Analysis of Associating Radiologic Parameters With Clinical Outcomes After Posterior C1–2 Fusion
Article information
Abstract
Objective
To evaluate which radiologic parameters affect clinical outcomes in patients underwent posterior C1–2 fusion for atlantoaxial dislocation.
Methods
From January 2014 to December 2017, among 98 patients underwent C1–2 posterior fusion, patients with previous cervical surgery or extending to subaxial spine or basilar invagination were excluded. Finally, 38 patients were included. O–C2, C1–2, C1–C7, C2–C7 cobb angle (CA), T1 slope, C1–7, C2–7 sagittal vertical axis (SVA), and posterior atlantodental interval (PADI) were measured at preoperative and postoperative 1 year. The difference between postoperative and preoperative values for each parameter was designated as Δvalue. Postoperative subaxial kyphosis (PSK) was defined to decrease ≥ 10° at subaxial spine. Visual analogue scale (VAS), Japanese Orthopedic Association (JOA) score, Neck Disability Index (NDI) were used to evaluate clinical outcomes.
Results
Mean age was 54.4 ± 15.9. Male to female was 14 to 24. Of radiologic parameters, C1–7 SVA and PADI were significantly changed from 26.4 ± 12.9 mm, 17.1 ± 3.3 mm to 22.6 ± 13.0 mm, 21.6 ± 3.4 mm. ΔC1–2 CA was correlated with ΔC1–7 CA and ΔC2–7 SVA. ΔPADI correlates with ΔO–C2 CA. VAS correlates with ΔC1–7 CA (p = 0.03). JOA score also correlates with ΔC2–7 SVA (p = 0.02). NDI was associated with ΔPADI (p < 0.01). The incidence of PSK was 23.7%, and not significant with clinical outcomes.
Conclusion
ΔC1–2 CA was correlated with ΔC1C7 CA, ΔC2–7 SVA. ΔC1–7 CA, ΔC2–7 SVA, and ΔPADI were the key radiologic parameters to influence clinical outcomes. Postoperative C1–2 angle should be carefully determined as a factor affecting clinical outcomes and cervical sagittal alignment.
INTRODUCTION
Atlantoaxial dislocation (AAD) can cause severe neurologic deficit which make patients disabled or neck pain results from kyphosis at upper cervical spine [1-6]. Pathologies of AAD were various such as trauma, inflammation, congenital anormaly, and iatrogenic causes [1-10]. The treatment of AAD has always been a major concern for spine surgeon. Since transarticular screw fixation and interlamina fusion was introduced by Mergel and Brook, posterior C1–2 fusion has been known as an effective treatment for AAD [11,12]. As Ham’s technique emerged, cervical polyaxial screw and rod fixation system is widely used because of its simplicity of the technique and low risk of vertebral artery injury [13].
As these surgical techniques have been popularized, some surgeons gradually interested in postoperative changes in cervical sagittal balance [14,15]. C1–2 fusion led to reciprocal changes in subaxial spine according to its angle, and it is considered to be an important factor for sagittal alignment in subaxial spine [16]. Moreover, some studies stated that these surgical techniques are associated with complex cervical deformities such as postoperative regional kyphosis, postoperative subaxial kyphosis (PSK), or hyperlordosis [17,18].
Previous studies focused on evaluating the relationship between postoperative C1–2 angle and subaxial sagittal alignment, but there were a few studies stated that the association between postoperative radiologic parameters related with sagittal balance and clinical outcomes [19,20]. In our study, we analyzed the correlation between radiologic parameters in cervical spine before and after posterior C1–2 fusion. Furthermore, we investigated how these changes in radiologic parameters affect clinical outcomes in patients treated posterior C1–2 fusion.
MATERIALS AND METHODS
1. Patient Selection
A retrospective analysis of medical records and radiologic data was performed on patients that had undergone posterior atlantoaxial fusion for AAD at a single center from January 2014 to June 2017. This study was approved by the Institutional Review Board of Catholic Medical Center (OC21RISI0008). Ninety-eight patients treated by posterior C1–2 fusion during this period. Patients had previous cervical surgery history or concomitant with basilar invagination were excluded. In addition, patients needed additional fusion extension to occipital or subaxial spine were also excluded. Finally, 38 patients were included in the study.
2. Surgical Techniques
The patient was placed in prone position with Mayfield head fixator under general anesthesia. The surgeon reduced C1–2 dislocation as much as possible by adjusting patient’s head by flexion or extension while pulling out the Mayfield head fixator. C1–2 reduction was confirmed under the C-arm fluoroscopy. When we cannot achieve acceptable reduction by adjusting patients’ position, we usually released C1–2 facet joint in order to reduce dislocation additionally by removing the capsule surrounded it during surgery. C2 roots were preserved by protecting root retractor during the releasing of C1–2 facet. All procedures were performed under intraoperative monitoring (IOM). Vascular anomalies were evaluated preoperatively to avoid neurovascular injury. Atlantoaxial fixation was accomplished using a variety of surgical constructs combined with C1 lateral mass to C2 pedicle screw, C2 pars screw, or to C2 laminar screw fixation. We controlled C1–2 angle by compressing or distracting the interspace between 2 polyaxial screws along the rods. Autograft bone harvested from the posterior superior iliac spine was inserted to the interlaminar space of C1–2 to enhance the fusion. Modified brook’s Wiring technique between C1 and C2 lamina was performed to fix the autograft bone and obtain additional biomechanical strength [11]. Finally, fluoroscopy was used to confirm the reduction and lordotic angle of C1–2 (Fig. 1).

Radiologic and surgical figures of the patient treated by C1 lateral mass–C2 pars and lamina screw construct wiring interlamina with autograft bone. Preoperative lateral (A) and postoperative lateral (B) and anteroposterior (C) radiographs. (D) Midsagittal image of computed tomography after surgery. (E) Intraoperative figure represents wiring interlamina (black arrow) with an autograft bone (white arrow).
3. Radiologic Parameters
Two neurosurgeons (JTH and JHP) measured all radiologic parameters on cervical standard lateral radiographs using INFINIT PACS (INFINIT Healthcare, Seoul, Korea) using an electrical caliper on 2 occasions. The 4 sets of radiologic parameters measured were then averaged for statistical analysis. Lateral radiographs were obtained in the neutral head position. A standard distance of 1.8 m was maintained between the tube and patients. The following parameters were measured on radiograph before surgery and at 1 year after surgery.
• C2 cobb angle (CA): The angle between the line connecting McGregor line and the inferior endplate of C2 (Fig. 2A).
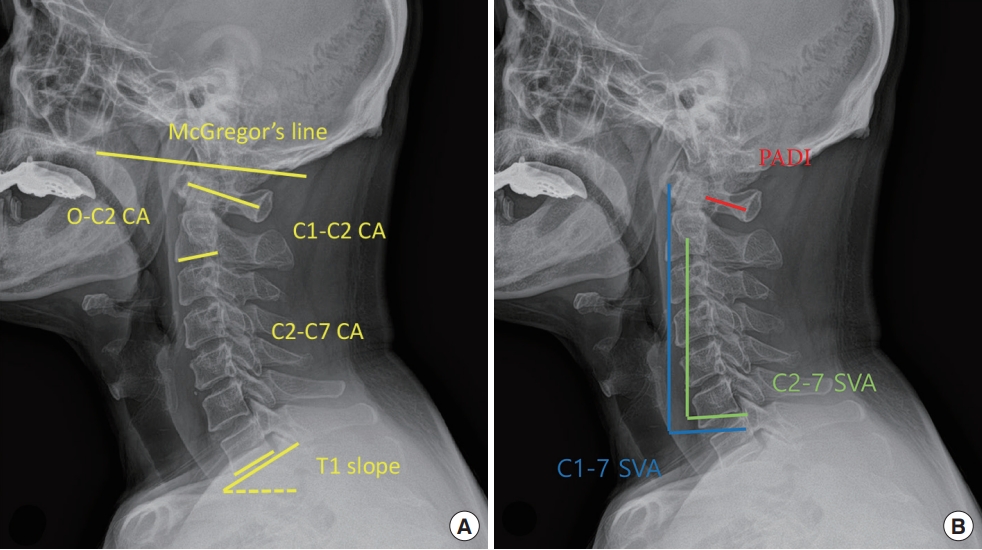
Radiologic parameters on a cervical lateral plain radiograph in patient with atlantoaxial dislocation. (A) O–C2, C1–2, C1–7, C2–7, T1 slope are measured between the lines on cervical lateral plain radiograph. (B) C1–7 (blue line), C2–C7 sagittal vertical axis (SVA; green line), and posterior atlantodental interval (PADI; red line) are measured on cervical lateral plain radiograph. CA, cobb angle.
• C1–2 cobb angle (CA): The angle between the line connecting the middle point of the anterior and posterior arch of C1 and the inferior endplate of C2 (Fig. 2A).
• C1–7 cobb angle (CA): The angle between the line connecting the middle point of the anterior and posterior arch of C1 and the inferior endplate of C7 (Fig. 2 A).
• C2–7 cobb angle (CA): The angle between the inferior endplate of C2 and C7 (Fig. 2A).
• T1 slope: The angle between horizontal line and the T1 superior endplate. (Fig. 2A).
• C1–7 sagittal vertical axis (SVA): The distance between the plumb line from the anterior margin of C1 and posterior superior corner of C7 (Fig. 2B).
• C2–7 sagittal vertical axis (SVA): The distance between the plumb line from the center of C2 and the posterior superior corner of C7 (Fig. 2B).
• Posterior atlantodental interval (PADI): The distance between the line connecting the middle point of the anterior and posterior arch of C1 and the dens of C2 (Fig. 2B).
The difference between preoperative and postoperative values for each parameter was designated as the Δvalue.
PSK was defined as the postoperative change of ≥ 10° at C2–7 CA.
4. Clinical Outcomes
Clinical outcomes were assessed using visual analogue scale (VAS) for neck pain, Neck Disability Index (NDI) [21] and Japanese Orthopedic Association (JOA) scores [22] at preoperative and postoperative one year. Improvements in VAS and NDI scores were also expressed as the difference between postoperative and preoperative values. The Δvalue was used for the difference between postoperative and preoperative values for each parameter.
5. Statistical Analysis
The Student t-test, the paired t-test, and Mann-Whitney Utest were used to analyze continuous and ordinal variables, as appropriate. Correlation test and a linear logistic regression model were used to evaluate the natures of correlations between the radiologic parameters and clinical outcome. p-values of < 0.05 (2-tailed) were considered statistically significant, and IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. The intra-inter reliabilities of radiologic parameters were calculated. Intraclass correlation coefficient values were rated as follows: 0 to 0.2 slight agreement, 0.21 to 0.4 fair agreement, 0.41 to 0.6 moderate agreement, 0.61 to 0.8 substantial agreement, and 0.81 to 1.0 excellent agreement.
RESULTS
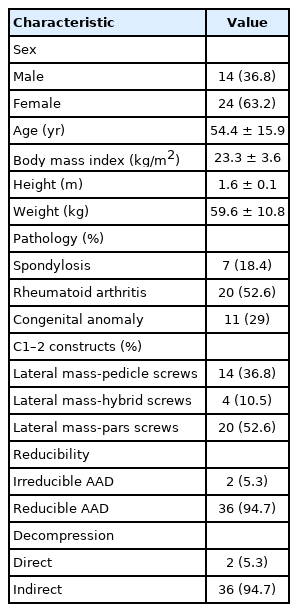
Clinical information is summarized in Table 1. There were 14 males and 24 females of mean age 54.4± 15.9 years and mean body mass index 23.3 ± 3.6 kg/m2. Mean height and weight were 1.6 ± 0.1 m and 59.6 ± 10.8 kg. Twenty patients (52.6%) had rheumatoid arthritis (RA), 11 (29%) had a congenital anomaly, and 7 (18.4%) had degenerative spondylosis as pathologies for AAD. Fourteen patients were fixed with C1 lateral mass –C2 pedicle screw construct, 4 with C1 lateral mass –C2 hybrid construct, and 20 with C1 lateral mass –C2 pars construct. Two patients were irreducible AAD, then we performed to release of C1–2 facet joint during surgery. There were no patients need anterior approaches for additional decompression. Thirty-six patients were reducible AAD and obtained sufficient reduction of C1–2 dislocation by pushing down the spinous of C2 during connecting the rod. Two patients needed C1 laminectomy for decompression, we usually inserted autograft bone chip into released C1–2 facet joint for using fusion-bed. The remaining 36 patients, only indirect decompression obtained the reduction of atlantoaxial joint was enough.
1. Radiologic Parameters
Radiologic parameters obtained at preoperative and postoperative are summarized in Table 2. The difference between preoperative and postoperative each CA except C1–7 SVA and PADI was not significant because the values of each CA in some patients were counteracted for each other when analyzing all of the values together. This feature may contribute to small difference between preoperative and postoperative each CA, and it was not significant. T1 slope seemed unchanged statistically at postoperative for the same reason. C1–7 SVA showed a tendency to decrease from 26.4± 12.9 to 22.6± 13 at postoperative (p= 0.03). C2–7 SVA showed slight increase at postoperative, but it was not significant. PADI dramatically increased about 4.5 mm comparing to preoperative value (p< 0.01). The intra- and intercorrelations of radiologic parameters were 0.94 and 0.88, respectively. Measurements of radiologic parameters showed excellent degree of agreement.
Correlations between radiologic parameters are presented in Table 3. ΔO–C2 CA correlated positively with ΔC1–2 CA and ΔPADI, and negatively with ΔC2–7 CA. ΔC1–2 CA correlated positively with ΔC1–7 CA and ΔC2–7 SVA. ΔC1–7 CA correlated positively with ΔC2–7 CA and ΔT1 slope. ΔC2–7 CA correlated positively with ΔT1 slope and negatively with ΔC1–7 SVA and ΔC2–7 SVA. ΔC1–7 SVA correlated positively with ΔC2–7 SVA.
2. Clinical Outcomes
VAS, NDI, and JOA score improved significantly at postoperative. Mean VAS decreased from 5.1±2.9 to 1.7±1.6 (p<0.01). Mean JOA scores increase from 13.2±2.7 to 15.3±2.6 (p=0.02). Mean NDI decreased form 22.2 ±11.0 to 6.7 ±5.8 (p < 0.01). However, 3 patients deteriorated neck pain at postoperative. One patient suffered severe neck pain at VAS 9. The patient showed that reciprocal kyphotic change from 34° to 1.5° in subaxial spine after surgery. The other presented neck pain at VAS 4. The patient represented slight change of ΔC2–7 CA from 32.5° to 34.4°, but we failed to make lordotic C1–2 angle (from 4.5° to 4.8°) intraoperatively. Another complained neck pain VAS 5. The patient also showed that reciprocal kyphotic change from 10.9° to 2.2° in subaxial spine even though we made kyphotic C1–2 angle from 22.2° to 13.1°. This patient complained neck pain and developed subaxial kyphosis although we tried to underreduce C1–2 angle.
3. Relationship Between Radiologic Parameters and Clinical Outcomes
VAS, NDI, JOA score were associated with several radiologic parameters. Δ VAS correlated with ΔC1–7 CA, ΔT1S negatively (r= -0.357, p= 0.03, r= -0.341, p= 0.04). ΔNDI correlated with ΔPADI negatively (r= -0.499, p= 0.01). ΔJOA score correlated with ΔC2–7 SVA positively (r= 0.354, p= 0.03). In linear logistic regression, ΔC1–7 CA represented negatively linear correlation with ΔVAS, ΔPADI also showed negatively linear correlation with ΔNDI, ΔC2–7 SVA represented positively linear correlation with ΔJOA score, respectively (Fig. 3).
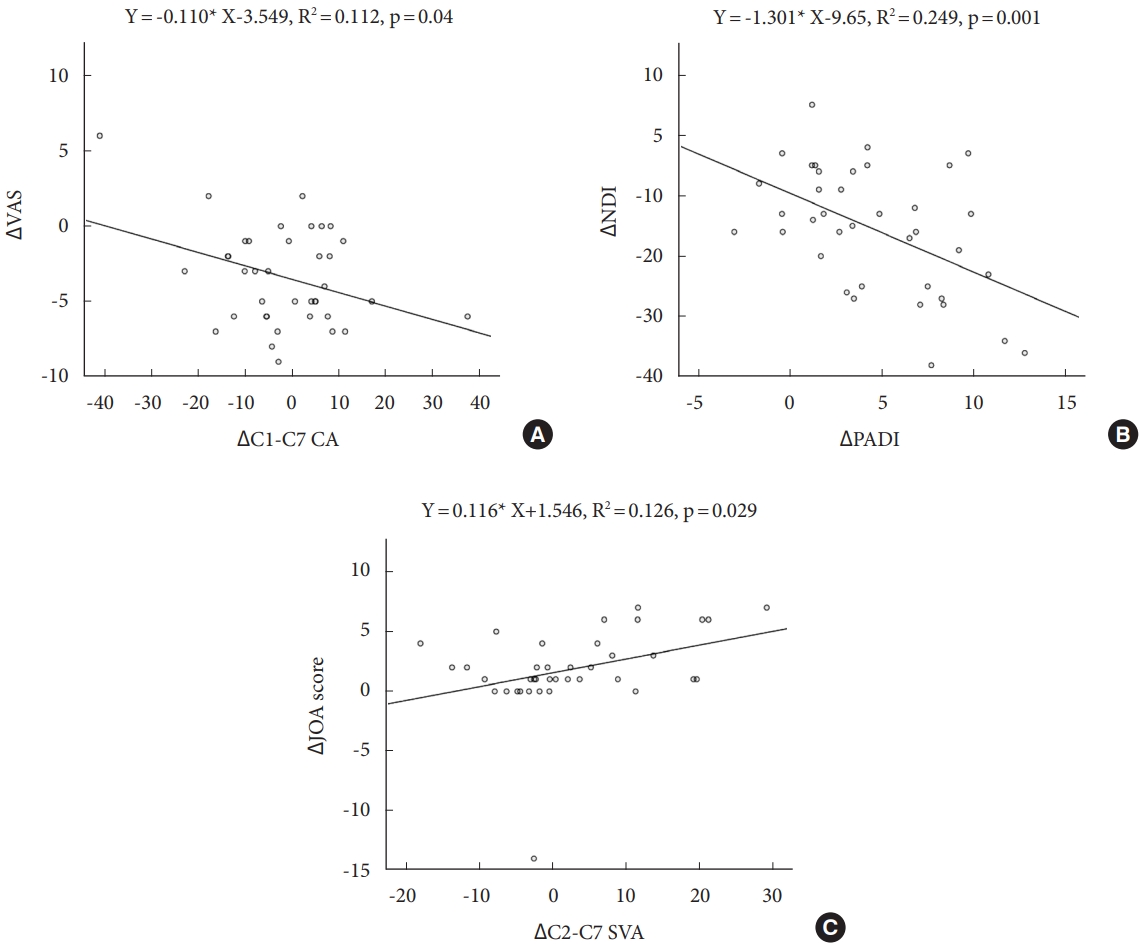
Linear logistic regression plots between radiologic parameters and clinical outcomes. A linear correlation with ΔC1–7 CA (A), ΔVAS a linear correlation with ΔPADI (B) and ΔNDI a linear correlation with ΔC2–C7 SVA and ΔJOA score (C). CA, cobb angle; VAS, visual analogue scale; PADI, posterior atlantodental interval; NDI, Neck Disability Index; SVA, sagittal vertical axis; JOA, Japanese Orthopedic Association. Asterisk (*) means multiple.
The incidence of PSK was 23.7%, it was not significantly associated with VAS (p = 0.26), NDI (p = 0.32), JOA score (p = 0.97).
DISCUSSION
Atlantoaxial fusion is frequently associated with sagittal realignment in subaxial spine, and many authors stated the negative correlation between ΔC1–2 CA and ΔC2–7 CA after surgery [14,15,17,18]. However, we observed different results of correlation between ΔC1–2 CA and ΔC2–7 CA. Postoperative sagittal realignment in subaxial spine was occurred in our study. However, the reciprocally negative correlation between ΔC1–2 and ΔC2–7 CA wasn't found although preoperative C1–2 and C2–7 CA represented the negative correlation each other. We found that the reciprocally negative correlation between ΔO–C2 and ΔC2–7 CA, instead of ΔC1–2 CA. We studied why this phenomenon happened in our study unlike other studies. First, ΔC1–2 CA was compensated by ΔC2–7 CA as well as ΔO–C1 CA to maintain the horizontal gaze. The preoperative range of motion (ROM) of C1–2 angle in normal people has about 6° when flexion and extension [23], and compensate for the change of subaxial spine in available ROM. However, postoperative C1–2 angle is fixed after surgery, and it plays the constant no room to change. It seems that the O–C1 angle plays as a buffer angle to maintain the horizontal gaze. ΔC2–7 CA was compensated by ΔO–C1 CA instead of the constant C1–2 angle. It seems that postoperative C1–2 CA as the constant does not work on cervical sagittal realignment. ΔO–C2 CA was actually the parameter obtained by adding a constant to ΔO–C1 CA. Therefore, ΔO–C2 CA was correlated with ΔC2–7 CA negatively, not ΔC1–2 CA excluding ΔO–C1 CA. Second, we observed a radiologic feature of patients in this study. The ratio of C1–2 CA and C2–7 CA was different to normal ranged patients. Some authors stated that normal values of C1–2 angle ranged from 25.6° to 28.9° and it accounted for 75%–80% of cervical standing lordosis [24,25]. However, our patients showed the proportion of cervical lordosis of C1–2 CA was about 52.5%, suggesting that C1–2 CA constitutes a relatively small proportion of cervical lordosis, and the proportion of C2-7 CA is predominant in cervical lordosis compared with others. Therefore, ΔC2–7 CA is increased relatively compared to other studies. There is the possibility that this feature might contribute that the reciprocal correlation between ΔC1–2 and ΔC2–7 CA was not significant.
The correlation of each radiologic parameter was summarized in Table 3. The important findings of this correlation test is described to 3 things. One thing is that the change of upper cervical spine (ΔO-C2 CA) is correlated with the change of subaxial cervical spine (ΔC2-C7 CA) and this relationship does not be affected before and after surgery. Second thing is that ΔC1–2 CA is associated with cervical kyphosis and flattening of cervical curvature. Third thing is that ΔC2–7 CA is a subjective radiologic parameter affected by various radiologic parameters.
ΔC1–2 CA was associated with ΔO–C2, ΔC1–7 CA, and ΔC2–7 SVA. Moreover, ΔC1–7 CA was negatively correlated with ΔVAS (Fig. 3A). Therefore, it can be expected if ΔC1–7 CA decreased after surgery, neck pain would be unchanged or worse. In addition, ΔC2–7 SVA had a positive correlation with ΔJOA score (Fig. 3C). Therefore, cervical sagittal alignment is significantly related to neck pain as well as cervical myelopathy. Some studies supported our results. Shimizu et al. [26] found that a significant correlation between the degree of cervical kyphosis and the amount of cord flattening leading to decreased vascular supply. Cervical sagittal malalignment is strongly related with neck pain. Tang et al. [27] also reported that C2–7 SVA directly correlated with NDI and cervical myelopathy. As a result, intraoperative C1–2 angle determined by surgeon was an important factor to affect not only cervical sagittal realignment but also VAS and JOA score.
ΔC1–7 CA was associated with ΔC1–2 CA, ΔC2–7 CA, ΔT1 slope. This correlation is taken for granted that C1–7 CA was the parameter including C1–2 and C2–7 CA. ΔT1 slope was changed according to ΔC1–7 CA.
ΔC2–7 CA also correlated with ΔO–C2 CA, ΔC1–7 CA, ΔT1 slope, ΔC1–7 SVA, and ΔC2–7 SVA. It was the most subjective radiologic parameter that was correlated with various others and also associated with VAS and JOA score such like ΔC1–2 CA. Nevertheless, surgeons can adjust ΔC1–2 CA as determining intraoperative C1–2 CA under C-arm fluoroscopy, but ΔC2–7 CA cannot be controlled intraoperatively and be predicted during follow-up. Therefore, surgeons should carefully observe the change of C2–7 CA in the patient after posterior C1–2 fusion.
ΔT1 slope was relative with ΔC1–7 CA, ΔC2–7 CA. This change in T1 slope explains that ΔT1 slope was complementary to the change of cervical spine.
ΔC2–7 SVA was correlated with ΔC1–2 CA, ΔC2–7 CA, ΔC1–7 SVA, JOA score. ΔC2–7 SVA was affected simply not only ΔC2–7 CA, but also ΔC1–2 CA. ΔC1–7 SVA was correlated with ΔC2–7 SVA each other. However, ΔC1–7 SVA showed the difference to ΔC2–7 SVA in that there was not correlated with ΔC1–2 CA. This different point interestingly affected that ΔC1–7 SVA was not significant with JOA score.
ΔPADI correlates with ΔO–C2 CA, and it was a factor associated with ΔNDI. ΔPADI increased significantly after surgery, it pointed out that most patients obtained enough reduction of AAD intraoperatively. This point explained why the reduction of AAD is important in the improvement of quality of life. Several Authors emphasized the importance of enough reduction of AAD, but this was still controversial. Jun et al. [28] suggested that complete reduction of AAD could obviate the need for direct decompression. Goel and Shah [29] introduced that facet manipulation and fixation in irreducible AAD facilitated reduction of AAD. Otherwise, Wang et al. [30] stated that sufficient decompression by laminectomy and solid fusion for AAD is more important than complete reduction for treatment of AAD. Lang et al. [31] also reported that incompletely reduced AAD had comparable clinical outcomes with those with complete reduction. Nevertheless, complete reduction of AAD without laminectomy can provide patients with sufficient fusion-bed for bone graft. Because of this advantage, we removed the capsule of C1–2 facet joint and distracted the facet joint by osteotome to release sufficiently in case of irreducible AAD. Finally, we obtained sufficient reduction of AAD in 94.7% of patients except 2 cases performed C1 laminectomy.
The PSK that occurred 23.7% of patients was not correlated with clinical outcomes. There are some studies that PSK was one of causes for postoperative neck pain [32]. We also agree that neck pain was associated with PSK. In the present study, patients with PSK showed the tendency to complain of severe neck pain. However, deterioration of neck pain was also observed in patients without PSK in our study. This point resulted in the failure to prove the statistical significance that PSK is related with neck pain. Yoshimoto et al. [18] also observed similar results of ours. The author stated that 12 patients among 44 patients without any progression of PSK complained neck pain aggravated after surgery. For this reason, there was no significance found between PSK and clinical outcomes.
Several studies reported the PSK between the changes in subaxial alignment and intraoperative C1–2 angle [17,18,33]. Toyama [34] investigated 75 cases of interlaminar bone grafting with wiring and reported that straight, kyphotic, and swan neck deformities occurred after surgery and recommended that the optimum postoperative C1–2 CA is 20°. Kato et al. [35] also recommended that the optimum postoperative C1–2 CA should be 20° in patients with the preoperative C1–2 angle of 0°–20° or < 0°, but perform an in situ angle in patients with a C1–2 angle of ≥ 20°. Some authors stated that surgical overreduction of C1–2 CA would be associated with PSK instead of optimal C1–2 angle [34-36]. These statements will be useful to recover the lordosis of subaxial spine and decrease the kyphosis of subaxial spine postoperatively. However, there is no consensus for optimal C1–2 fusion angle because physiological cervical sagittal alignment is different individually. The understanding of postoperative sagittal alignment is still insufficient. PSK is the result from multiple factors associative with cervical sagittal realignment such as age, postoperative C1–2 angle, the extent of surgical dissection, compensation of adjacent segmental angle. Therefore, it is careful to define the optimal postoperative C1–2 CA. Nevertheless, there are several things that spine surgeon should pay attention to obtain better clinical outcomes during surgery. At first, most patients with AAD have kyphotic C1–2 angle, and it is important that kyphotic C1–2 angle change to physiological lordotic angle. It is because a decrease in the C1–2 and C0–2 angle may likely induce a reduction in the pharyngeal space and can be a predictor of postoperative dysphagia, which is not compensated by the middle or lower cervical spine. At second, it is difficult to adjust C1–2 angle to target angle intraoperatively. We tried to fix postoperative C1–2 CA to 20° under C-arm fluoroscopy, but in some patients C1–2 CA were fixed more or less than 20°. Therefore, the greatest care must be taken to determine C1–2 fixation angle during surgery. Finally, C1 slope should be posteriorly slanted. It is not only because posteriorly slanted C1 slope is important to maintain the C1–2 segment lordosis, but also because the posteriorly slanted C1 slope and kyphotic angulation of the C0–1 segment allows some degree of freedom for neck extension as the space between the occiput and C1 posterior arch and allows some rooms for upper cervical extension to prevent the collision of occiput and implant.
The weaknesses of this study are its retrospective design and small sample size. In addition, patients had various pathologies, which included RA, congenital anomalies, and osteoarthritis. This study included 2 irreducible AAD patients. We performed the releasing of C1–2 facet in these patients, but we could not obtain sufficient reduction of AAD, which may have made a different sagittal realignment comparing to other patients. Moreover, C1–2 constructs for posterior fusion was not monotonous, it composed of hybrid structures such like C2 pedicle, lamina, and pars screws. Although we compressed or distracted the rod under C-arm fluoroscopy to control appropriate C1–2 CA, some patients obtained postoperative C1–2 CA showed a large deviation around 20°. Patient numbers 6 and 36 obtained kyphotic C1–2 angle of 4.8° and 9.4°. Patient numbers 12 and 38 obtained lordotic C1–2 angle of 34.3° and 36°. It is difficult for us to adjust intraoperative C1–2 CA closely as looking images in C-arm fluoroscopy. Finally, the long-term radiographic and clinical outcomes more than one year were not evaluated in the present study.
CONCLUSION
ΔC1–7 CA, ΔC2–7 SVA, and ΔPADI were the key radiologic parameters to influence clinical outcomes. Postoperative C1–2 angle relative to ΔC1–7 CA and ΔC2–7 SVA should be carefully determined as improving individual’s pain and neurologic improvement. Indirect decompression obtained by reduction of AAD is also important to increase ΔPADI and then decrease NDI.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: JTH; Data curation: JP, JTH; Formal analysis: JP, JTH; Methodology: JP, JTH; Project administration: JTH; Visualization: JP, JTH; Writing - original draft: JP; Writing-review & editing: JTK, ISK, JTH