The Role of Alginate Hydrogels as a Potential Treatment Modality for Spinal Cord Injury: A Comprehensive Review of the Literature
Article information
Abstract
Objective
To comprehensively characterize the utilization of alginate hydrogels as an alternative treatment modality for spinal cord injury (SCI).
Methods
An extensive review of the published literature on studies using alginate hydrogels to treat SCI was performed. The review of the literature was performed using electronic databases such as PubMed, EMBASE, and OVID MEDLINE electronic databases. The keywords used were “alginate,” “spinal cord injury,” “biomaterial,” and “hydrogel.”
Results
In the literature, we identified a total of 555 rat models that were treated with alginate scaffolds for regenerative biomarkers. Alginate hydrogels were found to be efficient and promising substrates for tissue engineering, drug delivery, neural regeneration, and cellbased therapies for SCI repair. With its ability to act as a pro-regenerative and antidegenerative agent, the alginate hydrogel has the potential to improve clinical outcomes.
Conclusion
The emerging developments of alginate hydrogels as treatment modalities may support current and future tissue regenerative strategies for SCI.
INTRODUCTION
Biomaterial engineers and physician-scientists have come together to create innovative solutions as the burden of chronic diseases rises worldwide [1]. While the application of injectables and enhanced medical device systems of synthetic materials has led to breakthrough outcomes, the biomaterial’s reactivity with the biological system can lead to cytotoxic immunological effects [1]. This has created a bottleneck in the development of biomaterial-based treatments that can promote optimal physiological functions while remaining chemically inert [2]. Recently, biomolecular and cell-based approaches have taken the spotlight in treating a wide variety of pathologies, with spinal conditions now at the forefront of biomedical research [3].
Spinal cord injury (SCI) is a traumatic life-changing pathology with substantial physical, emotional, and socioeconomic implications on the patient [3]. SCI outcomes often include partial or complete loss of sensory and motor function below the injury level. The therapeutic role of biomaterials, such as hydrogels, has previously been evaluated extensively as a regenerative modality. In general, hydrogels are separated into naturally derived and synthetic forms. The natural forms may be derived from chitosan, hyaluronan, collagen, agarose, or alginates [4]. Synthetic forms may be derived from polyethylene glycol, polyurethane, and poly(-ε-caprolactone) which have been U.S. Food and Drug Administration approved for use in humans subjects [5-7]. Hydrogel materials have also been found to occupy the injury site and take on a variety of shapes for in situ gelations. Once incorporated, their soft, highly porous, and 3-dimensional (3D) structure can mimic the extracellular matrix (ECM) environment to support damaged spine tissue [8].
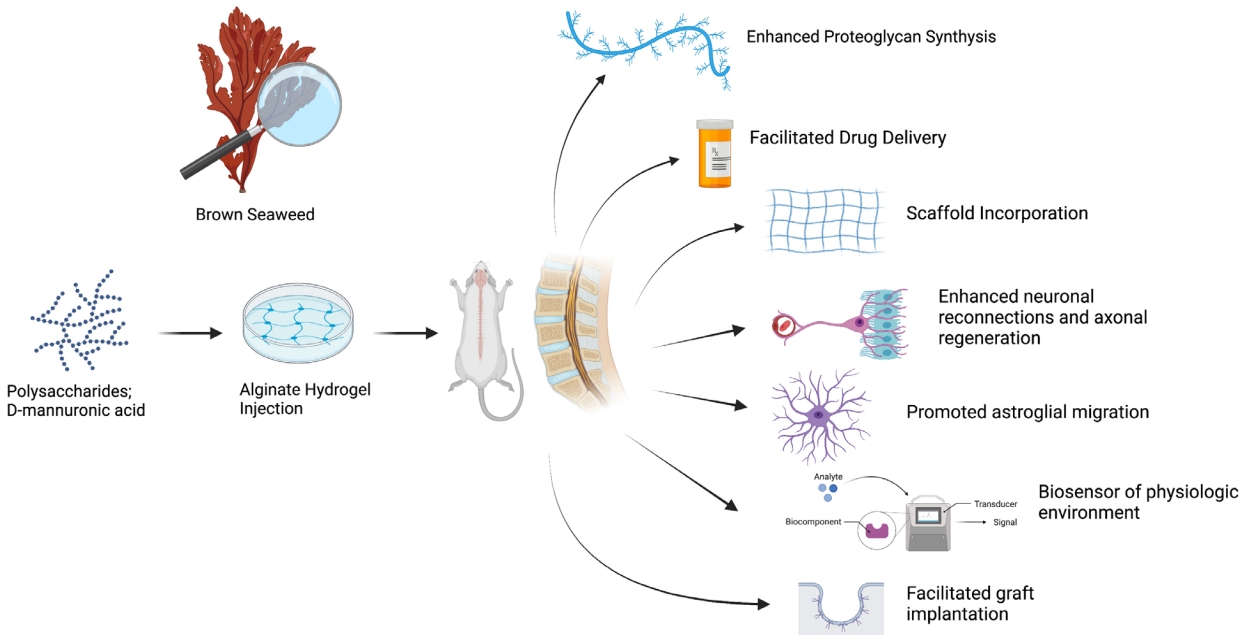
The introduction of alginate hydrogels into clinical spine research may drive the application of biomaterials in mainstream spinal care [9]. Alginates in their basic forms are a family of naturally occurring polysaccharides that are purified from brown algae [10,11]. Historically, alginates have been utilized in several industries. For example, in the food industry, it is used as an additive for food stabilization or as a binding agent [12]. When alginates are hydrated, they form into a viscous, hydrophilic, and biocompatible hydrogel [2,13]. This key feature is valuable due to the broad range of its applicability, particularly in tissue engineering in regenerative medicine, where it can be designed to mimic the mechanical integrity of natural-human tissue [2,14]. Herein, we describe alginate hydrogels’ properties while presenting their functional outcomes in SCI. We also aim to provide an overview of the current advances in spinal care related to this biomaterial and depict its value in future treatment considerations.
METHODOLOGY
A comprehensive search of the literature was performed using electronic databases such as PubMed, EMBASE, and OVID MEDLINE electronic databases. The keywords used were “alginate,” “spinal cord injury,” “biomaterial,” and “hydrogel.” Studies that did not discuss alginate hydrogels, conference abstracts, or non-English articles were omitted. Our search yielded 180 articles, of which 81 were selected for inclusion in our review.
GENERAL PROPERTIES
1. Composition
Alginates are polysaccharides extracted from brown algae; these include Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera [15]. D-mannuronic acid constitutes the primary component in alginate hydrolysate, which is made of guluronic acid [16]. Further characterization has shown that alginate is composed of homopolymeric blocks of (1, 4)-linked beta-D-mannuronic acid (M-residues) and alpha-L-guluronic acid (G-residues) arranged in diaxial links (Fig. 1) [17]. The ratio of the 2 polymers depends on the algae of origin [17,18]. Alginate readily forms a hydrogel in the presence of crosslinking agents such as divalent cations [2]. Crosslinking occurs through carbodiimide coupling or Schiff base reactions. Gelation could also occur as a result of a physical network stabilized from hydrophobic interactions within the alginate backbone [19]. This composition allows for a high water retention capacity that ranges between 20%–90% of its original mass, a characteristic that improves its biocompatibility for biomedical applications [20]. Alginate hydrogels can also be easily modified in their chemical composition; thus their molecular weights can vary. The molecular weights of alginate range from 32,000 and 400,000 g/mol [1,21]. Increasing the molecular weight of alginate can modify the hydrogel’s physical properties, however, it can also elevate the viscosity level, which is undesirable for biomedical applications [2,22]. By manipulating the molecular weight, the hydrogel’s chemical and physical properties can be directed toward various biomedical applications [23].
Alginate-derivatives such as cell-interactive alginate and amphiphilic-alginate create specific drug delivery vehicles while influencing cell-based behaviors [24]. For example, covalent coupling with biomolecules such as gelatin or tripeptides like arginyl glycyl aspartic acid can provide cell-specific binding sites, making them more suitable in drug delivery [25,26]. Krebs et al. [27] in 2010 demonstrated the use of an injectable system for localized gene delivery of calcium phosphate-DNA nanoparticles using alginate hydrogels containing proteo-blastic cells for in vivo osteogenesis. Another study by Lawson et al. [28] demonstrated the ability of alginate gels containing collagen type 1 and beta-tricalcium phosphate to enhance human cell growth and differentiation in vitro before implantation. The role alginate hydrogels may play in drug delivery has also previously been demonstrated. In a study by Gao et al. [29] in 2020, modified pH-responsive alginate hydrogel beads were demonstrated to effectively increase the concentration of berberine hydrochloride delivery within the gastrointestinal tract, thus showcasing their potential role in sustained drug delivery via oral administration.
Finally, synthetic polymers can be structurally altered to influence different degradation, mechanical or chemical properties. In comparison, however, natural hydrogels, such as alginates, display structural similarities like that of the human ECM. Alginates share structural similarity with hyaluronic acid which is a major component of the brain and spinal cord ECM. This biomechanical similarity allows natural-derived alginates to be incorporated within live tissues and reduce the inflammatory processes or immunological reactions often associated with synthetically derived hydrogels [30-33]. Alginate hydrogels can be delivered in vivo through minimally-invasive techniques, including direct injection, emphasizing the ease of clinical applications [34].
2. Hybrid Hydrogels
Hydrogels may be composed of a mixture of several biomaterials. These mixtures allow for specialized properties, depending on the desired characteristics. Alginates have been combined with polyacrylamide to achieve increased stiffness [35]. Hydrogels made of alginate and chitosan are also used in combination as biomaterials to generate a hybrid hydrogel. Chitosans are cationic polysaccharides produced by the deacetylation of chitin; this makes them favorable in combination with alginates for the delivery of anionic compounds such as nucleic acids [36-38]. When combined, the alginate-chitosan hybrid forms polyelectrolyte complexes with increased stability [39,40]. Hybrid hydrogels [NNM1] are being tested in the field of regenerative medicine for wound healing, bone healing, and tissue engineering [36,41-45]. Recently, Steinle et al. [46] explored the role of chitosan-alginate hybrid hydrogels for the continuous delivery of synthetic mRNA to obtain a sustained release of exogenous protein (humanized gaussia Luciferase). Results demonstrated the drug delivery potential of the hybrid hydrogel for the sustained release of synthetic mRNA into cells.
3. Regenerative Potential
The possibility of modifying the molecular weight and degree of crosslinking makes hydrogels, including alginate hydrogels, novel option for treating spinal pathologies [10,47,48]. Slight modifications to the polymeric chain structure, such as ionic, covalent and thermal crosslinking of the gels during the manufacturing process, have extended the hydrogels’ versatility as a bulking agent in rat models [2]. Alginates have been described as “antidegenerative” and “pro-regenerative” agents, making them a good candidates to be studied in spinal pathologies such as SCI [49]. Several studies have demonstrated the use of alginate hydrogels as a matrix for neural stem cell growth [41-43]. Ashton et al. [50] previously demonstrated a method for creating alginate hydrogel scaffolds incorporated with poly(lactide-co-glycolide) microspheres with adjustable degradation rates in stem cell cultures. The authors highlighted a significant increase in the rate of expansion of neuronal progenitor cells cultured in degrading hydrogel alginates. Another study by Novikova et al. [51] in 2005 showed that alginate hydrogels combined with fibronectin promoted olfactory ensheathing cells proliferation. This study suggested the use of ECM when engineering biosynthetic scaffolds based on alginate hydrogels. Furthermore a study by Banerjee et al. [52] in 2009 investigated the effects the modulus of alginate hydrogels has on the proliferation and differentiation of neural stem cells. Kataoka et al. [53] also demonstrated nerve outgrowth and astrocyte reactions at the stump of 2 transected spinal cords of young rats implanted with alginates at the site of the lesion. Significant growth was seen in comparison to collagen gels serving as controls. Altering the concentration of alginate and calcium ions, the authors demonstrated enhancement in expression of B-tubulin III within alginate hydrogel scaffolds [52].
THERAPEUTIC POTENTIAL
1. SCI Overview
SCI is a potential field for alginate hydrogel implementation. SCI is a complex disorder that affects over 180,000 people annually worldwide [10]. It commonly manifests into long-term impairments such as loss of motor/sensory function and loss of autonomous function of breathing, sexual function, and bladder control [19]. The pathophysiology of SCI is complex, where the trauma to the spinal cord can institute a cascade effect of biochemical and cellular responses that trigger apoptosis in neurons and glial cells that lead to lesion development [19]. The functional deficits due to SCI are typically permanent because affected neurons have limited regenerative ability and are often exposed to inhibitory molecules that prevent regeneration [54,55]. Rehabilitative approaches and epidural stimulation remain the only treatment modalities, despite the significant efforts to find alternative therapeutic strategies. Advances in polymer science have identified that biomaterial hydrogels, such as alginate hydrogels, may promote spinal regeneration of damaged tissues in animal models [56]. The properties of alginates, including their versatility, biocompatibility, lack of toxicity, ease of gelation, and biomechanical similarity to that of the ECM, may be valuable for spinal cord regeneration [31]. As such, the utility of alginate hydrogels in spinal care has been explored extensively (Table 1).
2. Hydrogel Scaffolding
Prang et al. [57] demonstrated the feasibility of alginate hydrogel scaffolds for axonal regrowth using in vitro and adult rat models after acute SCI. In an entorhinal-hippocampal slice culture model, anisotropic capillary hydrogels supported directed central nervous system axonal growth and permitted longitudinally oriented reinnervation in vitro and integration into the spinal cord without major inflammatory reactions in vivo [57]. In a study in 2015 by Günther et al. [58], 2-mm long alginate hydrogels seeded with bone marrow stromal cells (BMSCs) expressing brainderived neurotrophic factor (BDNF) or green fluorescent protein as control were implanted into the C5 hemisection lesion of a rat spinal cord. On the 4-week assessment, numerous BMSCs appeared in the scaffold channels along with macrophages, blood vessels, and Schwann cells. Moreover, axon numbers were 3-4x higher in the alginate group compared to the control. [58] Lesions filled with BMSCs without alginate hydrogels presented random axon orientation, compared to axons in alginate-based scaffolds, which showed axons in linear orientation concerning the hydrogel channel wall [58]. These indications show that alginate hydrogel scaffolds can play a crucial role in guiding axonal regeneration. Another study by Tobias et al. [59] in 2001 found that BDNF-producing fibroblast grafts encapsulated within an alginate-poly-L-ornithine scaffold survived, proliferated, and continued to secrete BDNF for at least one month in culture. Encapsulation further permitted retention of bioactivity and allowed graft survival in a spinal cord despite the absence of immunosuppression. This subsequently fostered an environment adequate for axonal growth. In a further study in 2005, Tobias et al. [59] examined the effects of the same alginatebased grafts on subtotal cervical hemisections. The study assessed forelimb and hindlimb function and axonal growth in the absence of immunosuppression. Results showed that the alginate graft led to improved partial recovery of forelimb and hindlimb function compared to the group without the alginate graft. Immunohistochemical examination in the alginate graft group revealed an abundance of axonal promoters, including neurofilament (RT-97), 5-HT, CGRP, and GAP-43 along the injury site [59]. Axonal reorganization and behavioral recovery was induced by a BDNF releasing alginate graft, suggesting that alginate grafts are a feasible strategy for therapeutic recovery of injured SCI [59,60]. A recent study in 2019 by Schackel et al. [61] grafted poly-L-ornithine and laminin-coated alginate hydrogels into a cervical hemisection of adult female rats immediately postinjury. The authors reported the implants to remain firmly integrated and to exhibit signs of host cell migration and neurite extension further reinforcing its potential in axonal regeneration.
3. Drug Delivery
Alginate hydrogels may also play a key role in drug delivery for SCI [51,62,63]. Drug delivery has also been studied in vivo SCI models [31,64]. Alginate hydrogels can serve as precise delivery vectors for these molecules to the desired target tissue [65]. Rolipram, a phosphodiesterase-4 inhibitor as neuroprotective agent, was prepared in microfibrous patches of alginate for controlled release in vivo delivery of high or low doses [31,66]. The results showed improvement in functional recovery of motor systems following the drug delivery of low-dose rolipram. When animals were given high-dose rolipram patch treatments, there was a 50% decline in survival rates. This outcome highlights the value of alginate hydrogel encapsulation of drugs as drug-delivery platforms [66]. The injection of RhoA inhibitor (RhoAi) was also facilitated using an alginate hydrogel vector. In a rat SCI model, Devaux et al. [67] assessed the use of an alginate hydrogel for the delivery of RhoA inhibitors. The drug was experimentally tested both in vivo and in vitro, however, the authors demonstrated the importance of a delivery regimen to facilitate the neuronal reconnections and axonal regeneration in vivo using tissue-secreted media and proteomic analysis. Wen et al. [68] analyzed the union of alginate hydrogels with an integrin ligand, a signaling receptor that plays a crucial role in regulating progenitor cell proliferation. The in vitro results showed that the alginate model enhanced the encapsulation and differentiation of neural progenitor cells, indicating a further potential regenerative property.
4. Stem Cell and Neurotrophic Factor Delivery
Alginate hydrogels can also be used for the delivery of cell and neurotropic factors. In a study by Ansorena et al. [69], the alginate hydrogel was used as a reservoir for glial-derived neurotrophic factor (GDNF) and injected into the hemisection of SCI rat models. After 6 weeks, the lesions of rats injected with the alginate hydrogel with GDNF had more key neurofilaments compared to controls [69]. The hydrogel group also had superior endothelial cell and nerve fiber infiltration at the lesion site, showing that the hydrogel can promote growth factor release better functional outcomes. Des Rieux et al. [70] investigated the use of vascular endothelial growth factor (VEGF)-containing hydrogels as a stimulating agent for a traumatized spinal cord. VEGF-loaded particles were mixed with fibrinogen and injected into the lesion of a spinal cord. Their results revealed that the local delivery of VEGF via an alginate-fibrinogen vector promoted plasticity in the injured spinal cord. Moreover, in a study by Liu et al. [71], researchers constructed sodium alginate and naloxone (an opioid receptor antagonist) loaded macrophage-derived microvesicles to assess functional recovery in mice with SCI. Their results showed that the microvesicles could decrease the concentration of free calcium, thereby alleviating inflammatory factors such as tumor necrosis factor-α, interleukin (IL)-1β, IL-6 and increasing the anti-inflammatory expression of IL-10. In addition, motor functional improvement in mice was significant after treatment. A recent study by Zhang et al. [72] also found that alginate hydrogels combined with basic fibroblast growth factors (bFGFs) can prevent blood-spinal cord barrier destruction. Researchers found that a single in situ injection of the hydrogel combined with alginate and bFGF can have significant therapeutic effects together, rather than when treated alone. The study demonstrated that the hydrogel improved the blood-spinal cord barrier and functional recovery in mice, thereby potentially introductive a therapeutic strategy to approach SCI. Alginate hydrogels can act as an implant in cell-based therapies. Mesenchymal stem cells, embryonic and neural cells have been studied to replace defective cells and facilitate regeneration; however, they risk cell mortality after their transport. Therefore, alginate hydrogels have been incorporated as implants that can act by adhering to and protecting neurological cells. Barminko et al. [73] investigated the efficacy of encapsulating an implantable device with alginates for the delivery of human mesenchymal stem cells (hMSCs) in the treatment of SCI. The authors demonstrated functional maintenance of the hMSCs, and a curbed inflammatory reaction, both in vivo and in vitro. Similarly, gel encapsulation of Wnt3a-secreting fibroblasts in alginates yielded enhanced axonal recovery in rats SCI models than alginates or Wnt3a proteins alone [74].
LIMITATIONS TO CLINICAL TRANSLATION
The benefits of alginate hydrogel in SCI may be limited by its long-term stability versus degradation [75]. Within the dynamic physiological environment, the hydrogel dissolves due to divalent ion release after exchange reactions with monovalent cations. The result is the release of homeostatic promoting calcium ions. As such, the gel may serve as a matrix for platelet and erythrocyte aggregation. Depending on the situation, this reaction may be desired or undesired. This could result in a cascade of negative reactions that could contribute to dysregulation within the intraspinal environment. This instability is one limiting factor in using alginate hydrogels as long-term survival in vivo is an important consideration in its clinical utility [75,14]. The alginate hydrogel has not been evaluated in clinical trials for its safety and potential risk to patients with SCI, despite being immunologically inert in animal studies. However, some commercially available alginates have been shown to contain cytotoxicity and mitogenic impurities that could elicit an unwanted immune response after transplantation [76]. This emphasizes the fundamental need for standardized cGMP-level purification and toxicity studies before use in the biotechnological setting.
Increasing the relevance of alginate hydrogels in clinical trials would require a greater understanding of the biomaterial properties to determine appropriate therapies for human subjects [31]. A common approach with alginate hydrogels is adjusting their composition and makeup to meet a particular application’s needs. Tailoring alginate hydrogels in human trials requires revisiting the different crosslinking strategies using molecules that are themselves safe for translation to humans. Similarly, the mechanism and biproducts of degradation in humans have yet to be fully characterized [10] (Table 2). In other fields of study, advances have been made that allow for the use of implantable alginate hydrogels in human subjects. Ongoing trials evaluating their role as a material for reconstructing the left ventricle in human subjects are currently ongoing (Clinicaltrail.gov ID: NCT04- 781660) [77]. Other clinical trials are exploring the feasibility and safety of alginate hydrogels to treat anal fistulas, diabetic foot ulcers, and other chronic wounds [78,79].
FUTURE DIRECTIONS
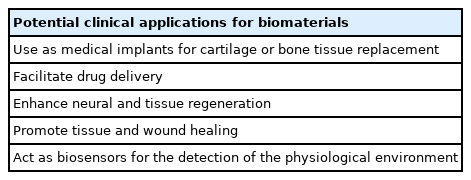
The utilization of alginate in medicine is expected to evolve considerably into a more active role, especially in drug delivery, wound healing and tissue regeneration. With alginate’s ability to be physically and chemically modified, we can expect future derivatives incorporated into a diverse range of biological systems, not just exclusively in the spine. The engineering of new alginate polymers with enhanced chemical and physical properties can develop various proteins with novel functions. Moreover, alginate hydrogels can have an advanced role in alternative technologies such as 3D bioprinting and microfluidics, increasing their versatility in the multiple spheres of biomedical research [80] Shang et al. [81] recently verified a gel growth model for 3D printing of hybrid calcium gluconate alginate hydrogels. The authors describe integrating collagen fibrils into the alginates scaffold to create cell-adhesions motifs within its chemical structure, creating ECM-like microenvironments. These 3D models will mitigate the complexity of designing cell growth, maturation and behavior. These prospects will pave the way for opportunities in drug treatments, cell therapies, and tissue transplantation.
CONCLUSION
Alginate hydrogels are efficient and promising substrates for tissue engineering, drug delivery, neural regeneration, and cell-based therapies for patients with SCI. With its ability to act as a pro-regenerative and antidegenerative agent, the alginate hydrogel has the potential to improve clinical outcomes. However, thus far, its translation to clinical practice has not been widely assessed. Nevertheless, with the current era of regenerative medicine, positive contributions may be anticipated in spine research and clinical care.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: RJ, MB; Formal analysis: SES, FM; Methodology: FM; Project administration: MB; Visualization: SES, CO; Writing - original draft: RJ, SES, CO, AKG, AB; Writing - review & editing: RJ, SES, CO, AKG, AB, AS, NM.