|
 |
- Search
| Neurospine > Volume 18(2); 2021 > Article |
|
|
Abstract
Objective
Midline lumbar interbody fusion is performed for treatment of various lumbar degenerative diseases, with good clinical outcomes and few complications. However, there are no large-scale or long-term studies regarding midline lumbar interbody fusion. Therefore, the purpose of this study was to evaluate the clinical results of midline lumbar interbody fusion and to compare the results according to surgical level.
Methods
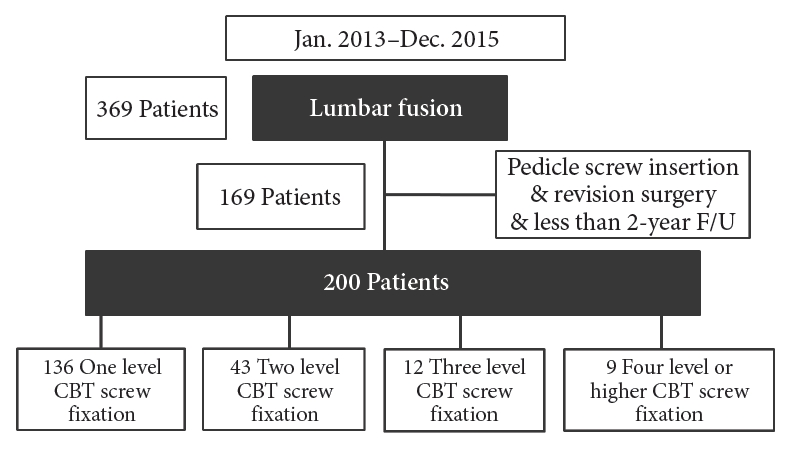
Between January 2013 and December 2015, 200 patients with lumbar degenerative disease undergoing midline lumbar interbody fusion surgery were enrolled. The mean patient age was 69.9 ± 15.8 years (range, 40–85 years). The patients were divided into groups according to surgical level: (1) level 1 operation (136 patients), (2) level 2 operation (43 patients), (3) level 3 operation (12 patients), and (4) level 4 or higher (9 patients). Clinical outcomes, fusion rates, and complications were compared among the 4 groups.
Results
All clinical outcomes significantly improved after surgery (measured at 3 years postoperatively) in all groups. Mean fusion rate was 90.5% ± 5.21%. Fusion rate was highest in group I (95.8%) and lowest in group IV (85.2%). There were complications in 17 cases (8.5%). Adjacent segment disease occurred in 16 cases, 5 of which required surgery. Group 1 had 1 case, and group 4 had 4 cases. Screw loosening occurred in 1 case in group 4. There were no cases of infection or mechanical complications.
Posterior lumbar screw fixation and fusion are conservative surgeries that are performed for treatment of degenerative diseases of the lumbar spine with instability [1,2]. Most lumbar fusions are performed using pedicle screw (PS) fixation. Lateral muscle dissection is required to insert the PS and requires a long surgical incision and retraction of the paravertebral tissue. These methods can cause severe postoperative pain at the surgical site and iatrogenic muscle damage. PS insertion also causes superior facet joint violation and injury to the medial branch of the posterior ramus of the spinal nerve. This procedure can cause mechanical pain around the screw insertion point and adjacent segment degeneration. The cortical bone trajectory (CBT) screw fixation method was first introduced by Santoni et al. [3] in 2009 to overcome the limitations of PS fixation. Since 2009, several articles and a meta-analysis have been published regarding cortical screw insertion [4-7].
However, there have been no large-scale or long-term studies performed at a single institution involving surgeries performed by a single surgeon. In addition, none of the prior studies have compared outcomes by surgical level. Therefore, the purpose of this study was to evaluate the clinical results of midline lumbar interbody fusion with CBT screw fixation for lumbar degenerative diseases and to compare the results based on surgical level.
This study was approved by the Institutional Review Board at National Health Insurance Service Ilsan Hospital (2019-044). Three hundred sixty-nine patients with lumbar degenerative disease underwent surgery between January 2013 and December 2015 at our institution. Among them, 80 patients were excluded because they had PS fixation. In addition, 20 patients were excluded due to need for a second operation, and 69 patients were excluded because they were lost to follow up (Fig. 1). Two hundred patients underwent conventional posterior lumbar interbody fusion with cortical screw fixation by a single surgeon. Clinical outcomes, radiologic studies, and surgical methods were reviewed and analyzed retrospectively. The inclusion criteria are described below. First, all patients were diagnosed with spinal stenosis, degenerative or spondylolytic spondylolisthesis, or degenerative disc diseases based on clinical symptoms, physical examination, and imaging (with x-ray, computed tomography [CT], and magnetic resonance imaging [MRI]). All of the included patients had received conservative treatments (including medication, physiotherapy, and injection therapy) for > 6 months prior to inclusion. We also included patients who required surgery due to significant clinical symptoms. Only cases that employed cortical screws with midline lumbar interbody fusion were included. Only patients with > 3 years of follow-up were included. The exclusion criteria are as follows: patients with tumors, congenital disease, fractures, or repeat surgeries; patients who underwent PS fixation; and patients who had < 3 years of follow-up. The mean patient age was 69.9 ± 15.8 years (range, 40–85 years). The patients were divided into 4 groups according to surgical level: (1) level 1 operation (136 patients), (2) level 2 operation (43 patients), (3) level 3 operation (12 patients), and (4) level 4 or higher operation (9 patients). The mean follow-up period was 48.9 ± 10.8 months (range, 38– 72 months).
The patients were placed under general anesthesia in the prone position. All operations were performed by a single surgeon (HYZ) using the same surgical technique. A midline skin incision was made, and posterior decompression was achieved by laminectomy and bilateral partial medial facetectomy. Discectomy was performed, and an interbody cage was inserted. We used 2 PEEK cages (CAPSTONE; Medtronic, Memphis, MN, USA) per disc level. The cortical screw was inserted into the pedicle under fluoroscopic guidance. We used a bilateral screw-rod system with CS (MIDLF; Medtronic Sofamor Danek, Memphis, TN, USA). If there were no surgical complications, the patient was allowed to sit upright and walk on the first postoperative day. Clinical and radiographic results were obtained by an independent observer for 6 days postoperatively. The patients were continuously followed in the outpatient clinic.
We examined the visual analogue scale (VAS), Oswestry Disability Index (ODI), 36-item Short Form health survey (SF-36) mental component summary score (MCS), and physical component summary score (PCS) at preoperative, postoperative, and final follow-up to determine the clinical outcomes. We also reviewed and analyzed the following parameters retrospectively: operative time, intraoperative bleeding, length of incision, days of hospitalization, and surgical complications.
Preoperatively, patients underwent x-ray, CT, and MRI imaging. Plain radiographs were obtained postoperatively at 6 months, 1 year, and at the final follow-up. Imaging with CT and MRI was performed in patients with adjacent segment disease (ASD) after surgery. The criteria for radiological ASD were as follows: (1) decrease of disc height > 10%; (2) translation > 3 mm and rotation changes > 10° on flexion and extension lateral x-rays; and (3) worsening by 2 or more grades as noted on postoperative lumbar lateral x-rays (based on the University of California, Los Angeles grading scale for intervertebral disk degeneration [8] at an adjacent level); and (4) identification of spinal stenosis or disc herniation at an adjacent level on follow-up MRI. The height of the intervertebral discs was measured on neutral lumbar lateral x-rays according to the Frobin method [9]. The surgical indications for ASD were extreme low back pain, severe radiculopathy, or limitation of daily activities caused by radicular or neurogenic intermittent claudication and that was refractory to at least 3 months of conservative treatment of at least 3-month duration. Fusion was determined at the final follow-up examination by the first author (SHN). Fusion was defined as absence of movement at the surgical level on dynamic x-rays and osseous continuity between the vertebra and the grafted bone on CT without loosening of the PSs [10].
The findings are expressed as mean value ± standard deviation or count, as indicated. One-way analysis of variance and chi-square tests were used to compare the results from the 4 groups after adjusting for age and sex. A p-value of < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA) and SAS ver. 9.2 (SAS, Cary, NC, USA).
Two hundred patients underwent midline lumbar interbody fusion with CBT screw insertion at the author’s institution. Table 1 shows the detailed demographics of the 4 groups of patients, which were comparable. This study comprised 63 male (31.5%) and 137 female patients (68.5%). Patient age ranged from 40 to 85 years (average age, 69.9 ± 15.8 years). The patients were followed for an average of 48.9 ± 10.8 months. The most frequent surgical site was L4/5, followed by L5/S1. The preoperative diagnoses were spinal stenosis without spondylolisthesis (102 patients), degenerative spondylolisthesis (76 patients), spondylolytic spondylolisthesis (19 patients), and deformity (3 patients). Radiographs of representative 2 cases are shown in Figs. 2 and 3.
Intraoperative blood loss, operation time, days of hospitalization, complications, and fusion rate of the 4 groups are shown in Table 3. Fusion rate was highest in group I (95.8%) and lowest in group IV (85.2%). Complications occurred in 17 cases (8.5%). ASD occurred in 16 cases, of which 5 required surgeries with ASD. Group 1 had 1 case of ASD, and group 4 had 4 cases. Screw loosening occurred in 1 case in group 4. There were no cases of infection or mechanical complications.
The new CBT method was initially supported by Santoni et al. [3]. In Matsukawa et al. [11], CBT screws provided a 30% increase in uniaxial yield pullout strength compared to that of conventional PS. In addition, in vivo insertion torque of the CBT screws increased by 1.71 times compared to that of conventional PS. Zhang et al. [12] found that CBT screws had better biomechanical performance in pullout strength and toggle tests than did conventional PS. It is known that CBT screw/rod structures provide almost the same stability as conventional PS-rod structures [13]. Prior studies have shown good results for CBT screws in the laboratory. However, prior to this study, there were no long-term or large-scale clinical studies. In addition, no prior studies regarding CBT screws addressed the results by surgical level. In this study, we will discuss the clinical efficacy of CBT fixation.
Most clinical outcomes, such as VAS, ODI, and Japanese Orthopaedic Association, improved after CBT screw fixation in prior studies [14]. There were no differences identified between CBT screw fixation and PS fixation. In our study, back VAS, leg VAS, ODI, and SF-36 improved at the final follow-up in all groups.
The CBT screw fixation technique of inserting a screw into the caudomedial entry point near the pars articularis has been widely used. This technique maximizes the interface between the screw and the cortical bone and provides enhanced screw bone bonding strength [15]. The paths from the inside to the outside and from the caudal to the cephalad portions of the cortical screw can reduce the risks of nerve damage and superior facet violation. This technique may also allow for shorter skin incisions, less muscle dissection, less intraoperative bleeding, shorter operation time, and shorter hospitalization. And this also reduced postoperative infection. Sakaura et al. [10,16] and Lee et al. [17] found that operative time, bleeding amount, hospital days, and incision length were all shorter/smaller with CBT fixation than with PS fixation. Although it was not discussed in this study, operative time, incision length, bleeding amount, and length of hospital stay were all lower with CBT fixation than with PS fixation in out institution. And there was no infection case.
Many studies have previously addressed the complication and fusion rates of CBT fixation [14,16,18]. The fusion rate of CBT fixation was not significantly different from that of PS fixation. In our study, the fusion rate was good at surgical levels 1–3 but poor above level 4. The potential complications of CBT fixation include intraoperative nerve injury, dura tear, screw malpositioning, postoperative surgical site infection, screw loosening, and adjacent segment degeneration. In Keorochana et al. [14], the intraoperative complication rate was lower with CBT fixation than it was with PS fixation, although the difference was not statistically significant. In our study, there was 1 case of screw malposition and 5 cases of dura tear. Shorter operative times improve a surgeon’s concentration and reduce the rate of intraoperative complications. Postoperative complications were significantly less frequent with CBT than they were with PS fixation. In Hu et al. [18], complications of CBT fixation were not significantly different from those of PS fixation. Among these complications, ASD occurred twice as often with PS fixation than it did with CBT fixation [16]. CBT fixation allows for a smaller incision of the superior facet and paraspinal muscles and less violation of the superior facet than does PS fixation [10]. Superior facet violations increase biomechanical stress and consequently cause instability in the adjacent segments [19]. In our study, ASD occurred in one case of level one and in 4 cases above level 4. Keorochana et al. [14] reported about the loss of reduction as a disadvantage of CBT. Compared to PS, it is difficult to obtain sufficient lordosis when surgery is performed with CBT level 3 or higher. PS is the most common and reliable tool for correcting spinal deformity [14]. So, in our cases, Smith-Petersen Osteotomy was performed to obtain sufficient lordosis through CBT.
However, screw loosening that occurs in patients with severe osteoporosis can be minimized by using CBT [20]. CBT increases pullout strength of screw by maximizing the contact surface between screw and cortical bone [11]. Biomechanical study reported that the pullout load of CBT was increased by 30% compared to PS [3]. In our study, there was 1 case of screw loosening in group 4. In case of severe osteoporosis, CBT is a good surgical method.
Our study has several limitations. It has inherent risk of selection bias given its retrospective design. In addition, because our study size was small, we were limited in our ability to make comparisons between the groups for several factors known to affect prognosis. Regardless of these limitations, the results of this study suggest that the operative results according to surgical level must be considered when performing CBT fixation. Prospective studies must be conducted using well-guided evidence-based protocols with adequate controls.
Fig. 2.
A case from one level cortical bone trajectory screw fixation group. (A-D) A 78-year-old woman had L4/5 stenosis. (E) She underwent L4/5 posterior interbody fusion with cortical bone trajectory screw fixation. (F-H) After 5 years, there were no specific complications on follow-up radiologic examination.
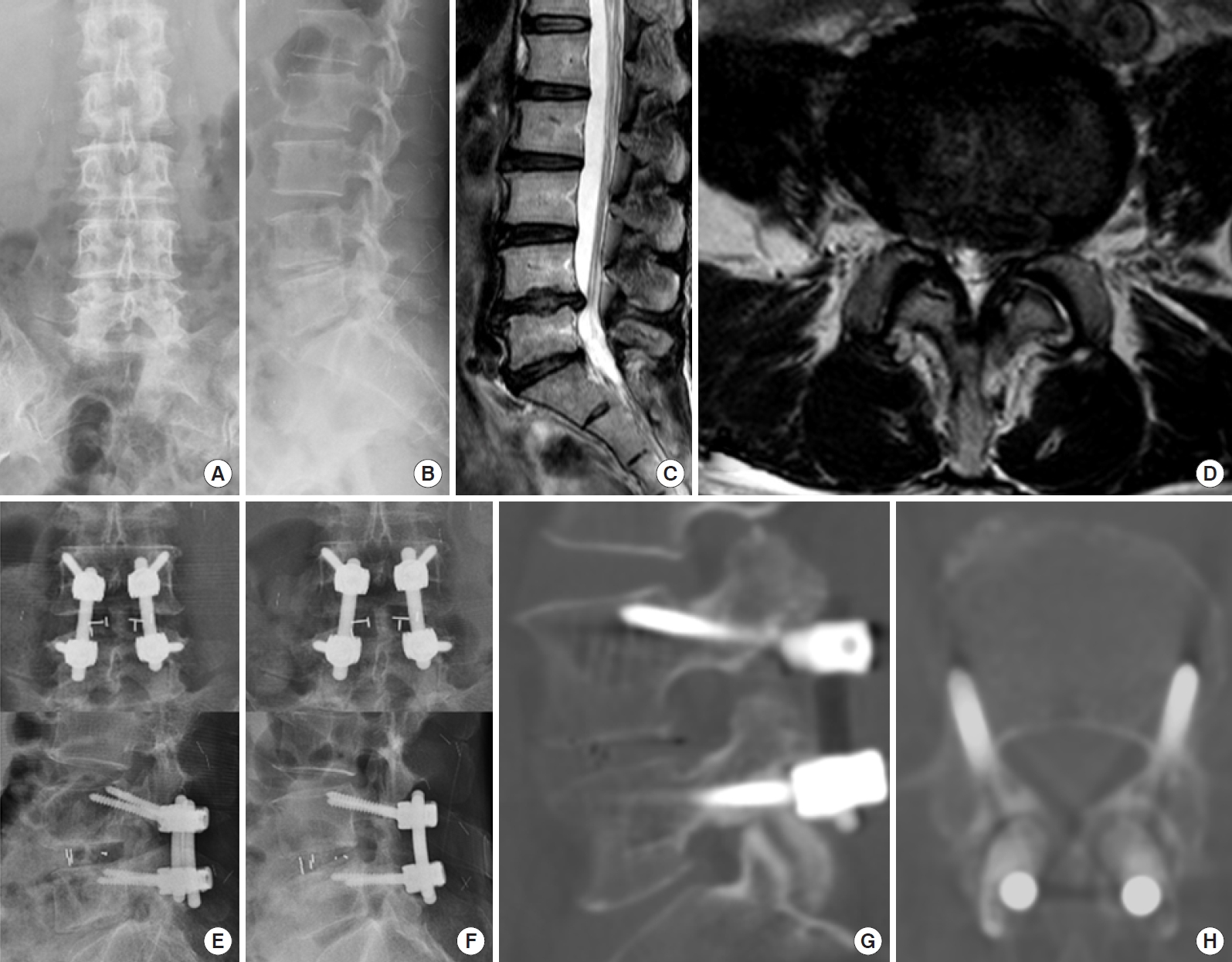
Fig. 3.
A case from 4 level cortical bone trajectory screw fixation group. (A-D) A 75-year-old man had multiple stenosis L2/3/4/5/S1. (E, F) He underwent L2/3/4/5/S1 posterior interbody fusion with cortical bone trajectory screw fixation. In the process of inserting the left L4 cortical bone trajectory screw, the pedicle was damaged, so the L4 screw could not be inserted. (G, H) After 5 years, there were no specific complications on follow-up radiologic examination.

Fig. 4.
All clinical outcomes improved significantly after 3 years in all groups. Back VAS (A), leg VAS (B), ODI (C), SF-36 MCS (D), and SF-36 PCS (E) improved significantly (in all groups) after 3 years (p < 0.05). VAS, visual analogue scale; ODI, Oswestry Disability Index; SF-36 MCS, 36-item Short Form health survey mental composite score; SF-36 PCS, 36-item Short Form health survey physical composite score.
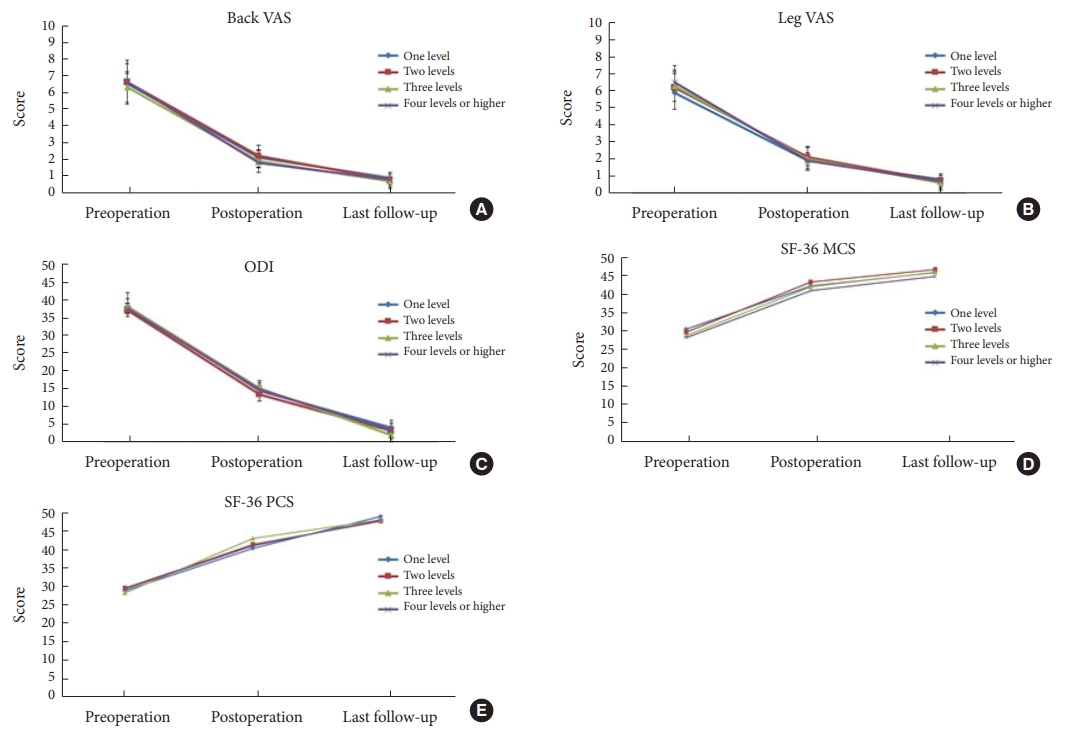
Table 1.
Patient demographics
Table 2.
Comparison of clinical parameters according to fusion levels
| Variable | One level (n = 136) | Two levels (n = 43) | Three levels (n = 12) | Four levels or higher (n = 9) | p-value | |
|---|---|---|---|---|---|---|
| Back VAS | ||||||
| Preoperation | 7.8 ± 0.36 | 7.5 ± 0.12 | 7.9 ± 0.15 | 8.2 ± 0.51 | 0.471 | |
| Postoperation | 2.7 ± 0.15# | 2.8 ± 0.18# | 2.8 ± 0.51# | 3.1 ± 0.48# | 0.687 | |
| Last follow-up | 1.9 ± 0.27# | 1.7 ± 0.39# | 1.8 ± 0.54# | 1.9 ± 0.37# | 0.269 | |
| Leg VAS | ||||||
| Preoperation | 8.2 ± 0.16 | 8.1 ± 0.27 | 8.3 ± 0.28 | 8.1 ± 0.62 | 0.271 | |
| Postoperation | 2.5 ± 0.24# | 2.4 ± 0.12# | 2.6 ± 0.73# | 2.5 ± 0.58# | 0.259 | |
| Last follow-up | 1.4 ± 0.71# | 1.3 ± 0.68# | 1.4 ± 0.71# | 1.5 ± 0.42# | 0.321 | |
| ODI | ||||||
| Preoperation | 43.9 ± 2.13 | 41.7 ± 2.32 | 45.2 ± 2.71 | 46.7 ± 1.75 | 0.461 | |
| Postoperation | 15.1 ± 2.01# | 14.6 ± 1.13# | 13.7 ± 2.27# | 14.5 ± 1.21# | 0.103 | |
| Last follow-up | 5.7 ± 1.54# | 4.9 ± 0.12# | 3.6 ± 1.12# | 5.1 ± 1.75# | 0.363 | |
| SF-36 MCS | ||||||
| Preoperation | 30.5 ± 9.12 | 29.7 ± 8.15 | 28.8 ± 5.17 | 28.2 ± 6.27 | 0.335 | |
| Postoperation | 42.1 ± 8.13# | 43.2 ± 7.22# | 41.9 ± 9.14# | 40.8 ± 10.1# | 0.825 | |
| Last follow-up | 45.7 ± 8.15# | 46.5 ± 9.13# | 45.8 ± 7.54# | 44.7 ± 9.77# | 0.433 | |
| SF-36 PCS | ||||||
| Preoperation | 28.8 ± 6.33 | 29.5 ± 7.27 | 28.2 ± 8.15 | 29.2 ± 4.89 | 0.541 | |
| Postoperation | 40.1 ± 8.12# | 41.2 ± 8.12# | 42.8 ± 7.19# | 40.8 ± 5.12# | 0.358 | |
| Last follow-up | 48.8 ± 7.59# | 47.5 ± 6.19# | 47.8 ± 5.85# | 47.8 ± 7.91# | 0.256 | |
Table 3.
Comparisons of intraoperative blood loss, operative time, hospital day, fusion rate, and complications
| Variable | One level (n = 136) | Two levels (n = 43) | Three levels (n = 12) | Four levels or higher (n = 9) | p-value | |
|---|---|---|---|---|---|---|
| Operation time (min) | 131.21 ± 22.46 | 152.74 ± 31.91 | 281.67 ± 57.57 | 332.67 ± 42.12 | 0.021* | |
| Bleeding loss (mL) | 153.17 ± 20.12 | 230.15 ± 31.75 | 812.24 ± 204.53 | 1,383.48 ± 257.32 | 0.001* | |
| Hospital day (day) | 9.15 ± 0.57 | 9.45 ± 0.28 | 12.37 ± 3.12 | 14.12 ± 3.78 | 0.037* | |
| Fusion rate (%) | 95.8 ± 1.21 | 95.3 ± 0.98 | 94.1 ± 2.71 | 85.2 ± 4.21 | 0.043* | |
| Complications | ||||||
| ASD | 1 | 0 | 0 | 4 | ||
| Screw loosening | 0 | 0 | 0 | 1 | ||
| Dura tear | 3 | 3 | 2 | 0 | ||
| Postoperative infection | 0 | 0 | 0 | 0 | ||
REFERENCES
1. Bridwell KH, Sedgewick TA, O’Brien MF, et al. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord 1993 6:461-72.


2. Dickman CA, Fessler RG, MacMillan M, et al. Transpedicular screw-rod fixation of the lumbar spine: operative technique and outcome in 104 cases. J Neurosurg 1992 77:860-70.


3. Santoni BG, Hynes RA, McGilvray KC, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J 2009 9:366-73.


4. Hung CW, Wu MF, Hong RT, et al. Comparison of multifidus muscle atrophy after posterior lumbar interbody fusion with conventional and cortical bone trajectory. Clin Neurol Neurosurg 2016 145:41-5.


5. Chin KR, Pencle FJR, Coombs AV, et al. Clinical outcomes with midline cortical bone trajectory pedicle screws versus traditional pedicle screws in moving lumbar fusions from hospitals to outpatient surgery centers. Clin Spine Surg 2017 30:E791-7.


6. Chen YR, Deb S, Pham L, et al. Minimally invasive lumbar pedicle screw fixation using cortical bone trajectory - a prospective cohort study on postoperative pain outcomes. Cureus 2016 8:e714.



7. Kasukawa Y, Miyakoshi N, Hongo M, et al. Short-term results of transforaminal lumbar interbody fusion using pedicle screw with cortical bone trajectory compared with conventional trajectory. Asian Spine J 2015 9:440-8.



8. Ghiselli G, Wang JC, Bhatia NN, et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am 2004 86:1497-503.


9. Frobin W, Brinckmann P, Biggemann M, et al. Precision measurement of disc height, vertebral height and sagittal plane displacement from lateral radiographic views of the lumbar spine. Clin Biomech (Bristol, Avon) 1997 12 Suppl 1:S1-63.


10. Sakaura H, Miwa T, Yamashita T, et al. Posterior lumbar interbody fusion with cortical bone trajectory screw fixation versus posterior lumbar interbody fusion using traditional pedicle screw fixation for degenerative lumbar spondylolisthesis: a comparative study. J Neurosurg Spine 2016 25:591-5.


11. Matsukawa K, Yato Y, Kato T, et al. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine (Phila Pa 1976) 2014 39:E240-5.


12. Zhang RJ, Li HM, Gao H, et al. Cortical bone trajectory screws used to save failed traditional trajectory screws in the osteoporotic lumbar spine and vice versa: a human cadaveric biomechanical study. J Neurosurg Spine 2019 Mar 8 1. -8. https://doi.org/10.3171/2018.12.SPINE18970. Epub.

13. Perez-Orribo L, Kalb S, Reyes PM, et al. Biomechanics of lumbar cortical screw-rod fixation versus pedicle screw-rod fixation with and without interbody support. Spine (Phila Pa 1976) 2013 38:635-41.


14. Keorochana G, Pairuchvej S, Trathitephun W, et al. Comparative outcomes of cortical screw trajectory fixation and pedicle screw fixation in lumbar spinal fusion: systematic review and meta-analysis. World Neurosurg 2017 102:340-9.


15. Kojima K, Asamoto S, Kobayashi Y, et al. Cortical bone trajectory and traditional trajectory--a radiological evaluation of screw-bone contact. Acta Neurochir (Wien) 2015 157:1173-8.


16. Sakaura H, Miwa T, Yamashita T, et al. Cortical bone trajectory screw fixation versus traditional pedicle screw fixation for 2-level posterior lumbar interbody fusion: comparison of surgical outcomes for 2-level degenerative lumbar spondylolisthesis. J Neurosurg Spine 2018 28:57-62.


17. Lee GW, Son JH, Ahn MW, et al. The comparison of pedicle screw and cortical screw in posterior lumbar interbody fusion: a prospective randomized noninferiority trial. Spine J 2015 15:1519-26.


18. Hu JN, Yang XF, Li CM, et al. Comparison of cortical bone trajectory versus pedicle screw techniques in lumbar fusion surgery: a meta-analysis. Medicine (Baltimore) 2019 98:e16751.