 |
 |
- Search
|
|
||
Abstract
Objective
This study compared the radiological and clinical outcomes with transforaminal lumbar interbody fusion (TLIF) to evaluate the effect of indirect decompression through oblique lumbar interbody fusion (OLIF) as revision surgery.
Methods
We enrolled patients who underwent single-level fusion with revision surgery at the same level as the previous decompression level. We retrospectively reviewed 25 patients who underwent OLIF from 2017 to 2018 and 25 who received TLIF from 2014 to 2018. Radiologic and clinical outcomes were evaluated by cross-sectional area (CSA) of the spinal canal, thickness and area of ligamentum flavum (LF), subsidence, disc height, fusion rate, Oswestry Disability Index (ODI), and visual analogue scale (VAS).
Results
Compared with OLIF, the thickness and area of the LF after surgery were significantly less in TLIF, and the resulting CSA extension was also significantly higher. However, both groups showed improvement in ODI and VAS after surgery, and there was no difference between the groups. Complications related to the posterior approach in TLIF were 4 cases, and in OLIF, there were 2 cases that underwent additional posterior decompression surgery and 6 cases of transient paresthesia.
Lumbar spinal stenosis (LSS) with or without low-grade spondylolisthesis in degenerative spinal disease is found in a large portion of the elderly population and is a major cause of chronic low back pain, neurogenic claudication, and radiculopathy. It has already been reported that surgical posterior decompression is more effective and superior to conservative treatment in LSS, and it is performed as the primary surgical method when conservative treatment is no longer effective [1-3].
Postoperative progressive spondylolisthesis, recurrent stenosis, and recurrent herniated nucleus pulposus are the main indications for reoperation, which has been reported to have a rate of 9.5% at 4 years postoperatively and up to 19% at 11 years postoperatively [4,5]. Reoperation at the same level as the previous decompression is preferably treated with fusion because laminectomy had been performed previously and the remaining facet joint is smaller [5]. Fusion is therefore often performed in consideration of mechanical back pain and destabilization occurring postlaminectomy good results have been reported in long-term outcomes [6].
The posterior lumbar interbody fusion (PLIF) or transforaminal lumbar interbody fusion (TLIF) is a direct decompression method that removes the facet and ligamentum flavum (LF). However, performing PLIF or TLIF as revision surgery is challenging for surgeons. Anatomical landmarks are obscured due to previous surgery, and the risk of complications such as incidental durotomy, neural injury, and surgical site infection may be increased due to epidural adhesions [7-10].
Oblique lumbar interbody fusion (OLIF) is a minimally invasive surgical technique using an oblique retroperitoneal approach which differs from PLIF and TLIF [11]. OLIF uses a relatively large cage compared to PLIF and TLIF cages, so it is advantageous when forming solid intervertebral stability. The taller cage used with OLIF also reduces disc bulging and increases the LF to induce indirect decompression [12-14]. Additionally, compared to PLIF and TLIF, the surgical time is shorter, blood loss is less, and paravertebral muscles are preserved [15,16].
When revision surgery is considered, OLIF takes an oblique retroperitoneal approach rather than using the previous surgical site, so dural adhesions or altered anatomical landmarks are not obstacles which reduces the risk of related complications. When OLIF is performed at the same level as the previous surgery, insufficient information exists on how adhesions caused by previous decompression surgery affect indirect decompression, and on whether indirect decompression revision surgery is sufficient compared to traditional direct decompression. This study compares the radiological and clinical outcomes of TLIF and OLIF as revision surgery in recurrent stenosis that occurred at the same level after previous posterior decompression surgery and will help resolve these concerns about this technique.
All patients were provided with written informed consent and the relevant Institutional Review Board of the Kyungpook National University Hospital approved this study (KNUH 2022-02-010). Patients were enrolled who had undergone previous posterior lumbar decompression surgery (decompression laminectomy, hemilaminectomy and discectomy, and a unilateral approach with bilateral decompression) and who underwent revision fusion surgery at the same level. Previous posterior decompression surgery was performed for spinal stenosis or herniated nucleus pulposus (HNP) without improvement in symptoms despite conservative treatment. Revision fusion surgery was performed if recurrent or new-onset neurological symptoms and/or leg pain were present during follow-up and spondylolisthesis with segmental instability, recurrent stenosis, and recurrent HNP were diagnosed at the same level. The decision criteria for TLIF and OLIF in revision fusion surgery were the same. After explaining the pros and cons of each surgical procedure, the surgeon decided on the surgical procedure according to the patient’s request and the surgeon’s discretion.
OLIF was introduced in 2016 in this institution with TLIF being the primary technique prior to that. Cases in which TLIF was performed as revision surgery were therefore selected from 2014 to 2018, and cases in which OLIF was performed were selected from 2017 to 2018 to minimize the effects of the learning curve. All surgeries were performed by 2 spine surgeons with more than 10 years of experience.
For revision surgery, cases that received single-level fusion from L3 to S1 were grouped into 2 groups and compared: TLIF, 25 cases and OLIF, 25 cases. High-grade spondylolisthesis, combined sequestrated disc herniation, infection, trauma, and tumor cases were all excluded.
Age, sex, symptom duration, diagnosis prior to revision surgery, surgical level, bone mineral density (BMD), subsidence, and additional posterior decompression surgery were investigated in all of the 50 enrolled patients. BMD was defined using a T-score as follows: greater than -1.0, normal; greater than -2.5 but less than -1.0, osteopenia; and less than -2.5, osteoporosis.
Patients who underwent TLIF, had the procedure through a midline skin incision in the prone position after induction of general anesthesia, and bilateral subperiosteal dissection to expose the facet joint while preserving the posterior midline structures. After bilateral facetectomy, discectomy, and endplate preparation, 2 cages were inserted, one from each side, and autobone was used as a fusion material, followed by pedicle screw placement.
For OLIF, it performed through a left side retroperitoneal approach in the right lateral decubitus position. At the L3–4 and L4–5, levels the cage was inserted through the corridor between the psoas muscle and the aorta, and at theL5–S1 level, it was inserted through the corridor between the right iliac artery and the left iliac vein. In all OLIF cases, the OLIF system (Medtronic, Memphis, TN, USA) was used, allobone (Grafton, Medtronic) was used for the cage as a fusion material, and percutaneous pedicle screw placement (longitude system, Medtronic) was performed. Cage height was determined by preoperative computed tomography (CT) in all patients: If intervertebral disc height was greater than 6 mm, a cage 4 mm higher than the intervertebral disc height was used and if the intervertebral disc height was less than 6 mm, a 10-mm cage was inserted. Additional posterior decompression surgery was performed if the patient’s symptoms did not improve after surgery, or if the improvement was insufficient. In our series, 2 patients were underwent the additional posterior decompression at 6 and 8 days after OLIF, respectively.
Magnetic resonance imaging (MRI) was taken using a 1.5-T EXCITE whole-body imaging system (General Electric, Milwaukee, WI, USA). The axial localizing sequence was taken from the surgical site to identify the disc space gap, and 4 slices were obtained with 4.0-mm slice thickness and 1.0 mm spacing between slices per level. Images were displayed and analyzed through PiView (INFINITT, Seoul, South Korea) digital image viewing software. The cross-sectional area (CSA) of the spinal canal and LF measured at the surgical site was measured using a graphic cursor to measure the outline of the spinal canal. The thickness of the LF was defined as the average of the longest thicknesses of both ligaments (Fig. 1A). Spinal canal area and LF thickness were evaluated by MRI scans performed immediately before and after surgery, and at 12 months after surgery. Intervertebral disc height was defined as the distance between the superior and inferior endplates, vertically connected from the center of the anteroposterior diameter of the inferior vertebral body (Fig. 1B). Subsidence was classified according to postoperative disc height loss as follows: none, < 10%; mild, 10%– 24%; moderate, 25%–49%; and severe, 50%–100% (Fig. 1C) [17]. Fusion rate was evaluated by radiography and CT scan. Solid fusion was determined when there was continuous trabecular bone in the cage and/or bone union of the facet joint was observed without screw loosening (Fig. 2).
Clinical outcomes were evaluated preoperatively, 6 months, 12 months, and 24 months postoperatively using the Oswestry Disability Index (ODI) and the visual analogue scale (VAS) for both back and dominant leg pain.
The ages of the TLIF and OLIF surgery groups were similar. There were 13 males and 12 females in the TLIF surgery group, and 9 males and 16 females in the OLIF surgery group. Symptom duration was 7.6± 6.9 months in the TLIF surgery group and 7.6± 2.6 months in the OLIF surgery group, and as such, there was no difference between the 2 groups. The mean follow-up durations of the TLIF and OLIF groups were 32.4± 8.5 months (range, 24–58) and 28.9± 5.8 months (range, 24–43), respectively, and there was no statistical difference between the 2 groups (p= 0.094). In the TLIF surgery group, there were 10 cases of spondylolisthesis, 8 cases of recurrent stenosis, and 7 cases of recurrent HNP. In the OLIF surgery group, there were 13 cases of spondylolisthesis, 10 cases of recurrent stenosis, and 2 cases of recurrent HNP. As for the operation level, 3 cases were at L3–4, 20 were at L4–5, and 2 were at L5–S1 in the TLIF surgery group, and 2 cases were at L3–4, 20 cases were at L4–5, and 3 cases were at L5–S1, in the OLIF surgery group. There was no significant difference in BMD between the TLIF surgery group and the OLIF surgery group, however, there was a significant difference in subsidence (p= 0.047) (Table 1). In the OLIF surgery group, 2 cases did not show sufficient symptom improvement, so additional posterior decompression surgery was subsequently performed.
Clinical outcomes between the TLIF and OLIF surgery groups were compared except for the 2 cases who underwent additional posterior decompression surgery. In the TLIF surgery group, the ODI score improved from 28.0± 7.7 preoperatively to 16.0 ± 7.3 at 12 months postoperatively and 17.1± 6.3 at 24 months postoperatively. VAS for leg and back pain also improved from 7.3± 1.7 and 5.0± 2.5 preoperatively to 2.9± 2.2 and 2.5± 2.0 at 12 months postoperatively and 3.1±1.9 and 2.7±1.7 at 24 months postoperatively.
In the OLIF surgery group, the ODI score improved from 25.6± 5.9 preoperatively to 12.6± 4.5 at 12 months postoperatively and 13.1± 4.4 at 24 months postoperatively. VAS for leg pain improved from 6.4 ± 1.4 preoperatively to 3.4 ± 1.2 at 12 months postoperatively and 3.3± 1.0 at 24 months postoperatively, and VAS for back pain improved from 5.8± 1.2 preoperatively to 2.9± 1.1 at 12 months postoperatively and 2.8± 1.4 at 24 months postoperatively.
There were no significant differences in ODI score, VAS for leg and back pain between the 2 groups before, 6 months, 12 months, and 24 months after surgery (Table 2).
The preoperative CSA of the spinal canal was 108.9± 54.3 mm2 in the TLIF surgery group and 99.1± 45.2 mm2 in the OLIF surgery group. At 12 months after surgery, the CSA of the spinal canal was 173.5± 38.6 mm2 and 125.62± 46.7 mm2 in TLIF surgery and OLIF surgery, respectively. The increase was higher in the TLIF surgery group than in the OLIF surgery group, and there was a statistically significant difference between the 2 groups (p= 0.023 and p< 0.001). There were no significant differences in LF thickness between the 2 groups, with the TLIF surgery group at 4.54±1.75 mm and the OLIF surgery group at 3.53±1.4 mm before surgery. At 12 months postoperatively, the LF thickness was significantly decreased in the TLIF surgery group compared to the OLIF surgery group to 1.76± 0.68 mm and 2.5± 1.0 mm, respectively (p< 0.001 and p< 0.001). There were no significant differences in LF areas between the 2 groups preoperatively, 78.4 ± 46.6 mm2 for TLIF surgery and 75.8 ± 44.6 mm2 for OLIF surgery. At 12 months postoperatively, LF areas were significantly decreased to 20.6± 10.7 mm2 in the TLIF surgery group and less so in the OLIF surgery group which decreased to 53.9± 29.9 mm2 (p< 0.001 and p< 0.001) (Table 3). Subsidence was compared by evaluating the decrease in disc height after surgery between the 2 groups. In the TLIF surgery group, mild (32.0%), moderate (24.0%), and severe (12.0%) subsidence was observed, and in the OLIF surgery group, mild and moderate subsidence was present at 13.0% and 21.7%. There was no significant difference in disc height between the 2 groups before surgery, but after surgery, the disc height of the OLIF surgery group was 12.7± 2.0 mm and that of the TLIF surgery group was 10.7± 2.2 mm, showing a more significant increase than that of the TLIF surgery group (p< 0.006 and p< 0.001). There were no significant differences in the fusion rates between the 2 groups at 1 year and 1.5 years after surgery. The fusion rates at 1.5 years in the TLIF and OLIF surgery groups were 80% (20 of 25) and 87% (20 of 23), respectively (Table 4).
The TLIF surgery group had 4 cases of posterior approachrelated complications, 2 cases of wound dehiscence, 3 cases of cerebrospinal fluid (CSF) leakage, and 1 case of nerve damage. Two of the CSF leakage cases were accompanied by wound dehiscence and nerve damage, respectively. The OLIF surgery group had 6 cases of transient paresthesia on the approach side, which was significantly different from that in the TLIF surgery group (p= 0.022) (Table 5). There was no ureteral injury, vascular injury, sympathetic trunk injury, or superior hypogastric plexus injury in the OLIF surgery group. Although it is not a surgery-related complication, 2 cases in the OLIF surgery group underwent additional posterior decompression surgery because the symptoms did not improve sufficiently after surgery.
This study compares the radiological and clinical outcomes between 2 surgical groups: direct decompression with TLIF and indirect decompression with OLIF, for revision surgery at the same level as previous posterior decompression. Traditional posterior approach revision surgery, such as PLIF and TLIF, is a burden on the surgeon because of the high risk of incidental durotomy and nerve root injury due to scar tissue and dural adhesions from previous surgeries.
In the literature comparing primary surgery and revision surgery in open TLIF, it was reported that the risk of inadvertent dural tears increased by 3.2 times when one or more previous lumbar decompressions were performed [7]. Similarly, the overall complication rate of the group that underwent primary TLIF surgery and the group that underwent revision TLIF surgery was 59 of 287 (20.6%) and 76 of 244 (31.1%), respectively (odds ratio [OR], 1.75; p< 0.01), incidental durotomy was 32 of 287 (11.1%) and 44 of 244 (18%), respectively (OR, 1.75; p= 0.03) [8]. In a study that performed PLIF in 50 patients as revision surgery, neurologic complications (leg pain or motor loss) occurred in 32% and permanent cases occurred in 8% [18].
Similar results were reported in studies on primary and revision surgery of minimally invasive (MIS) TLIF. According to Kang et al. [9], 4% of dural tears occur during primary surgery and 19% occur during revisional surgeries, Selznick et al. [10] reported that CSF leakage per level was 5.9% in primary surgery and 21.4% in revision.
In a meta-analysis study comparing OLIF and MIS TLIF, there was no significant difference in overall complication rates between the 2 groups (13.8% in MIS TLIF, 16.3% in OLIF, p=0.45) [19]. In approach-related complications, dural tear and root injury occurred in 4 cases (1.7%) of 232 patients in the MIS TLIF group, and none in 240 patients in the OLIF group. Sympathetic chain injury occurred in 3 cases (1.3%) in the OLIF group and none in the MIS TLIF group. In the OLIF and MIS TLIF groups, different complications may occur depending on the approach, so it is difficult to determine which surgical procedure is better.
As revision surgery, OLIF surgery is a retroperitoneal oblique approach rather than an approach to the previous surgical site, so altered anatomical landmarks and dural adhesions do not interfere during surgery. In our study, there were 4 cases of posterior approach-related complications in the TLIF surgery group, whereas none occurred in the OLIF surgery group. In the OLIF surgery group, there were 6 cases of transient paresthesia and the incidence was significantly higher than that of the TLIF surgery group, but all symptoms resolved within a few weeks after surgery. Therefore, in terms of complications, OLIF can be a safer alternative surgical technique compared to TLIF.
In this study, the preoperative CSA of spinal canal in the TLIF and OLIF surgery groups was 108.9± 54.3 mm2 and 99.1± 45.2 mm2, respectively. Considering that the symptomatic LSS of the CSA of spinal canal from previous studies is ≤ 77.5 mm2 [20], it is considered that the preoperative CSA of spinal canal in both groups is larger than 77.5 mm2 because our subjects underwent previous posterior decompression (Figs. 3A, 4A). In another report, 3 weeks after OLIF surgery, the median CSA extension ratio was confirmed to be 30.2%, and all subjects showed clinical improvement [13]. In this study, the OLIF surgery group showed a CSA extension of 41.7%± 50.3%, and the TLIF surgery showed a CSA extension of 90.9%± 87.6%. The reason CSA extension showed greater results in the TLIF surgery group is thought to be due to posterior direct decompression (Figs. 3C, 4C). This is supported by our results that the TLIF surgery group showed a greater reduction in LF thickness (57.8%± 19.4% in TLIF, 26.5% ± 17.2% in OLIF) and LF area (70.7%± 12.8% in TLIF, 24.6%± 17.3% in OLIF) before and 12 months after surgery than the OLIF surgery group. Although the CSA extension was greater in the TLIF surgery group than in the OLIF surgery group, there were no significant differences between the 2 groups in clinical outcomes, including ODI, VAS for leg, and VAS for back. Our results suggest that even in revision, the CSA extension necessary for clinical improvement can be sufficiently obtained by indirect decompression.
In this study, both the number and grade of subsidence showed worse outcomes in the TLIF surgery group than in the OLIF surgery group. Subsidence was observed in 17 cases in the TLIF surgery group, among which 8 cases were mild, 6 cases were moderate, and 3 cases were severe, On the other hand, in the OLIF surgery group, 3 cases were mild, 5 cases were moderate, and there were no severe cases. This is thought to be advantageous for subsidence because the OLIF cage can use a longer length than the TLIF cage, and the cage can be placed on the cortical rim of the vertebral body to support it. Although there was no statistical significance, VAS of leg preoperatively was higher in the TLIF surgery group than in the OLIF surgery group (7.3± 1.7 in TLIF, 6.4± 1.4 in OLIF, p= 0.075), and at 6 months postoperatively, the TLIF surgery group was lower than the OLIF surgery group (3.0 ± 1.6 in TLIF, 3.8 ± 1.0 in OLIF, p = 0.056). This suggests that although the TLIF surgery group may be at a disadvantage compared to the OLIF surgery group in terms of subsidence, the TLIF surgery group was better in terms of nerve root decompression. In a study evaluating radiographic outcomes according to cage type in TLIF reported that the increase in foraminal height obtained through TLIF was small, but that the more important key was direct decompression [21]. This study provides evidence to support our claim.
In a recent report on revision OLIF surgery performed on 34 patients at the same level after previous lumbar decompression, the CSA extension increased from 136.4± 57.9 mm2 to 194.1± 58.6 mm2 before and after surgery, the clinical improvement rate was 59.0%, and fusion rates of 93.0% were reported [22]. These results suggest that indirect decompression is sufficient in revision surgery when performed with OLIF and that OLIF can be a good alternative for revision surgery. However, this study is different from our study in that the L5–S1 level, which has a relatively high frequency of LSS, was excluded from the study, and 2 level, not a single-level, was included in the study object.
Prior to this study, we compared the clinical and radiological outcomes of primary OLIF surgery and revision OLIF surgery [23]. In previous studies, OLIF as revision surgery showed acceptable clinical outcomes, but the effect of indirect decompression was less than that of primary OLIF, which is thought to be due to perineural adhesions and scar formation from previous surgery. In this study, the clinical and radiological outcomes of TLIF and OLIF performed as revision surgery were compared, and there were no differences in clinical outcomes between the 2 groups. These results suggest that perineural adhesion and scar formation caused by previous surgery may have slightly less improvement in clinical outcomes during revision surgery compared to primary surgery, but this suggests that there is no difference in the effects of perineural adhesion and scar formation on both direct decompression and indirect decompression.
The disadvantage of indirect decompression through OLIF compared to TLIF, is that if symptoms do not improve after surgery, additional posterior decompression surgery may be required. In our study, 2 cases in the OLIF surgery group underwent additional posterior decompression surgery. They did not show any improvement in symptoms after surgery, and both patients complained of dominant radiculopathy on the same side as the previous decompression side. They underwent additional posterior decompression surgery within 2 weeks after OLIF surgery, and their symptoms improved after the subsequent surgery. These results are the basis for supporting the content of our previous study that, as revision surgery, OLIF reduced the effects of indirect decompression due to perineural adhesion and scar formation in the same side as the previous decompression compared to the virgin side [21].
If symptoms persist or residual symptoms remain on the same side as the previous decompression side, additional posterior decompression surgery may be required after OLIF, which is a limitation of OLIF. On the other hand, good outcomes can be expected when revision OLIF is considered as a symptom of the virgin side. Therefore, if appropriate indications are applied, OLIF can be a good alternative to TLIF in revision surgery. However, to support our claim, a comparative study on the risks of additional posterior decompression surgery on the same side and virgin side in revision OLIF is indicated.
First, it is a single-center, retrospective study, and the number of subjects is small. In this study, there were no posterior approach-related complications in the OLIF surgery group, but there were no statistically significant differences compared to the TLIF surgery group, which is expected to be significant if the number of subjects increase. Moreover, the subsidence between the 2 groups was worse in the TLIF surgery group than in the OLIF surgery group. However, in this study, when 2 patients who underwent additional posterior decompression were included, there was a significant difference in subsidence between the 2 groups, but there was no significant difference between the 2 groups when excluded. It is also expected that there will be a significant difference between the 2 groups as the number of subjects increases. Second, selection bias may occur in the method of determining surgical procedures. We considered the following 3 factors in determining the surgical procedure. (1) After fully explaining the pros and cons of each surgical procedure, the patient’s request was given priority. (2) If there was a history of previous abdominal surgery, TLIF was performed. (3) TLIF was performed when the corridor between the right iliac artery and the left iliac vein was narrow at L5–S1 level. Even if sufficient explanation is provided to the patient for each surgical procedure, there may be other factors that may influence the decision of the surgical procedure. These factors must be adjusted appropriately to obtain generalizable results, but since these factors were not analyzed, they could compromise the results. Finally, this study lacks information on the pathophysiological effects of perineural adhesions and scar formation caused by previous surgery on outcomes after revision surgery. This needs to be elucidated through additional research.
Since complications associated with the posterior approach can be avoided, OLIF is a safer and useful minimally invasive surgery. Transient paresthesia after OLIF surgery may occasionally occur, but is acceptable as it usually improves. However, due to perineural adhesions or scar formation, there may not be sufficient symptom improvement, and in some cases, additional posterior decompression surgery may be required, which is a limitation of OLIF. Therefore, appropriate indications are applied, OLIF is a good alternative to TLIF when revision surgery is considered.
NOTES
Funding/Support
This work was supported by the Technology development Program (S3301849), funded by the Collabo R&D between Industry, Academy, and Research Institute and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HR22C1832).
Author Contribution
Conceptualization: SP, DC, HK, KK; Data curation: JH, SL, HK, KK; Formal analysis: SP, JH, DC, SL, CHK, IH, KK; Funding acquisition: DC, HK; Methodology: SP, KK; Project administration: JH, DC, SL, DP, CHK, IH, HK, KK; Writing - original draft: SP; Writing - review & editing: CHK KK.
Fig. 1.
(A) The cross-sectional area (CSA) of the spinal canal (red arrow and text) and the thickness (yellow arrow and text) and area (green arrow and text) of the ligamentum flavum (LF) were measured at the mid-disc level of the axial sequence using T2-weighted magnetic resonance imaging. (B) Intervertebral disc height (DH) was measured in lateral radiography as the length of the line from the midpoint of the inferior endplate to the superior endplate vertically. (C) Subsidence was measured by the degree of cage subsidence into the vertebral endplates on lateral radiography.
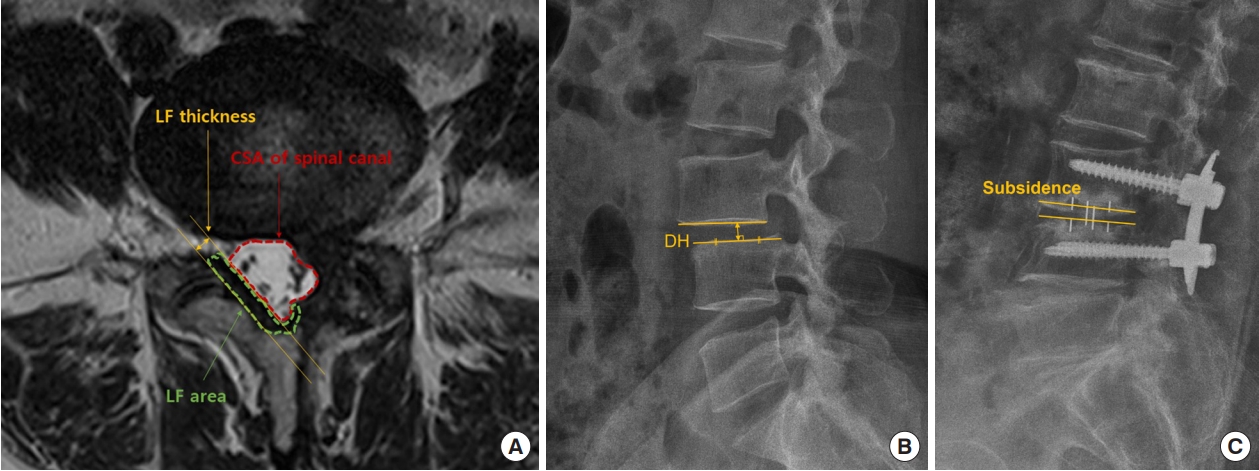
Fig. 2.
Evaluation of the fusion state through computed tomography (CT). CT scans of transforaminal lumbar interbody fusion (TLIF) (A, B) and oblique lumbar interbody fusion (OLIF) (C, D) cases, showing fusion through coronal and sagittal images.
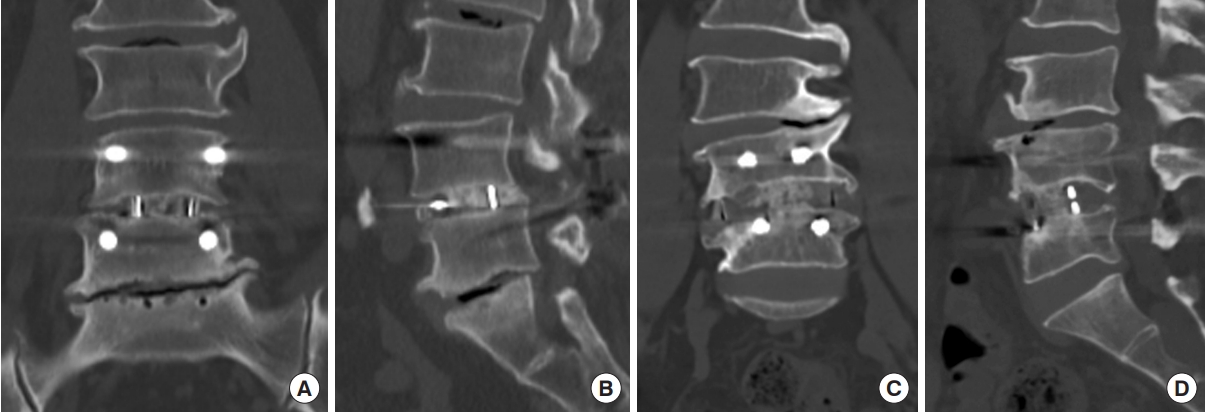
Fig. 3.
Illustrative case of revision transforaminal lumbar interbody fusion (TLIF). (A) Axial image of T2-weighted magnetic resonance imaging (MRI) showed spinal stenosis with herniated nucleus pulposus in the left subarticular zone at the L4–5 level. (B) MRI performed after left side decompression via a posterior approach. Restenosis was observed in the left lateral recess. (C) Cross-sectional area extension and decompression of the left lateral recess were observed after TLIF.

Fig. 4.
Illustrative case of revision oblique lumbar interbody fusion (OLIF). (A) Axial image of T2-weighted magnetic resonance imaging (MRI) showed spinal stenosis with herniated nucleus pulposus (HNP) in the right subarticular zone at the L4–5 level. (B) An axial image of MRI performed after right side decompression via a posterior approach. Restenosis with HNP was observed in the right lateral recess. (C) Cross-sectional area extension and decompression of the right lateral recess were observed after OLIF.
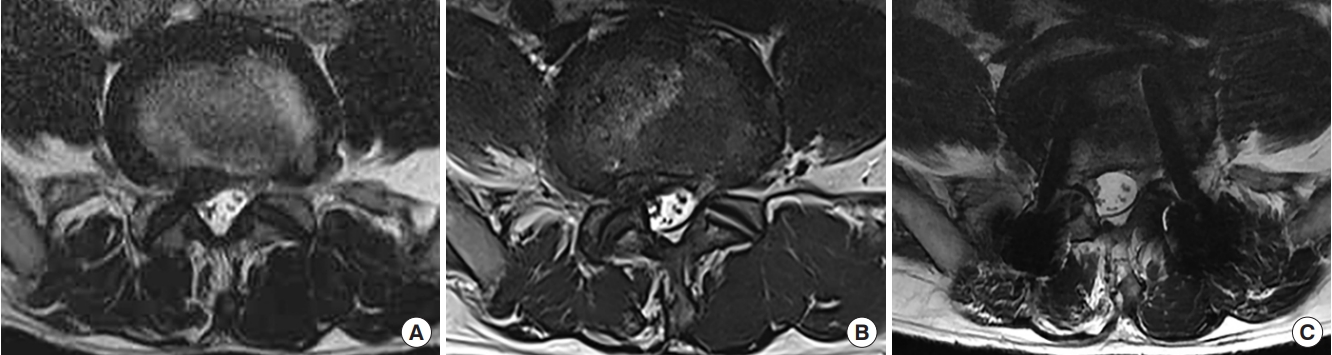
Table 1.
Demographics of the enrolled patients
| Variable | TLIF surgery group (n = 25) | OLIF surgery group (n = 25) | p-value |
|---|---|---|---|
| Age (yr) | 63.6 ± 9.9 | 66.6 ± 8.65 | 0.254 |
| Sex, male:female | 13:12 | 9:16 | 0.393 |
| Symptom duration (mo) | 7.6 ± 6.9 | 7.6 ± 2.6 | 0.978 |
| Follow-up duration (mo) | 32.4 ± 8.5 (24–58) | 28.9 ± 5.8 (24–43) | 0.094 |
| Diagnosis | 0.183 | ||
| Spondylolisthesis | 10 | 13 | |
| Recurrent stenosis | 8 | 10 | |
| Recurrent HNP | 7 | 2 | |
| Operation level | 0.819 | ||
| L3–4 | 3 | 2 | |
| L4–5 | 20 | 20 | |
| L5–S1 | 2 | 3 | |
| BMD | |||
| Normal | 15 | 8 | |
| Osteopenia (-2.5 < T- score < -1.0) | 7 | 12 | |
| Osteoporosis (T-score ≤ -2.5) | 3 | 5 | 0.139 |
| Subsidence | |||
| None | 8 | 16 | |
| Mild | 8 | 3 | |
| Moderate | 6 | 6 | |
| Severe | 3 | 0 | 0.047* |
| Additional posterior decompression surgery | 0 (0) | 2 (4.0) | 0.490 |
Table 2.
Comparison of clinical outcome between TLIF and OLIF surgery group (exclusion of additional posterior decompression surgery)
Table 3.
Comparison of radiologic outcome between TLIF and OLIF surgery group (exclusion of additional posterior decompression surgery)
| Variable | TLIF surgery group (n = 25) | OLIF surgery group (n = 23) | p-value | |
|---|---|---|---|---|
| Cross-sectional area of spinal canal (mm2) | ||||
| Preoperative | 108.9 ± 54.3 | 99.1 ± 45.2 | 0.502 | |
| 12 Months after surgery | 173.5 ± 38.6 | 125.62 ± 46.7 | < 0.001* | |
| Increasing percentage [(12 months – preop)/preop × 100] | 90.9 ± 87.6 | 41.7 ± 50.3 | 0.023* | |
| Increasing value (mm2) (12 months – preop) | 64.6 ± 45.4 | 26.5 ± 14.2 | < 0.001* | |
| LF thickness (mm) | ||||
| Preoperative | 4.54 ± 1.75 | 3.53 ± 1.4 | 0.033 | |
| 12 Months after surgery | 1.76 ± 0.68 | 2.5 ± 1.0 | 0.003* | |
| Decreasing percentage [(preop – 12 months)/preop × 100] | 57.8 ± 19.4 | 26.5 ± 17.2 | < 0.001* | |
| Decreasing value (mm) (preop – 12 months) | 2.77 ± 1.47 | 1.0 ± 0.82 | < 0.001* | |
| LF area (mm2) | ||||
| Preoperative | 78.4 ± 46.6 | 75.8 ± 44.6 | 0.847 | |
| 12 Months after surgery | 20.6 ± 10.7 | 53.9 ± 29.9 | < 0.001* | |
| Decreasing percentage [(preop – 12 months)/preop × 100] | 70.7 ± 12.8 | 24.6 ± 17.3 | < 0.001* | |
| Decreasing value (mm2) (preop – 12 months) | 57.7 ± 38.5 | 21.9 ± 21.9 | < 0.001* | |
| Subsidence | 0.186 | |||
| None | 8 | 15 | ||
| Mild | 8 | 3 | ||
| Moderate | 6 | 5 | ||
| Severe | 3 | 0 | ||
| Disc height (mm) | ||||
| Preoperative | 9.0 ± 2.3 | 8.1 ± 1.9 | 0.134 | |
| 12 Months after surgery | 10.7 ± 2.2 | 12.7 ± 2.0 | 0.002* | |
| Increasing percentage [(12 months – preop)/preop × 100] | 28.5 ± 46.4 | 63.3 ± 35.9 | 0.006* | |
| Increasing value (mm) (12 months – preop) | 1.7 ± 3.2 | 4.6 ± 2.0 | < 0.001* | |
Table 4.
Comparison of radiologic fusion rate between TLIF and OLIF surgery group (exclusion of additional posterior decompression surgery)
| Variable | TLIF surgery group (n = 25) | OLIF surgery group (n = 23) | p-value |
|---|---|---|---|
| Fusion at 1 year after surgery | 0.687 | ||
| Yes | 14 | 10 | |
| No | 11 | 13 | |
| Fusion at 1.5 year after surgery | 0.661 | ||
| Yes | 20 | 20 | |
| No | 5 | 3 |
Table 5.
Complication related with surgical approach between TLIF and OLIF surgery group
| Variable | TLIF surgery group (n = 25) | OLIF surgery group (n = 25) | p-value |
|---|---|---|---|
| Posterior approach-related complication | 4 Cases† | 0 Case | 0.117 |
| Wound dehiscence | 2 Cases | 0 Case | 0.490 |
| CSF leakage | 3 Cases | 0 Case | 0.235 |
| Nerve damage | 1 Case | 0 Case | 1.000 |
| Transient paresthesia (approach side) | 0 Case | 6 Cases | 0.022* |
REFERENCES
1. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008;358:794-810.



2. Malmivaara A, Slätis P, Heliövaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine (Phila Pa 1976) 2007;32:1-8.

3. Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine (Phila Pa 1976) 2000;25:1424-36.

4. Radcliff K, Curry P, Hilibrand A, et al. Risk for adjacent segment and same segment reoperation after surgery for lumbar stenosis: a subgroup analysis of the spine patient outcomes research trial (SPORT). Spine (Phila Pa 1976) 2013;38:531-9.


5. Martin BI, Mirza SK, Comstock BA, et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976) 2007;32:382-7.


6. Mendenhall SK, Parker SL, Adogwa O, et al. Long-term outcomes after revision neural decompression and fusion for same-level recurrent lumbar stenosis: defining the effectiveness of surgery. J Spinal Disord Tech 2014;27:353-7.

7. Khan IS, Sonig A, Thakur JD, et al. Perioperative complications in patients undergoing open transforaminal lumbar interbody fusion as a revision surgery. J Neurosurg Spine 2013;18:260-4.


8. Tormenti MJ, Maserati MB, Bonfield CM, et al. Perioperative surgical complications of transforaminal lumbar interbody fusion: a single-center experience - clinical article. J Neurosurg Spine 2012;16:44-50.

9. Kang MS, Park JY, Kim KH, et al. Minimally invasive transforaminal lumbar interbody fusion with unilateral pedicle screw fixation: comparison between primary and revision surgery. Biomed Res Int 2014;2014:1-6.




10. Selznick LA, Shamji MF, Isaacs RE. Minimally invasive interbody fusion for revision lumbar surgery: technical feasibility and safety. J Spinal Disord Tech 2009;22:207-13.

11. Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J 2012;6:89-97.



12. Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35(26 Suppl):S331-7.


13. Fujibayashi S, Hynes RA, Otsuki B, et al. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976) 2015;40:E175-82.


14. Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J 2017;26:671-8.



15. Joseph JR, Smith BW, Marca F La, et al. Comparison of complication rates of minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion: a systematic review of the literature. Neurosurg Focus 2015;39:E4.

16. Walker CT, Harrison Farber S, Cole TS, et al. Complications for minimally invasive lateral interbody arthrodesis: a systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J Neurosurg Spine 2019;30:446-60.

17. Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8.


18. Yamashita T, Okuda S, Aono H, et al. Controllable risk factors for neurologic complications in posterior lumbar interbody fusion as revision surgery. World Neurosurg 2018;116:e1181-7.


19. Zhang Q-Y, Tan J, Huang K, et al. Minimally invasive transforaminal lumbar interbody fusion versus oblique lateral interbody fusion for lumbar degenerative disease: a meta-analysis. BMC Musculoskelet Disord 2021;22:802.




20. Majidi H, Shafizad M, Niksolat F, et al. Relationship between magnetic resonance imaging findings and clinical symptoms in patients with suspected lumbar spinal canal stenosis: a case-control study. Acta Inform Medica 2019;27:229-33.



21. Gelfand Y, Benton J, De la Garza-Ramos R, et al. Effect of cage type on short-term radiographic outcomes in transforaminal lumbar interbody fusion. World Neurosurg 2020;141:e953-8.






























