Effects of D-Serine and MK-801 on Neuropathic Pain and Functional Recovery in a Rat Model of Spinal Cord Injury
Article information
Abstract
Objective
Neuropathic pain is a common secondary complication of spinal cord injury (SCI). N-methyl-D-aspartate (NMDA) receptor activation is critical for hypersensitivity in neuropathic pain. This activation requires the binding of both glutamate and the D-serine co-agonist to the NMDA glycine site. We evaluated the effects of D-serine on neuropathic pain after SCI and explored the underlying molecular mechanisms.
Methods
Anesthetized rats underwent T9 spinal cord contusion (130 kdyn). D-serine (500 and 1,000 mg/kg) and MK-801 hydrogen maleate (2.0 mg/kg) were injected daily for 2 weeks, starting the day after SCI. Functional outcomes were assessed according to the Basso, Beattie, and Bresnahan scale, while histological outcomes were evaluated based on lesion volume and spared tissue area. Mechanical allodynia and thermal hyperalgesia were evaluated by measuring the withdrawal threshold of a von Frey filament and hot/cold plate latency. Western blotting was performed to determine the expression levels of Trpv1, Nav1.9, calcitonin gene-related peptide (CGRP), and β-actin in damaged tissue.
Results
The withdrawal threshold values and latency of the D-serine group were significantly lower than those of the noninjection group. The MK-801 group showed higher threshold values and latencies than the other groups. Western blotting showed increased Nav1.9 and Trpv1 levels and lower CGRP levels in the D-serine group, whereas the MK-801 group showed the opposite results.
Conclusion
D-serine increases neuropathic pain after traumatic SCI by mediating the NMDA receptor. NMDA receptor antagonists alleviate neuropathic pain after traumatic SCI.
INTRODUCTION
Neuropathic pain is a common complication of traumatic spinal cord injury (SCI) occurring in 37.8% to 60.7% of cases following acute SCI [1-3]. In peripheral neuropathy, the peripheral terminals of pain-processing unmyelinated C fibers and thinly myelinated Aδ-fibers can spur the development of neuropathic pain after being affected by metabolic damage, toxins, medications, cytokines, and other inflammatory mediators, resulting in fiber density changes and neuronal hyperexcitability [4,5]. Peripheral nerve damage provides an opportunity for maladaptation at every point in the pain pathway. After peripheral nerve injury, subsequent central nervous system changes contribute to central sensitization. In central neuropathy, SCI causes direct and indirect spinal damage that leads to changes in neurochemical features in the nervous system, including sodium ion channels; voltage-gated calcium channels; glutamate and gammaaminobutyric acid metabolism; and serotonergic, noradrenergic, opioid, and N-methyl-d-aspartate (NMDA) receptors [6,7]. Neurochemical and excitotoxic changes can cause the release of excitatory amino acids such as glutamate, produce free radicals and reactive oxygen species, and cause an imbalance in ionic gradients [8]. These maladaptive changes in neurons along the nociceptive pathway can lead to neuropathic pain following SCI.
NMDA receptor activation causes the spinal cord neurons to become more responsive to all inputs, including those from damaged or sensitized nociceptors and low-threshold mechanoreceptors, resulting in central sensitization [9]. Central sensitization plays a fundamental role in the development of neuropathic pain, in which NMDA receptor activation is a key mechanism. This activation requires the binding of not only glutamate but also the coagonist D-serine for efficient receptor opening [10]. D-serine plays an important role as a gliotransmitter released from astrocytes and contributes to the development of neuropathic pain [11-13]. Evidence to date supports the hypothesis that D-serine is involved in neuropathic pain. However, most studies reported so far have focused on peripheral neuropathy induced in animal models.
Based on these previous results, we hypothesized that D-serine plays an important role in the development of neuropathic pain after traumatic SCI. It is possible that D-serine-induced pain after SCI was caused by activation of NMDA receptor and calcium inflammation of nociceptors and neuronal calcification [14,15]. However, few studies have reported that D-serine causes neuropathic pain following traumatic SCI. Thus, this study analyzed whether D-serine induces neuropathic pain after traumatic SCI and tested whether the mechanism is mediated by NMDA receptors (Fig. 1A).
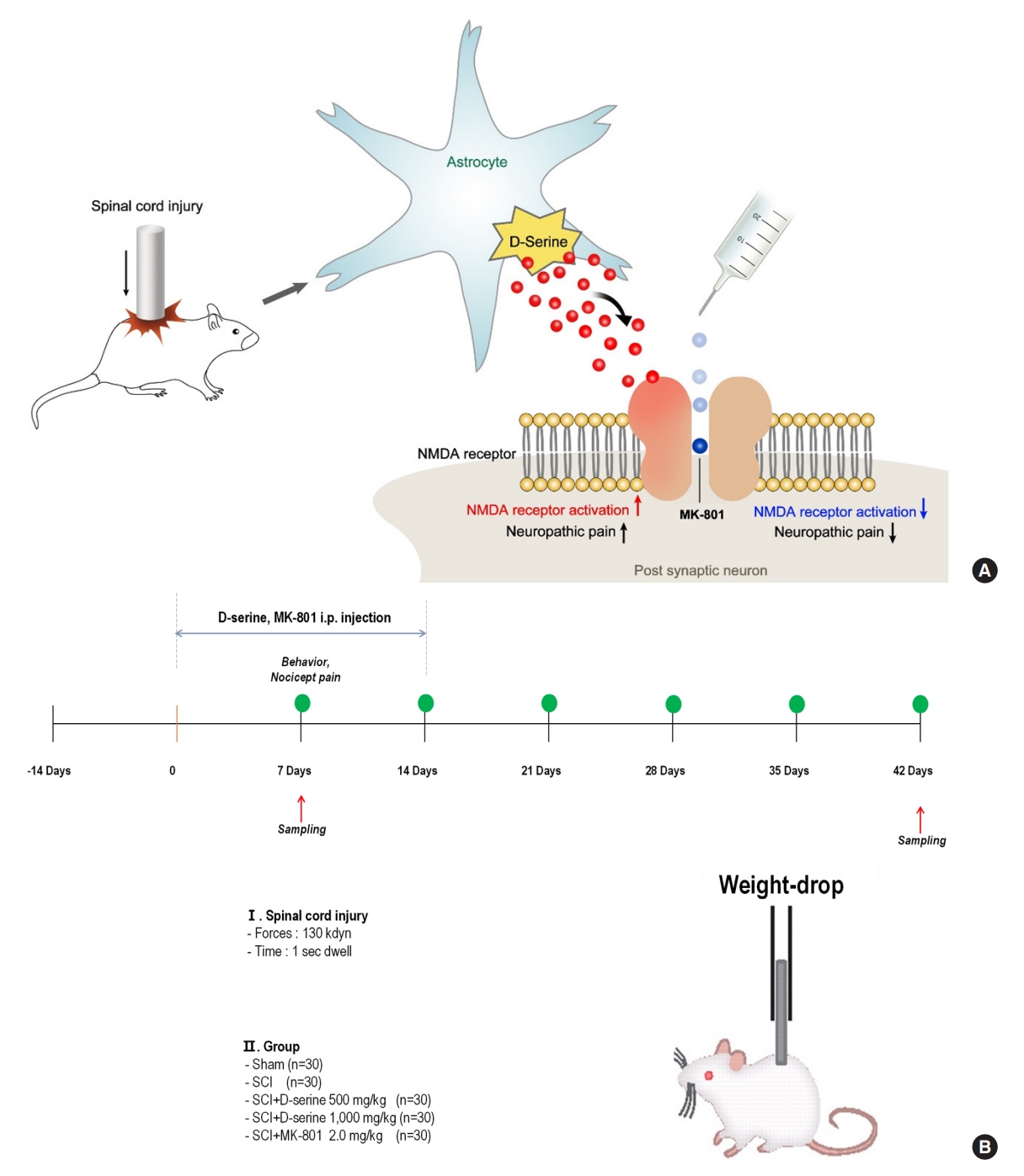
(A) Experimental animal design. The rat spinal cord is damaged at the T9 site using 130 kdyn of force on an infinite horizon impactor. Intraperitoneal D-serine (500 or 1,000 mg/kg) or MK-801 hydrogen maleate (MK-801; 2.0 mg/kg) is injected at the same time every day for 2 weeks, starting 1 day after surgery. (B) Schematic diagram of the proposed mechanisms for neuropathic pain after spinal cord injury. Increased D-serine production after the injury contributes to the development of neuropathic pain by inducing N-methyl-D-aspartate (NMDA) receptor activation. Exogenous MK-801 administration attenuates the spinal cord injury-induced development of neuropathic pain by inactivation of NMDA receptors. i.p., intraperitoneal; SCI, spinal cord injury.
MATERIALS AND METHODS
1. Animals and Cord Injury Model
All animal testing in this study was conducted at our university, which follows the National Institute of Health Guide for the Care and Use of Laboratory Animals (KNU-2020-0024). The animals were randomly assigned to 5 groups (n=30 per group): sham group, SCI group, SCI with D-serine 500 group (intraperitoneal [i.p.] injection of 500 mg/kg D-serine for 14 days beginning 1 day after SCI), SCI with D-serine 1000 group (i.p. injection of 1,000 mg/kg D-serine for 14 days beginning 1 day after SCI), and SCI with MK-801 group (i.p. injection of 2.0 mg/kg MK-801 for 14 days beginning 1 day after SCI). The rats were anesthetized via i.p. injection of a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg). After anesthesia, the spinal column was exposed from T8 to T10 and damaged at T9 with a 130 kdyn force applied using an infinite horizon impactor. Sham surgery was performed by exposing the spinal column in the same manner but without cord injury. Postoperatively, the urine was expelled by applying pressure to the abdomen twice daily for 6 weeks.
2. Drug Administration
The following drugs were injected i.p.: D-serine (500 mg/kg, 1,000 mg/kg) and MK-801 hydrogen maleate (MK-801; 2.0 mg/kg, a selective antagonist that binds to sites located within NMDA-associated ion channels) purchased from Sigma-Aldrich (St. Louis, MO, USA). The drugs were injected at the same time every day for 2 weeks, starting from the day after surgery. Thirty minutes before the evaluation of functional outcomes, all compounds were administered intraperitoneally. The choice of dose and pretreatment time for each drug were derived from previously reported studies [14,16-20]. The experimental design is illustrated in Fig. 1B.
3. Behavioral Assessments
At 1, 7, 14, 21, 28, 35, and 42 days following cord injury or sham surgery, the functional outcome of the rats was assessed using the Basso, Beattie, and Bresnahan’s open-field locomotor rating scale (BBB score, 0–21 points) [21]. The functional scores of the hind limbs observed for each animal for 1 minute were recorded and averaged by 2 investigators who were blinded to the group assignments. The ladder rung test was performed simultaneously with the BBB test. This test assesses the animal’s ability to accurately position itself on a step with its hind foot on a metal bar placed at 1.5 cm within a total length of 1.2 m while crossing the ladder [22]. The test also evaluates fore- and hindlimb coordination. The animals were videotaped at 3 intersections. The video data were analyzed by counting the positions of the correct hind paws on the bar. Each item was scored out of 10. In all experiments assessing functional outcomes, the rats underwent stabilization and training for 2 weeks before surgery. During the follow-up behavioral assessments, a blinded experiment was carried out with the experimental conditions of the individual animals blinded.
4. Processing of Spinal Cord Tissue
We prepared 3 sections per animal and sacrificed a total of 3 rats for each target. All animals used in this study were deeply anesthetized, perfused with phosphate-buffered saline (PBS) through the left ventricle to remove blood, and then perfused with 4% paraformaldehyde dissolved in 0.1 M PBS (pH 7.4) solution and fixed. After fixation, the spinal cord, approximately 15 mm above and below the epicenter, was collected, immersed in paraffin, and cut into 4-μm-thick sections. The cut tissue was deparaffinized and rehydrated for hematoxylin and eosin (H&E) and alizarin red S (ARS) staining. H&E staining was performed according to the manufacturer’s instructions (ab245880; Abcam). For calcium staining, the slides were incubated in 2% ARS (Sigma A-5533) at pH 4.3 (adjusted with ammonium hydroxide) and stained to remove excess dye. The slides were then soaked 20 times in acetone and 20 times in acetone-citrate solution. Section images taken with a Nikon Eclipse 80i were traced using ImageJ software ver. 1.53 (National Institutes of Health, Bethesda, MD, USA) to delineate and quantify the lesions. We identified the lesion site based on the results of H&E staining and confirmed it after merging the 3 based on the lesion site. Targets are merged with different colors by differentiating the 2nd antibody to proceed with fluorescent staining, and then quantification is performed after classifying the colors in which each target in the specified area appears in the Image J program, and then merging again. We performed ARS staining to highlight and detect calcium deposits in tissues or vasculature by binding to calcium through a chelation process, primarily to visualize the results at the cellular level.
5. Nociceptive Behavioral Tests
Von Frey filament and hot and cold plate experiments were performed every week to measure the degree of mechanical allodynia and thermal hyperalgesia after the development of the animal model. In this study, the hot plate test was based on methods in published paper [23]. Before proceeding with the von Frey filament experiments, the rats were placed in an acrylic box installed on a wire mesh test bench measuring 2× 2 mm and allowed to acclimatize for at least 15 minutes. When rat movements quieted, a medium-thick von Frey filament (Stoelting Co., Wood Dale, IL, USA) was used to make vertical contact with the affected sole and held in place for 5–6 secodns. A positive reaction was defined as avoidance, flinching, or licking of the sole. The stimulation was performed using a weak filament if a positive reaction was observed; otherwise, the stimulation was performed with a strong filament, and the continuous response was evaluated using the up-down method. The hot plate test is another classic test in the field and is typically set up to observe a response between 5 and 15 seconds. The temperature is often set at 52°C or 55°C and rarely at 48°C. A baseline latency of 5–15 seconds for paw licking is generally observed at 52°C or 55°C. In contrast to tail flick test and other tests that apply thermal stimulation to the hind paws, rats with SCI cannot remove their hind limbs from the stimulation. So, the results were limitingly gathered the nociceptive response time of the rat from the upper spinal pathway by placing on a metal surface maintained at a constant temperature [23,24].
6. Western Blot Analysis
Under anesthesia, the rats were bled through transcardial perfusion with warm 1× PBS. The thoracic spinal cords of the rats were then rapidly dissected and immediately homogenized on ice in cold RIPA buffer (Cell Signaling) containing a protease inhibitor cocktail (GenDEPOT). The homogenate was centrifuged at 13,000 rpm and 4°C for 30 minutes. Protein quantification of the homogenates was performed using the BCA Protein Assay (Thermo Scientific, Rockford, IL, USA). The proteins were separated by molecular weight by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 10% gel and transferred to a nitrocellulose membrane. The membrane was blocked using 5% nonfat milk powder in Tris-buffered saline containing 0.1% Tween-20 and incubated at room temperature for 1 hour. To determine the expression levels of Trpv1, Nav1.9 (Alomone Labs, 1:200), calcitonin gene-related peptide (CGRP; Cloud-Clone Corp, 1:200), and β-actin (Sigma-Aldrich, 1:1,000) in the damaged tissue, the membrane was incubated overnight at 4°C in a buffer with diluted antibody and then incubated with a secondary antibody (all Cell Signaling) bound to horseradish peroxidase for 1 hour at room temperature. The proteins were detected on a LAS 4000 instrument (GE Healthcare, Chicago, IL, USA) using a chemiluminescent reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA) and quantified using ImageJ software (National Institute Health, Bethesda, MD, USA).
7. Statistical Analysis
All values are presented as means±standard error of the mean. The statistical significance of the differences between groups was assessed using 1-way analysis of variance (ANOVA), followed by a post hoc least-significant difference range test. The differences in behavioral scores between the groups at each time point were analyzed using repeated-measures ANOVA with post hoc Tukey and Kruskal–Wallis tests. A p-value of < 0.05 and p<0.01 were considered statistically significant. All statistical comparisons were performed using IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA).
RESULTS
1. Effects of Drugs on Functional Outcomes in the SCI Rat Model
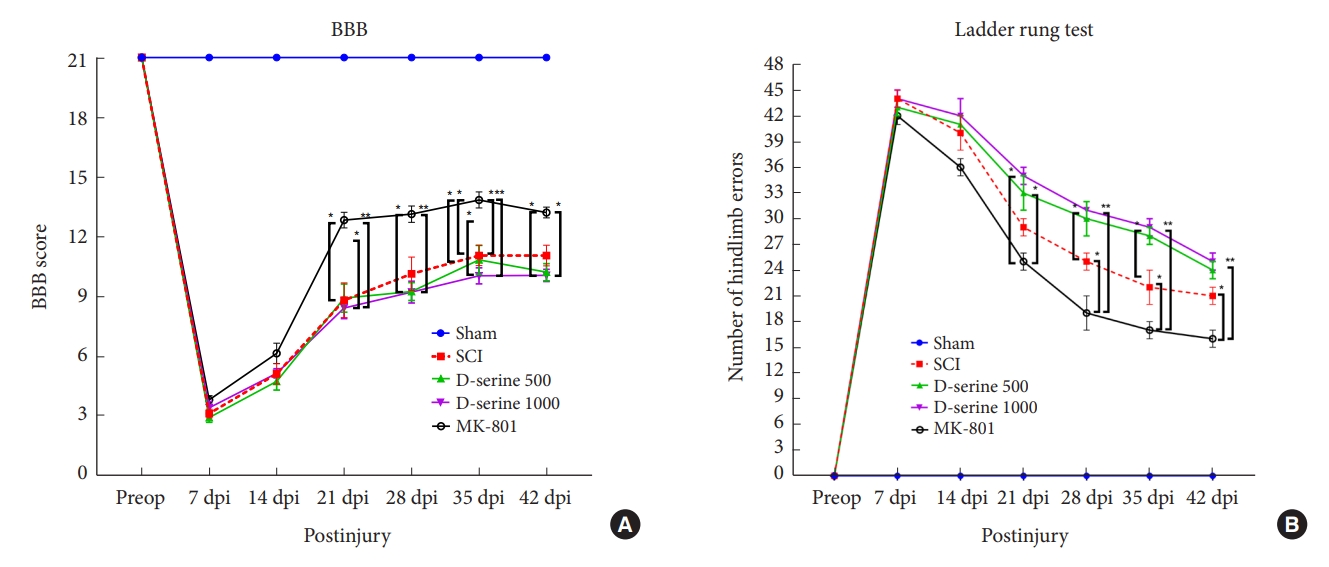
The rats in the sham operation group showed normal gait (score 21) after surgery. The MK-801 group showed significant functional recovery 21 days postinjury (dpi) compared to the SCI and D-serine groups. The mean BBB scores did not differ significantly at any time point in the SCI, D-serine 500, and D-serine 1,000 groups (Fig. 2A). In ladder rung test conducted at 21, 28, 35, and 42 dpi, D-serine group had significantly more foot faults than MK-801 group (Fig. 2B).
2. Histopathological Evaluations of the Spinal Cord
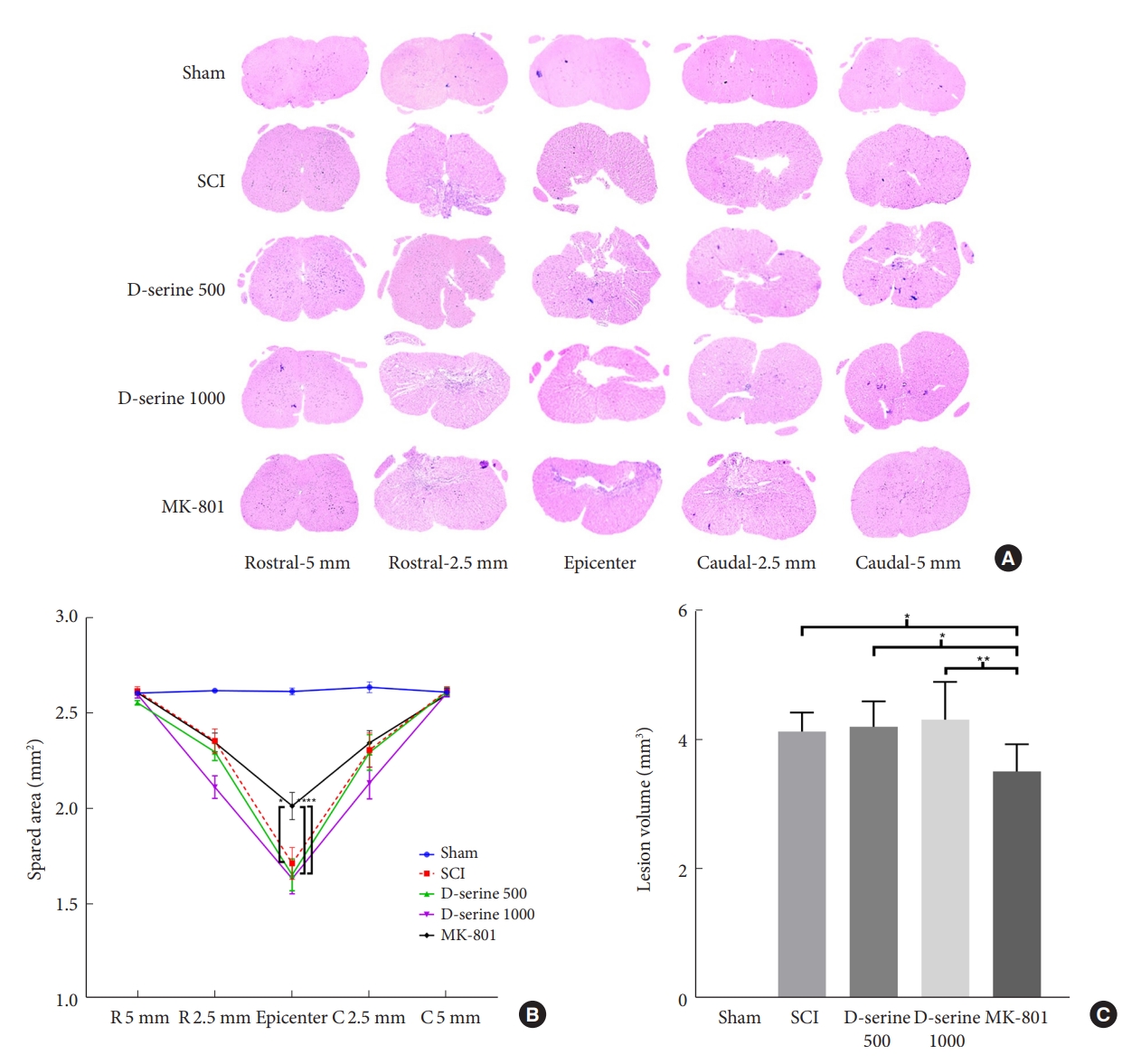
To investigate the effects of D-serine and MK-801 on the spared tissue area and lesion volume in the spinal cord after SCI, the rats were euthanized. The MK-801 group showed a lower lesion volume and a higher spared tissue area compared to those in the SCI and D-serine groups (Fig. 3). These results were consistent with the functional outcomes.
3. Effects of Drugs on Mechanical and Thermal Allodynia
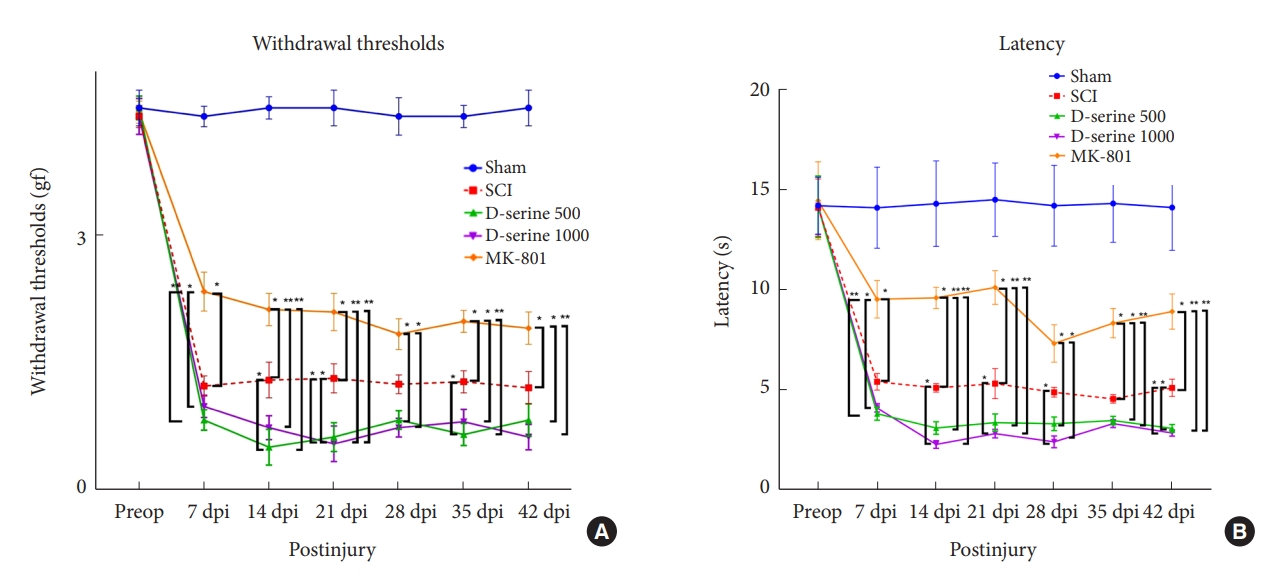
The results showed that cord injury-induced mechanical allodynia and thermal hyperalgesia postoperatively. At 14, 21, and 35 dpi, rats with SCI in the D-serine 500 group showed a significant decrease in the withdrawal threshold to mechanical stimulation compared to rats in the SCI group. The withdrawal thresholds in the MK-801 group were significantly higher than those in the SCI, D-serine 500, and D-serine 1,000 groups at 7, 14, 21, 35, and 42 dpi, respectively (Fig. 4A). We observed significant decreases in the withdrawal latency of the D-serine 1,000 group compared to those in the SCI group at 14, 21, 28, and 42 dpi. However, the MK-801 group showed a significant increase in withdrawal latency compared to those in the SCI, D-serine 500, and D-serine 1,000 groups at 7, 14, 21, 35, and 42 dpi (Fig. 4B).
4. Expression Levels of Nav1.9, Trvp1, and CGRP
We examined the expression levels of members of the pain signaling pathways, including Nav1.9, Trpv1, and CGRP, in Western blot assays of samples collected at 1, 7, 14, and 42 days after SCI (Fig. 5A). The expression levels of Nav1.9 and Trpv1 in the spinal cord were significantly higher in the D-serine group than those in the MK-801 group (Fig. 5B, C). Conversely, CGRP levels in the spinal cord were significantly higher in the MK-801 group than those in the D-serine group (Fig. 5D).
5. Calcium Deposition in Injured Spinal Cords
ARS staining was performed to investigate calcium deposition in the spinal cord after SCI. The D-serine group showed a larger area stained with alizarin S compared to that in the MK-801 group (Fig. 6).
DISCUSSION
The 2 important results of this study were as follows: first, the i.p. administration of D-serine significantly increased neuropathic pain in the SCI rat model. Second, rats that received an i.p. injection of MK-801, an NMDA receptor antagonist, showed significantly decreased neuropathic pain compared to the SCI and D-serine groups. In addition, i.p. injection of MK-801 promoted functional recovery. Although the estimated incidence of neuropathic pain after traumatic SCI is up to 60%, few studies have reported on neuropathic pain after traumatic SCI, especially on the effects of D-serine and MK-801. Thus, this study investigated the effects of D-serine and MK-801 on neuropathic pain after traumatic SCI in a rat model.
NMDA are heteromeric protein complexes. The 3 families of NMDA subunits are NR1, NR2, and NR3 [25]. The pathological role of NMDA receptors in neuropathic pain has been primarily studied in animal models of peripheral nerve injury. Isaev et al. [26] reported that the bath application of NMDA induced a greater increase in whole-cell currents and calcium influx in spinal lamina II neurons in nerve-ligated rats compared to that in control rats. Ultenius et al. [27] reported a significantly increased phosphorylation rate of the NR1 subunit of the NMDA receptor in the dorsal horn of the spinal cord after sciatic nerve ligation in rats. NMDA receptors in the dorsal horn play a critical role in nociceptive transmission and synaptic plasticity [28]. The activation of NMDA receptors requires the simultaneous binding of glutamate and the coagonists D-serine or glycine, which increases the affinity of glutamate binding and facilitates excitatory transmission [29]. D-serine is a potent coagonist of the NMDA glutamate receptor and appears to play a major modulatory role in NMDA receptor-mediated neurotransmission, neurotoxicity, synaptic plasticity, and cell migration [30,31]. Choi et al. [11] reported that D-serine increased glutamate receptor phosphorylation in a protein kinase C-dependent manner, contributing to the development of mechanical allodynia. Our results showed that the i.p. administration of D-serine significantly increased mechanical allodynia and thermal hyperalgesia. These results suggested that the exogenous administration of D-serine can increase glutamate receptor phosphorylation and activate NMDA receptors, leading to spinal neuron sensitization. Thus, we propose that D-serine plays a role in the aggravation of mechanical allodynia and thermal hyperalgesia in a rat model of SCI.
MK-801 is a noncompetitive antagonist of NMDA receptors. In the present study, the i.p. administration of MK-801 significantly reduced established mechanical and thermal allodynia in SCI rats. Thus, the exogenous administration of MK-801 may prevent pain perception by decreasing NMDA receptor activation to prevent the development of central sensitization. Our results regarding mechanical allodynia are consistent with those of other reports on the role of NMDA antagonist administration in a neuropathic pain model but discordant with those on thermal hyperalgesia. Thus, the role of NMDA receptors in thermal hyperalgesia remains controversial. Bennett et al. [32] reported that the intrathecal application of D-AP5, a competitive NMDA receptor antagonist, attenuated mechanical allodynia, but not thermal hyperalgesia, in a rodent spinal hemisection model of SCI. Ren et al. [33] reported that intrathecal administration of MK801 significantly attenuated thermal hyperalgesia in a carrageenan model of acute inflammation. The effectiveness of NMDA receptor antagonists differs in various neuropathic pain models. In this study, both mechanical allodynia and thermal hyperalgesia increased after the i.p. administration of D-serine. Therefore, exogenous administration of an NMDA antagonist attenuated the SCI-induced development of mechanical and thermal allodynia.
In this study, the i.p. administration of MK-801 was effective in the functional recovery of SCI in rats. Traumatic SCI causes an excessive accumulation of glutamate outside cells and leads to an increased flux of calcium ions into the cells via NMDA receptors [16]. Excessive glutamate release may damage oligodendrocytes in SCI [34]. Calcium dysregulation is a key step in the secondary injury cascade after SCI. Therefore, NMDA receptors may be effective therapeutic targets for preventing secondary injury in SCI. Esposito et al. [16] reported that the i.p. administration of MK-801 in SCI rats significantly improved the recovery of locomotor function. Gaviria et al. [35] reported that gacyclidine, a noncompetitive NMDA receptor antagonist, attenuated spinal cord damage in rats with contusive SCI. Our results are consistent with those of other studies on the effectiveness of NMDA antagonists in functional recovery after traumatic SCI.
In this study MK-801 was administered systemically, it could be affected off-target effects about cognitive function or behavior. It is well known that systemic administration of MK-801 causes enhanced locomotion in rodents [36]. Wisnewski and Lauwereyns [37] reported that systemic administration of a relatively small dosage of MK-801 facilitates performance. Similarly, systemic administration of MK-801 may have affected functional recovery due to the off-target effect in this study.
This study had some limitations. First, we did not study about the neuropathic pain and functional recovery by a specific D-serine antagonist. Because of MK-801 is not a specific D-serine antagonist, to determine whether the D-serine participate in excitotoxic neurotransmitter-induced central sensitization needs additional study. Second, we did not assess the differences in effects according to the dose of MK-801 administered. As we only administered a dose of 2.0 mg/kg, additional experiments on the effects of different doses are needed. Also, since the concentration was not measured at each time point, the difference in the result value depending on the concentration could not be explained. Third, we only administered drugs via intraperitoneal injection. Systemic injections may interfere with peripheral mechanisms. Fourth, there is a lack of a definite mechanism for the effect of NMDA antagonists on thermal hyperalgesia in the rat model of SCI. In many rat models of peripheral neuropathy, the administration of an NMDA receptor antagonist reduces mechanical allodynia but does not affect thermal hyperalgesia [32,38-40]. However, in the present study, MK-801 inhibited both SCI-induced mechanical allodynia and thermal hyperalgesia. Further studies are needed to characterize the discordant results between SCI and peripheral nerve injury models.
CONCLUSION
In our study, D-serine injection increased neuropathic pain following traumatic SCI. This mechanism was mediated by the NMDA receptor, and NMDA receptor antagonist alleviated neuropathic pain after traumatic SCI.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study was financially supported by Korean Spinal Neurosurgery Society (grant number: CGbio 2020-0001).
Author Contribution
Conceptualization: DCh CHK, IH, SL, SWL, KK; Data curation: SAM, KK; Formal analysis: SAM, KK; Funding acquisition: SL; Methodology: SAM, SWK, KK; Project administration: DY, SAM, SWK, SL, KK; Visualization: DY, SAM; Writing - original draft: DY, SWK; Writing - review & editing: DY, DC, CHK, KK.