss [2,24]. In addition, no difference in JOA score improvement between CLF and CLP was detected in 2 meta-analyses [24,27] or reoperation rate in another [25].
Only Lin et al. [23] have reported increased blood loss in CLF compared to CLP. Additionally, only Lin et al. [23] and Liu et al. [24] report that CLF has a longer operation time compared to CLP. Liu et al. [24] found that CLF was associated with a higher incidence of C5 palsy. Other authors also report a higher rate of C5 nerve palsy after CLF [2,23,25,26]. Lee et al. [28] analyzed cases of C5 palsy from their cohort of 90 patients, and found that, compared to C5 palsy patients who underwent CLP, those who underwent CLF demonstrated more severe weakness and longer recovery time.
Additionally, Lee et al. [27] found that CLF may show favorable long-term results with regard to preserving lordosis. Furthermore, Lin et al. [23] and Li et al. [26] report that CLF displayed a decreased postoperative ROM (preoperative ROM was similar between groups). Phan et al. [2] found that CLF exhibited a significantly higher complication rate than expansive laminoplasty (EL) (CLF, 26.4% compared to EL, 15.4%). Yuan et al. [25] also found that CLF was associated with a higher rate of total complication. Lin et al. [23] also detected a higher rate of overall complications for CLF.
As such, the general consensus within included meta-analyses suggests that, while many perioperative variables are not significantly different between CLF and CLP, CLF may be associated with higher complication profiles, recovery times, and neurological injuries when compared to the outcomes following CLP. Further longitudinal studies are necessary to fully understand the context in which these assessments can be validated.
2) Systematic reviews
We found 3 systematic reviews without meta-analyses comparing CLF and CLP. In an extensive systematic review of 15 studies, Lao et al. [29] compared clinical and radiographic outcomes between CLP and different laminectomy methods. Of 7/15 studies that reported operative time and blood loss, 5 reported that operative time for CLF was longer than that of CLP. No significant difference in kyphosis, C5 paresis, infection, subluxation, instability, CSF leakage, wound dehiscence, urinary retention, chronic pain, restenosis, nonunion, or hardware failure was found. Postoperative ROM was found to be significantly lower in CLP than in standard CLF. However, 6 of 15 studies provided radiographic data after CLP and CLF, and 5 of these studies found that there was a significantly greater decrease in ROM and greater increase in dural sac area following CLF, as compared to LP. Five of 15 studies reported no significant difference in clinical outcome between CLP and cervical laminectomy, another 5 found that clinical outcome of CLP was superior, and the final 5 found that clinical outcomes were worse for CLP than for cervical laminectomy. Additionally, 6 studies compared CLP and CLF, and CLP was found to be inferior in 2 studies, superior in 2 studies, and similar in 2 studies. One study provided an economic comparison between techniques which demonstrated that implant costs in CLF were almost 3 times greater than those of CLP operations. After correcting for longer constructs used in CLF, implants were still more than twice as costly [29].
In a 2016 systematic review, Singhatanadgige et al. [30] collected data from 4 studies examining cervical myelopathy caused by ossification of the posterior longitudinal ligament (OPLL). Of significance, it was noted that all 4 studies reported the incidence of C5 nerve palsy to be higher following CLF than CLP. One of the studies included reported significantly greater ROM in flexion, extension, and side bending for patients who underwent CLP. Interestingly, one study did not find any significant difference in recovery between groups, while another reported a significantly higher recovery for CLP as compared to CLF. However, there was no significant difference in VAS-neck pain or NDI scores between treatment groups. Yoon et al. [31] selected 4 retrospective cohort studies for a systematic review. Of these studies, 3 of 4 reported no significant difference in pain outcomes between CLP and CLF groups. Two of 3 studies reporting rates of reoperation found these rates to be lower following CLP. One study reported a higher incidence of severe neck pain after CLP.
DISCUSSION
1. Cost
Although there is a plethora of literature comparing clinical outcomes and complications for CLP and CLF, there are fewer studies that investigate potential differences in cost between these operative techniques. Here we report the available data related to the hospital costs and charges of CLP and CLF. In a literature review of cost, it was reported by Blizzard et al. [18], Lao et al. [29], Highsmith et al. [17], and Manzano et al. [14] that greater costs were associated with CLF than CLP. Notably, Blizzard et al. [18], found that median costs, which included all surgical, instrumentation, and hospitalization fees, were greater for CLF ($128,664) than for CLP ($105,431) (p < 0.001). Additionally, Highsmith et al. [17], reported that the implant costs in CLF were nearly triple those of CLP cases, and even after correcting for longer constructs used in CLF, implants were still found to be twice as costly.
In a recent retrospective cost analysis of CLP versus CLF for CSM, data from 81 cases at a single institution were analyzed. It was noted that the double-door technique, which does not require implants, was used for CLP (n = 55), and that CLF (n = 26) was employed using metallic instrumentation. 10,682 individual costs and charges were analyzed, and physicians’ fees were estimated using Current Procedural Terminology codes (these fees were not reported on hospital billing records). The authors found that CLP resulted in reduced LOS, total cost (hospital cost+estimated physicians’ fees), and hospital charges, and concluded that CLP is overall a less costly procedure. They did note, however, that CLF may be required in certain cases of neck pain, kyphotic deformity, and gross instability [32].
Looking at open-door CLP alone (a technique involving alternative levels centerpiece miniplate fixation), Wang et al. [33] found the average cost from admission to discharge among 56 patients was $9,817.9. The authors’ previous method, all-level plate fixation, resulted in an average cost from admission to discharge of $16,279.4. As such, the alternative levels plate fixation method, in which the plate is applied at alternating levels (C3, C5, C7), reduced costs by nearly 40%. Overall, Wang and colleagues concluded that their alternating levels miniplate fixation method is safe, effective, and economical.
There are few studies reporting costs and charges in CLP versus CLF for patients with CSM. From the data that is currently available, it seems possible that CLP is less costly than CLF. However, it is also acknowledged that there are certain cases where CLF is strongly indicated, regardless of cost. In these cases of preoperative cervical misalignment, kyphosis, and instability, CLF may be required. Ultimately, to confirm these results, additional studies with larger patient cohorts and more institutions should be conducted in order to examine differences in cost between CLP and CLF.
2. Preference for Laminectomy With Fusion
Manzano et al. [14] found that 70% of surveyed physicians in North America reported CLF to be their preferred surgical technique for CSM, which is interesting and begs the question: why do North American surgeons prefer CLF? Is CLF generally preferred because there are more cases in which it is indicated? This is unclear because, according to the current literature, CLF and CLP both have their own indications, advantages, and complications. It has been said that spine surgeons in Asia developed laminoplasty and tend to prefer it [27]. It would be interesting to survey physicians to determine the reasons behind their having a preferred technique, and to investigate whether or not there is a relationship between the frequency of specific indicators and frequency of CLF versus CLP usage. The literature so far suggests that CLP can potentially reduce costs [14,17,18,29,32,33] and CLP is sometimes associated with superior cervical ROM [15,17,18,23,26], lower complication rate [2,18,20,23,25], lower length of hospital stay [18,20,32], lower operation time [23,24], lower blood loss [20,23], less PMA [22], and lower rate of nerve palsies [2,18,23-26,30].
3. Indications and Contraindications
In regards to indications for CLF or CLP, it has been proposed that preoperative sagittal alignment and other conditions can have an effect on postoperative outcomes in CSM depending on which technique is used; thus, it is likely that there can be variation in which surgical techniques produce better results owing to differences in the preoperative state of the cervical spine. For example, the K-line, an imaginary line that connects the midpoints of the anteroposterior spinal canal at C2 and C7 and assesses the alignment of the ossification foci, can be used to determine which technique should be performed in patients with OPLL. The K-line is (+) when the OPLL peak exceeds the K-line and (-) when it does not. In a recent study, it was found that CLP is contraindicated in patients for whom the K-line is (-) [34].
Despite having contraindications such as the (-) K-line, it is generally accepted that CLP has shown relatively low complications rates while minimizing postoperative kyphosis. And yet, this partially points to the core of the issue in resolving whether CLP or CLF is the superior technique. At present, the main limitation in literature analyses of studies comparing CLP and CLF lies within the large variations of surgical techniques that have been implemented over the years [35]. Taken together, the various subtypes of CLF and CLP, as well as the great variation in clinical presentations in patients with CMS, make it very difficult to ascertain which is the better surgical approach.
4. Limitations in Current Literature
As suggested by Blecher et al. [35], the range of CLF and CLP subtechniques used across studies makes it difficult to standardize outcomes for meta-analyses. According to Phan et al. [2], such analyses may be limited by selection bias stemming from the inclusion of both randomized and nonrandomized observational studies as well as underlying differences in these same studies (such as presence of preoperative kyphosis and segmental stability). Potential confounds can arise when different surgical techniques are used across studies, with an example being that the French door and open-door LP procedures were employed at different centers in the study of Phan et al. [2]. Furthermore, factors such as preoperative state of cervical misalignment are often taken into consideration when it is decided whether patients receive CLP or CLF. Among CLP patients, increased cervical lordosis (> 20°) has been an indicator of better pain outcomes. Future studies should focus on differences in preoperative cervical lordosis and its effects on clinical outcomes in both CLP and CLF. Additionally, CLF is preferred for patients with high (> 40 mm) baseline SVA distances, as CLP has been shown to increase neck pain in these patients [34]. These factors could explain why, even though CLP might reduce costs, 70% of surveyed American spine surgeons still reported CLF to be their preferred surgical method. Finally, when outcomes analyzed, reporting for outcome satisfaction, and follow-up period are not standardized across all cases, this confers a potential source of bias to meta-analyses comparing CLF and CLP for CSM [2].
5. Future Directions
It is possible that future improvements will help shed light on the answer to this question of why the majority of spine surgeons prefer CLF over CLP. In order to determine the optimal treatment method for CSM, more sensitive outcome instruments are needed to detect and monitor the progression of pathological changes associated with myelopathy. Theoretically, future developments in the area of quantitative microstructural magnetic resonance imaging techniques as well as advances in serological biomarkers could make personalized medicine a reality for CSM. For example, in terms of radiographic measures, diffusion tensor imaging (DTI) is associated with proper clinical assessment of severity in CSM and improved outcomes after surgery [36]. According to Wen et al. [37], DTI is superior to current clinical and radiologic assessments in its predictive capabilities. In the future, perhaps better and more precise prognostication of CSM will allow for more clear cut differences in the efficacy of CLF versus CLP to be detected.
CONCLUSION
Both CLP and CLF are satisfactory treatments for CSM. CLP may provide benefits such as superior cervical ROM and reduced cost, charge, length of hospital stay, operation time, blood loss, PMA, and rate of nerve palsies as compared to CLF. However, there is not a consensus as to whether CLP or CLF provide significantly different clinical outcomes. This could be because there are different preoperative criteria in some cases that make CLF preferred to CLP, and vice versa. Future studies should survey physicians to determine the reasons underlying this preference. Even though CLF remains popular and has historically been the treatment standard, CLP certainly shows promise and will likely continue to be improved as new techniques, such as Wang and colleagues’ alternative level miniplate fixation method, continued to be fine-tuned. Up to this point, a major limitation of many CLF versus CLP comparison studies has been the diversity in CLF and CLP techniques implemented across studies as well as the variation in clinical presentations of CSM. Ultimately, we believe that future studies are needed to compare different CLF and CLP methods (open-door, French door, etc.), costs between CLP and CLF, and predictors of outcome for CLP and CLF in multilevel CSM.
Notes
The authors have nothing to disclose.