INTRODUCTION
Cerebrospinal fluid (CSF) leakage is a potential complication of cranial and spinal surgery11). Postoperative CSF leakage can induce delayed healing, wound infection and meningitis5,7.11). DuraSeal® (Covidien, Waltham, MA, USA) is a synthetic product which has been increasingly used to facilitate watertight repair of dural defects after cranial and spinal surgery. The DuraSeal® has been demonstrated to be both safe and effective in clinical studies2,4,7,10) and was approved in 2005 by the Food and Drug Administration (FDA). The authors report a patient who developed cord compression following the use of DuraSeal® in cervical spine surgery in which the expansion of the DuraSeal® was believed to be the causative factor.
CASE REPORT
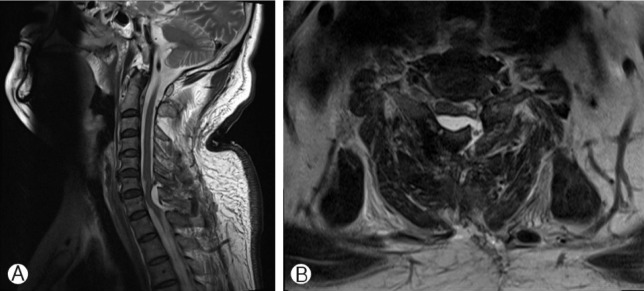
A 59-year-old man presented to the outpatient department with a 2-year history of progressive leg numbness. MRI (Magnetic resonance image) had shown intradural mass broad-based on the left side of the dura that compresses the cord severely, displacing it to the right posterior aspect at C7 level (Fig. 1). Opening the lamina and dura at C7 level, the mass removal was performed. The mass was totally removed and the histopathologic findings of frozen biopsy were consistent with those of a meningioma. When repairing dura, DuraSeal® was applied to dural surface that was incompletely approximated with sutures. Eight hours after surgery, the patient developed left-side weakness to motor grade I. A MRI scan was urgently performed, which demonstrated a large extradural mass which was causing severe cord compression at the level of tumor removal (Fig. 2). The patient underwent an emergency wound exploration. On reoperating wound, a thick layer of gelatinous material consistent with DuraSeal® was initially encountered. The layer of hydrogel compressed the dural sac severely. The hydrogel layer was excised. Also the minimal amount of surgicel was removed, which was separate and not interfering with the DuraSeal®. After surgery the patient is showing slightly improvement with his power increasing to motor grade II.
DISCUSSION
CSF leak is a potential complication after cranial and spinal surgery7,11). Leakage of CSF can lead to delayed healing of surrounding tissues such as skin, muscle, and bone due to its caustic effect11). Other potential complications include meningitis and severe headaches from CSF depletion7,11). The aim therefore once a CSF leak is identified, is to stem the leak.
Hydrogel sealant (DuraSeal®) is a synthetic sealant approved by the FDA, which has been increasingly used to facilitate watertight repair of dural defects after cranial and spinal surgery, and has been demonstrated to be both safe and effective in clinical studies2,4,7,10). DuraSeal® is a self-polymerizing agent which rapidly forms a firm, watertight hydrogel layer within several seconds of application over the dural surface. In comparison to fibrin glue, there is "stronger tissue adherence strength and burst strength". The firm layer is robust enough to withstand irrigation and gentle suction maneuvers without threat of dislodgement. These properties are advantageous in affecting a watertight barrier to minimize the risk of CSF leakage8).
Boogaarts et al. reported no evidence of postapplication CSF leaks or adverse events in 46 patients who had a spontaneous dural tear treated with DuraSeal®2). Cosgrove et al. reported similar findings with no evidence of postapplication CSF leaks in 95.5% of patients and no adverse events with the use of DuraSeal®4). After these reports, the use of DuraSeal® was extended as an adjunct to suture closure of an iatrogenic dural tear.
DuraSeal® is believed to be an ideal sealant for spine procedures because it is a nontoxic bioabsorbable synthetic hydrogel composed of 90% water with similar properties to tissue2,9). However, one of the drawbacks of using DuraSeal® is its potential to swell up to 50% and slow absorption rate. According to the manufacturers (Covidien, Waltham, MA, USA), product application to "confined bony structures where nerves are present is contraindicated." Few adverse events have been reported with the use of DuraSeal®1,3,9,11). A study in dog models has demonstrated that hydrogel shows in vivo expansion, and mass effect within the first 2 weeks, after insertion characterized by computerized tomography (CT) and MRI imaging6,11). The first reported complication of DuraSeal® was reported when used as a dural sealant after a posterior fossa decompression for a Chiari malformation1). A 13-year-old girl developed a worsening quadriparesis after the mass effect of hydrdogel sealant on the cervicomedullary region following foramen magnum decompression. A case of cervical cord compression has also been reported in a patient who underwent a C5-C6 anterior cervical decompression and fusion who sustained a CSF leak when excising the posterior longitudinal ligament with DuraSeal® being used as the primary sealant11). Three hours postoperatively, the patient developed progressive upper and lower extremity motor weakness. During emergent exploration and decompression, the surgeons determined the expanded hydrogel was the cause of the compression. Also a case has been reported of DuraSeal® causing cauda equina compression after a patient underwent a lumbar laminotomy and discectomy complicated by a dural tear9). This patient's dural tear was treated with DuraSeal® and the patient developed symptoms of cauda equina compression 9 days postoperatively. The patient was taken emergently to the operating room and during exploration and decompression, a large amount of blue DuraSeal® was visualized. The volume of DuraSeal® removed was measured as 10mL, which is more than three times the amount used primarily, suggesting considerable hydrogel expansion after implantation.
DuraSeal® has now been approved for spinal usage by the FDA. To date, the time profile and radiographic appearances of this dural sealant has previously only been systematically characterized in a dog craniotomy model6,8). In a dog model, DuraSeal®'s volume expansion was reported to peak between day 3 and 2 weeks after implantation6,9). To avoid such a complication, we would recommend judicious application of the DuraSeal® with thin-layer application to the defective area only. We would also caution against applying DuraSeal® in a manners such that it extends over and beyond the bone edges bordering the dural surface after bony decompression.
CONCLUSION
Our case demonstrates the use of DuraSeal® over a site of a dural repair is associated with the risk of developing postoperative cord compression as a result of swelling of the DuraSeal®. DuraSeal® tendency to expand is documented both in the instructions for its use and published case reports, and this is also confirmed by our experience. A very thin layer of DuraSeal® should be delivered over the site of dural repair and the possibility of postoperative expansion of it should be recognized by the surgeon.