Surgical Considerations to Improve Recovery in Acute Spinal Cord Injury
Article information
Abstract
Acute traumatic spinal cord injury (SCI) can be a devastating and costly event for individuals, their families, and the health system as a whole. Prognosis is heavily dependent on the physical extent of the injury and the severity of neurological dysfunction. If not treated urgently, individuals can suffer exacerbated secondary injury cascades that may increase tissue injury and limit recovery. Initial recognition and rapid treatment of acute SCI are vital to limiting secondary injury, reducing morbidity, and providing the best chance of functional recovery. This article aims to review the pathophysiology of SCI and the most up-to-date management of the acute traumatic SCI, specifically examining the modern approaches to surgical treatments along with the ethical limitations of research in this field.
INTRODUCTION
Spinal cord injury (SCI) affects more than 27 million people worldwide and is the second leading cause of paralysis in the United States [1,2]. Among the 329 million people in the United States, approximately 299,000 persons live with SCI with nearly 18,000 new cases occurring each year [3]. Most commonly the result of motor vehicle accidents and falls, SCI greatly affects life outcomes from personal, social, and economic standpoints. Health care costs and living expenses associated with SCI vary depending on education levels, the severity of neurological impairment, and preinjury employment history. Limited ability to return to work may exacerbate the financial burden that SCI patients experience, as only 18% of persons with SCI are employed 1-year postinjury. Although employment rate nearly doubles over time, the majority of those affected by SCI are left without stable forms of income [3].
In addition to the functional disabilities and economic impact associated with acute injury, individuals with SCI face high rates of rehospitalization. One study found that 36.2% of SCI patients were hospitalized at least once within the first-year postinjury [4]. Younger age, female sex, unemployment or retirement, and Medicaid coverage were all associated with increased odds of rehospitalization. Surgical, medical, and rehabilitative interventions, especially when used together, have been shown to improve neurological recovery, lead to higher postoperative sensorimotor function, and reduce rehospitalization after SCI [4-7].
Given the inability to modify the damage caused by primary injury and the poor regenerative capacity of central nervous system (CNS) neurons, recovery from SCI is often incomplete. However, timeliness of surgical intervention and spinal hemodynamic management could limit secondary damage [8-11]. Optimal timing, approaches, and parameters for acute surgical and medical interventions are understudied. Moreover, there are distinct ethical considerations for performing clinical trials in this population and setting. This gap must be addressed, as increased mobility improves quality of life, demonstrating the need to improve the efficacy of early interventions and enhance outcomes [12]. Further research can improve point of injury care and management, as well as facilitate neuroprotective strategies to optimize functional outcomes.
SPINAL CORD INJURY PATHOPHYSIOLOGY
To determine the best plan of care, it is essential to consider and understand the pathophysiology of acute, traumatic SCI. The acute phase of SCI is highly dynamic and associated with primary and secondary damage [13-15]. Primary damage, or frank disruption of axons and vasculature, results from one or more of 4 primary mechanisms: (1) impact plus persistent compression, (2) impact alone with transient compression, (3) distraction, and (4) laceration/transection. Impact plus persistent compression is the most common type of SCI observed in humans, with damage rarely affecting the entirety of the cord [16-18]. Upon injury, some spared and damaged axons traverse the lesion site, resulting in a subpial rim of myelinated and demyelinated axons [16]. The ability of these axons to effectively transmit signals across the injury site ultimately determines clinical classifications of complete versus incomplete injuries [14,19]. Neurological outcomes can often be predicted as soon as 72 hours after injury based on American Spinal Injury Association Impairment Scale scores, the presence of motor evoked potentials, or via emerging magnetic resonance imaging (MRI) biomarkers [20-23]. Recovery is greatest during the first 3 months postinjury, with progress plateauing at around 9 months in the absence of continuing rehabilitation [19,24]. However, long-term outcomes are closely related to the level and severity of the initial injury [19,20].
Primary damage from SCI triggers a cascade of biochemical, mechanical, and physiological change within affected tissues. This is known as secondary damage, beginning within minutes following primary impact and continuing for months postinjury. Secondary damage mechanisms can be subdivided into acute, subacute, and chronic phases, and extend beyond the area of injury to adjacent segments and throughout the neuraxis [13-15]. These mechanisms include vascular disruption, infarction, lipid peroxidation, ionic imbalance, oxidative stress, cell death, axon degeneration, demyelination, matrix remodeling, glial barrier formation, and neuroinflammation, which are expertly reviewed by Alizadeh and colleagues [15].
Briefly, frank disruption of vasculature causes infiltration of blood products and peripheral immune cells into the damaged spinal cord parenchyma as well as intact distal regions resulting in glial activation [25-29]. Microglia are among the first CNS cells to become activated in response to injury participating in phagocytosis and clearing of myelin debris [30-32]. They also contribute to the inflammatory response resulting in further blood spinal cord barrier breakdown and immune cell infiltration [31,33,34].
Similarly, astrocytes react quickly to SCI and in a severity-dependent manner with proliferation, migration, exaggerated hypertrophy, overlapping physical domains, increased cytokine production, and border formation [35]. Like microglia, astrogliosis has beneficial and maladaptive functions, regulated by specific signaling mechanisms in specific contexts. Reactive astrocytes limit the spread of inflammation and pathology, but they also secrete toxic factors that contribute to neuron and oligodendrocyte death [36], recruit peripheral leukocytes [37], and may later impair regeneration via inhibitory molecules such as chondroitin sulfate proteoglycans [38-46]. Additionally, extracellular levels of glutamate increase rapidly following impact and are likely linked to apoptotic mechanisms [47], which causes a neurotoxic increase in excitatory amino acid concentrations and further cell death [48-50].
Oligodendrocytes are particularly vulnerable excitotoxicity and are lost in tremendous numbers as early as 15 minutes post-injury [51-53]. This is especially detrimental as the loss of a single oligodendrocyte can compromise the functioning of numerous myelinated axons [54-58]. Facilitated in part by the inflammatory response, oligodendrocyte progenitor cells undergo robust proliferation in response to injury contributing to both the glial border and remyelination [59-70], which is a slow but largely complete process [71-73].
Axonal dieback is another clinically relevant consequence of SCI, occurring in 2 stages. During stage 1, the proximal and distal ends of the axon begin to diverge. The proximal end undergoes immediate axonal degeneration within an hour upon primary damage, and slower dieback over the next 48 hours [74,75]. During stage 2, axon bulbs continue to swell and retract from the injury site, roughly doubling in distance. This process closely corresponds with inflammatory cell infiltration [74,76,77]. Macrophage-mediated axotomy progresses dieback as well as a phenomenon known as Wallerian degeneration [76], which is the progressive degeneration of distal axon tracks [47]. The processes associated with Wallerian degeneration are mediated by pathways like those observed during apoptosis [47,78].
Each of these cellular and molecular responses occur in a severity-dependent manner with greater severity leading to greater tissue damage and worsened functional outcomes. Importantly, the timing of acute interventions for SCI has the potential to alter secondary injury cascades, decrease secondary pathology, and enhance postinjury recovery.
ETHICAL CONSIDERATIONS FOR RANDOMIZED TRIALS IN ACUTE SURGICAL MANAGEMENT OF SCI
Randomized controlled trials (RCTs) for surgical interventions raise several ethical issues [79]. By setting a null hypothesis, it may seem investigators are hypothesizing that one treatment is superior to the other, which means in theory that some patients will be randomized to a less favorable treatment [79]. Randomization also limits a surgeon’s ability to make surgical treatment decisions based on their patient’s individual situation [79], which may raise conflict of interest concerns and challenge the principles of autonomy and beneficence [80]. Placebo groups, such as sham surgery, pose significant risk to patients, such as general anesthesia and infection, without possibility of benefit [79-81]. Finally, trauma patients—which represent a subset of patients with SCI—pose additional challenges with informed consent. Patients with acute SCI who need surgery eminently would need to decide about joining a prospective research study in a narrow time window. For patients with concomitant head injury or decreased level of consciousness (e.g., shock), this puts surrogate decision-makers under pressure to decide unexpectedly if the patient lacks decision-making capacity [79,81-83]. Patients or their decision-makers during vulnerable situations, such as following traumatic injury, may make decisions out of desperation which can beget eagerness to participate and blur the lines of informed consent, particularly when the time window for the intervention is narrow [79,81-84].
Several proposals have been made to mitigate these ethical issues. Freedman proposed the widely accepted idea that RCTs are ethical if there is truly clinical equipoise between the 2 treatments—that is, there is no consensus between experts in the field on which treatment is superior [85]. This rationale provided the basis for a recent RCT of early surgical intervention for acute thoracic SCI [86]. Another way of thinking about this is having the control group receive the standard of care (usual care), rather than withholding a therapy with known benefit [87]. Conflict of interest can be reduced by having the patient’s surgeon be a different person than the study investigator—termed parallel care [79]. Finally, in research in emergency settings, such as traumatic acute SCI, the U.S. Food and Drug Administration has granted a waiver of consent if a study meets certain criteria [82,88]. Still, a waiver of consent raises concerns about patient’s rights and autonomy, as well as impact on vulnerable groups. Other ideas include studying how to better consent trauma patients and obtaining consent from a patient’s surrogate decision maker after detailed discussions of risks versus benefits [81,88].
ACUTE SURGICAL MANAGEMENT
1. Initial Diagnosis and Hospital Presentation
Once a patient is stabilized in the trauma bay, a trauma series computed tomography (CT) is performed, which includes a CT brain/cervical spine without contrast and CT chest, abdomen and pelvis with maximum intensity protocol with and without contrast [89]. If there is an injury pattern suspicious for a vascular injury, a CT head and neck angiogram is also ordered. If a spinal fracture, dislocation, or abnormality is identified on imaging, the spine surgery team is consulted for further recommendations. If surgery is applicable, the stabilization procedure should be performed as soon as possible; if the patient is not a surgical candidate, medical and therapeutic treatment is initiated instead for optimum recovery potential [89]. Interestingly, a recent study aimed to understand how the center type a patient presents to can influence their management and outcome after a SCI. Williamson et al. [90] reported that among a total of 11,744 incidents of SCI, those patients who were admitted directly to level I trauma centers had significantly higher odds of receiving a decompressive surgery compared to those who were either transferred to a level I center or went directly to a level II/III/IV center. As with all major health issues, social factors always play in role in accessibility of care and should be kept in mind when generalizing the results for SCI treatment.
2. Timing of Acute Decompression for SCI
With respect to preventing and mitigating the secondary injuries after SCI, there has been increased discussion regarding the advantages of acute surgical decompression. Before there were any large-scale clinical or retrospective studies, basic science groups investigated the potential effects of surgical decompression to improve neurological outcomes in clinically relevant animal models. From rat to beagle models, there is a plethora of laboratory evidence indicating significant benefits of acute surgical decompression with a varying postinjury time frame. For example, Dimar et al. [91] showed that longer periods of spinal cord compression worsened the prognosis of neurologic recovery in their rat model. Carlson et al. [92] found that the degree of early hematologic reperfusion after decompression was inversely proportional to the duration of spinal cord compression and proportional to neurologic recovery in dogs. Further, Carlson et al. [93] showed in the same dog model, longer durations of spinal cord compression produced larger lesions, which also corresponded to decreased long-term functional outcome. Consistent with these results, small single institution cohort reviews revealed similar results in patients who had undergone urgent decompression after SCI [94,95]. These initial studies showed mixed results, none of which were standardized across institutions. However, La Rosa et al. [96] showed in their systematic review that early decompression resulted in better outcome compared with both conservative and surgical treatment after 24 hours.
In 2012, Fehlings et al. [97] performed a large, multi-institutional retrospective study evaluating 313 patients across 6 hospital systems. Every patient in their cohort received both decompression and instrumented fusion procedures of their cervical spine either within an early (<24 hours) or late (>24 hours) time frame following self-reported SCI. Measurements of postoperative neurological improvement were based on the change in American Spinal Injury Association Impairment Scores (AIS) from baseline (within 24 hours of presentation). The main statistically significant result showed that patients in the early surgery group were almost 3 times more likely to have a 2 grade AIS improvement at the 6-month postoperative follow-up mark. Since that article was published, there have been a multitude of other corroborating cohort studies indicating that patients undergoing early surgery after SCI have a significantly better neurological outcome as compared with patients who underwent surgery after 24 hours [98-101].
Despite the clinical evidence suggesting that early surgical intervention is associated with improved outcomes, there remain a number of poorly defined issues regarding optimal timing. For example, the 24-hour cutoff point in some studies is somewhat arbitrary, and other studies have evaluated postsurgical intervention at 8 hours, 72 hours, or even 4 days [102-104]. Why then, if the animal models and surgeon preferences clearly show a time-dependent effect on the postoperative neurological benefits, would studies not aim to have their cutoff at less than 24 hours? [105] Multiple studies have addressed this discrepancy to show that only between about 20% to 50% of SCI patients can feasibly undergo an emergency decompression within the first 24 hours after injury due to practical factors like transportation and other life-saving measures [97,106,107]. A more recent study by Aarabi et al. [108] revealed that in patients with postoperative MRI confirmation of a complete acute decompression following cervical SCI, preoperative intramedullary lesion length, not the timing of surgery, was the only significant determinant for longterm neurological outcome. Their study also looked at ultraearly (less than 12 hours), early (between 12 and 24 hours), and late (after 24 hours) decompressive surgeries. In total, the relevance of acute decompression as a surgical management for SCI has quickly evolved over the past decades and has shown significant promise towards improving postoperative neurological function in patients across many studies. At the same time, there are still questions that need further investigation to prepare the most accurate, efficient, and beneficial guidelines for treating individuals with SCI.
3. Anterior Versus Posterior Approach for Acute Cervical Decompression
For cervical injuries, surgical technique can vary depending on the nature of the injury, patient factors, surgeon skill level [109]. The anterior approach generally offers easier access to any anterior compression or disc herniation while also utilizing a safer supine patient position. It is also less invasive when compared to the posterior or combined approaches, but there are limitations and contraindications. Johnson et al. [110] reported a 13% failure rate for superior endplate compression fractures following an anterior approach. These cases then require a switch to a posterior approach which extends time under anesthesia and increased risk for neurologic deterioration [111]. Wang et al. [112] further corroborated these results by showing increased risk for graft migration for patients who only underwent anterior fixation with posterior hardware. If a posterior approach is chosen for addition stability, it brings its own set of potential pitfalls. For one, pedicle screw placement in the cervical spine has been associated with increased rates of vertebral artery injury given its close proximity [113]. Mainly, studies have also focused on the relative instability at the cervicothoracic junction. If fixation does not cross C7 into T1, Nagashima et al. [114] noted up to 40% risk of hardware failure. However, Huang et al. [115] demonstrated that crossing the cervicothoracic junction during posterior decompression and fusion was associated with increased surgical time, estimated blood loss, and rates of wound dehiscence. These counteracting results further illustrate the need for larger cohort studies to clearly elucidate the necessity of crossing the junction.
Of note, situations exist where a combined approach may be beneficial, especially if a ventral decompression is needed or the anterior column’s integrity is compromised [116]. Recent case reports have even proposed a 3-staged surgical approach consisting of cervical laminectomy, posterior fixation, and anterior corpectomy and fusion [117]. While these singular demonstrations are intriguing and require further testing, the combined approach may increase surgical trauma via positional changes, nerve injuries, and incidence of emergency airway management [118-120]. Therefore, anterior alone and posterior alone approaches have dominated the current management of SCI.
In the setting of a SCI, only a handful of studies have directly examined the short and long-term outcomes of each approach for cervical decompression. The most recent study was conducted by Ren et al. [121] in 2020, in which almost 200 patients underwent either anterior reduction with interbody fusion or posterior reduction with short-segmental pedicle screw fixation. They followed these patients for 10 to 17 years and concluded that the posterior approach was associated with greater loss of alignment by 2 years and at final follow-up. The posterior approach group also had significantly more blood loss, longer operation times, increased risk for infection, and longer hospital stays [122]. In comparison, the anterior approach was associated with better long-term neurological recovery and preserved cervical alignment [121]. However, the majority of the current literature have come to conflicting conclusions. In the review of Verlaan et al. [123], the posterior pedicle screw fixation method was preferred over both anterior and combined posteroanterior approaches [123-125].
Looking forward, the debate between which surgical approach is superior will likely depend on timing and individual patient presentation. For example, the role of MRI in surgical decisionmaking is still under investigation. Imaging can prove crucial when planning either approach, but generally, an MRI is only required for the anterior technique to visualize any impeding structures along the way to the spine [126]. Importantly, getting an MRI requires substantial cost and time. As mentioned previously, timing of surgery is vital for patient recovery, so every second that can be saved, should be saved. Additionally, with recent innovations in robotic navigation and advancements in minimally invasive surgeries, there will be more data on the safety and efficacy of these enabling technologies. For these reasons, more large cohort prospective studies will be needed to accurately differentiate the benefits of each approach.
4. Anterior Versus Posterior Approach for Acute Thoracolumbar Decompression
There are a variety of causes for SCI in the thoracic and lumbar region including distraction, burst, compression, rotational, and tension band each of which requiring a slightly different surgical approach [127]. However, this discussion will focus primarily on fracture dislocations since they are by far the most common cause of SCI in the acute emergency setting given their high-impact nature [128]. Fracture dislocations are generally associated with severe neurological dysfunction on presentation and require immediate surgical decompression via posterior pedicle screw fixation after the patient has been medically stabilized [129]. Unlike in the cervical region, the posterior approach is considered the primary choice for acute thoracolumbar decompression for a number of reasons: less vascular/abdominal structures to navigate around when approaching posteriorly, better visualization of the spine, and surgeons generally are trained more in this approach compared to anteriorly [130,131]. Recent studies have begun examining whether the anterior approach is actually inferior to the posterior. They have shown no neurologic recovery difference between anterior and posterior approaches [124,132]. Other studies have implemented a sole anterior approach in patients that do not have any neurologic deficient on presentation; their results have been promising but still have not been tested in cohorts with severe high-impact injuries [133,134].
5. Hemodynamic Stability for Spinal Cord Injuries
As described above, there can be multiple barriers that could prohibit a patient from receiving an early acute decompression surgery in the setting of a SCI. Therefore, it is crucial for medical management to also be part of the treatment protocol. Initially, multiple studies revealed that corticosteroids were thought to be beneficial if given within 8 hours of the injury [135-137]. The pathophysiology behind this hypothesis had been studied prior and showed 2 possible pathways by which methylprednisolone may improve neurological recovery. First, methylprednisolone likely suppresses membrane degradation by inhibiting lipid peroxidation and hydrolysis at the site of injury. Additionally, the breakdown of cellular membranes peak within 8 hours of injury, which correlated with the literature at the time [138,139]. The second pathway is that vasoreactive by-products of arachidonic acid metabolism are reduced when treated with corticosteroids, which improve blood circulation to the injury site [140]. Over time, however, new data showed that continuous treatment of steroids increased risk for severe sepsis, infection, respiratory distress, and mortality. Sultan et al. [141] showed in their meta-analysis that there was not only a nonsignificant improvement in the neurological recovery but also a significant increased risk for pneumonia. Therefore, corticosteroids are no longer first line agents in the setting of an acute SCI.
Interestingly, the second pathway by which corticosteroids were hypothesized to help with SCI was the basis for the more recent approach to the medical management of SCI: hemodynamic stability and the impact of mean arterial pressure (MAP). The importance of MAP, blood flow, and perfusion relate back to the secondary effects of SCI, which have been thoroughly studied in the literature [142-144]. The 2013 guidelines for SCI management indicated that an ideal MAP was between 85–90 mmHg and should be targeted for the first 7 days following a SCI via supplementation of vasopressors and intravenous fluids [145]. Following suit, other studies began putting the guidelines into play, and the results were mostly positive. For instance, Hawryluk et al. [146] examined a cohort of 100 patients and showed that higher average MAP values correlated with improved recovery in the first 2–3 days after a SCI. Dakson et al. [147] showed that there was an 11x better chance for neurologic recovery in patients with MAP > 85 mmHg. The next steps for researchers were to elucidate potential methods to accurately monitor MAP continuously, discover the mechanisms by which MAP actually leads to improved outcomes, and refine the technique of maintaining a constant blood pressure range.
Recently, Brian Kwon and his laboratory addressed these questions and expanded the idea of hemodynamic stability for SCI [148]. In his work, Kwon discusses measuring intraparenchymal blood flow and oxygenation using laser Doppler blood flow/oxygen probes. Using this technique, they were also able to show that decompressive surgeries in combination with MAP augmentation significantly increase PO2 levels close to or above preinjury values, thereby preventing ischemia. They also looked for downstream metabolic changes when using decompression and MAP augmentation, discovering a decrease in the lactate/pyruvate ratio, which is a surrogate for decreased tissue damage. Lastly, they drew more attention to the importance of the previously studied intraspinal pressure (ISP) and spinal cord perfusion pressure (SCPP; SCPP = MAP−ISP) [149-151]. Along with the other recent literature, SCPP seems to be almost, if not more important than the MAP range. Squair et al. [152] showed that a SCPP within 60–65 mmHg, not MAP, was the best indicator of improved neurologic function in humans.
Of note, the literature has also described limitations to maintaining hemodynamic stability in older patients with more extensive neurologic injury. Even when the ideal SCPP and MAP are controlled for, confounding and uncontrollable variables can affect recovery chances for patients. For instance, Coleman and Geisler [153] found that among 760 patients, AIS score was the strongest predictor for positive outcomes. Furthermore, the World Federation of Neurosurgical Societies Spine Committee stated in their 2020 guidelines that factors such as older age and more severe neurological damage are associated with a lower likelihood of neurological recovery [154]. Much of the literature is in agreement with these statements, recommending surgery and medical management for the majority of acute SCI [155]. Studies have yet to prove that nonoperative management for any severity of acute SCI with boney injury or spinal instability is better than surgery. Interestingly, although elderly patients are at greater risk for deterioration, they generally wait longer for surgery and have higher inpatient mortality rates than younger patients [156]. More research is needed to fully elucidate the difference in surgical recommendations for patients with only instability, mild or severe deficits. However, based on the current literature, medical management followed by early decompression is recommended as the standard of care for all patients with signs of boney instability.
In total, the medical management of SCI has continued to evolve over the past decade. From corticosteroids to measuring the oxygenation of spinal cord blood flow, the treatment guidelines will continue to reflect the basic science, pharmacological, and clinical research breakthroughs being made [157]. Of note, a recent study in 2022 has begun to test the feasibility of not only measuring the oxygen tension of the acute SCI site, but also attempting to alter that tension by increasing the fraction of inspired oxygen, or FIO2 [158]. With the combination of both surgery and innovative medical treatment, neurological recovery following a SCI is more of a possibility than ever before (Fig. 1).
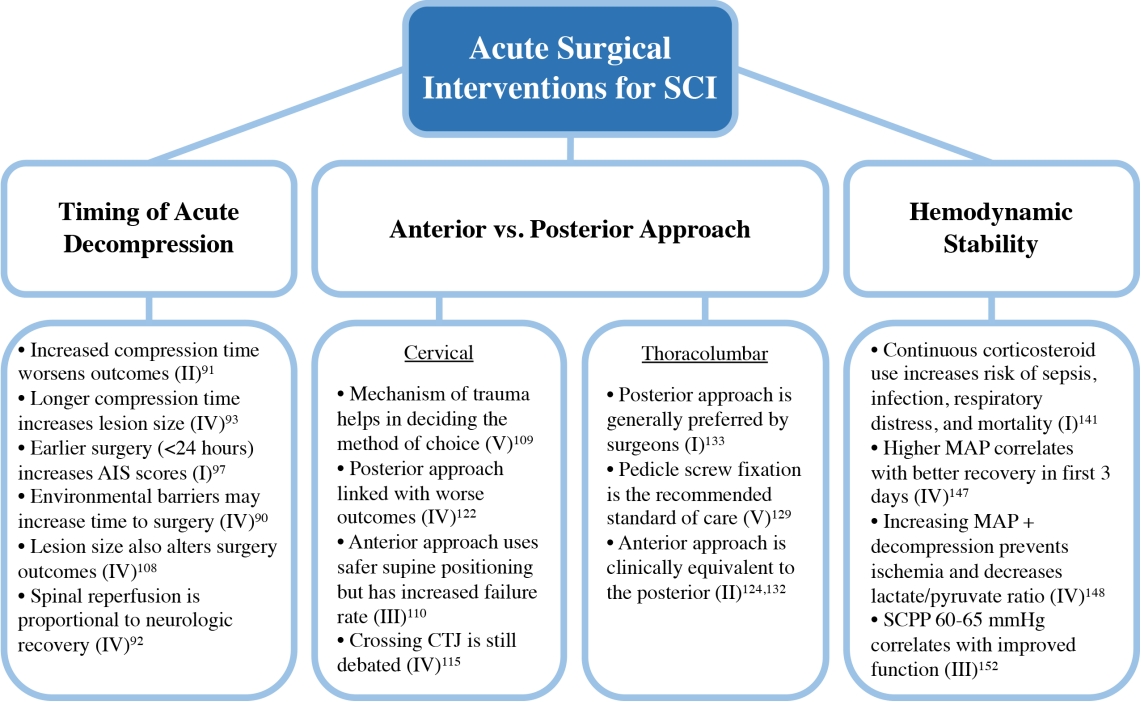
Acute surgical interventions for spinal cord injury (SCI). Interventions were broken down into 3 primary areas: timing of acute decompression, anterior versus posterior approach, and hemodynamic stability. Key aspects from each category are highlighted. The Oxford Center for Evidence Based Medicine Level of Evidence (I–V) for each key point is shown in parentheses followed by corresponding references. AIS, American Spinal Injury Association Impairment Scale; CTJ, cervico-thoracic junction; MAP, mean arterial pressure; SCPP, spinal cord perfusion pressure.
CONCLUSION
Acute traumatic SCI can leave a lasting impact on the overall well-being of an individual. For urgent cases associated with severe neurological dysfunction, it is crucial to provide emergency, rapid, and specialized therapies to minimize secondary injuries. After initial evaluation, surgical candidates should undergo timely decompression, ideally within 24 hours of injury. For both surgical and nonsurgical candidates, medical management with an emphasis on hemodynamic stability and optimizing cord perfusion should be started regardless of surgical status to maximize chances of recovery.
Informed by the numerous preclinical and clinical research studies evaluating the pathophysiology and treatment of SCI, management strategies are constantly evolving, with promising interventions just on the horizon. As described in this review, there are still numerous unanswered questions, new drugs funneling through clinical trials, and fluid protocols hinging on the results of breakthrough studies. In particular, the ongoing debate between ultra-early versus early decompression and when to begin intensive rehabilitation should be significant areas of focus for large-scale trials moving forward. Just like with the effects of initiating rehabilitation after SCI, timing of interventions is clearly important, and researchers have yet to fully define the complete pathophysiological impact of decompression timing on outcomes. Along the same line, are there additional preoperative patient variables that need to be considered when deciding on the most appropriate management plan? These factors could potentially include genetic factors, comorbidities, demographics, imaging, or inflammatory biomarkers – all of which require better understanding.
Looking ahead, we hope this review of the current literature surrounding the acute management of SCI brings to light not only how far treatment has progressed, but also the gaps of knowledge that remain unfilled. With the continuous introduction of neuroregenerative therapeutics like stem cell targeting, hydrogel scaffolding, and monoclonal antibodies, surgical/medical management will need to be tested ethically both alone and in conjunction with pharmacologic treatment to determine which methodology yields the best outcomes for patients. With more comparative data and large-scale cohorts, universal guidelines will begin to reflect these novel treatments once they have been thoroughly tested in the clinical setting.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was funded in part by a Research Incubator Award from the Duke Institute for Brain Sciences (TDF, MMA, DTL, HW).
Author Contribution
Conceptualization: TT, NL, MKH, HW, DL, TF, MAEB; Data curation: TT, NL, MKH, NZ, BC, EM, TW, HW, DL, TF, MAEB; Formal analysis: TT, NL, MKH, NZ, BC, EM, TW, HW, DL, TF, MAEB; Funding acquisition: HW, DL, TF, MAEB; Methodology: TT, NL, MKH, HW, DL, TF, MAEB; Project administration: HW, DL, TF, MAEB; Visualization: TT, NL, MKH, NZ, BC, HW, DL, TF, MAEB; Writing - original draft: TT, NL, MKH, NZ, BC, EM, TW, HW, DL, TF, MAEB; Writing - review & editing: TT, NL, MKH, NZ, BC, EM, TW, HW, DL, TF, MAEB.
