|
 |
- Search
Abstract
Objective
Recently, surgical outcomes of patients with intramedullary spinal cord tumors (IMSCT) have been improved due to advances of medicine. The purposes of our study were to evaluate the recent neurological outcomes after surgical treatment of IMSCTs.
Methods
We retrospectively reviewed 69 patients who underwent surgical treatment for IMSCT in our hospital between 1998 and 2013. Patient's age, sex, histological origin and grade, tumor location, tumor extension, preoperative neurological state, initial presenting symptom, and extend of tumor resection were analyzed to evaluate predictive factors that affect postoperative functional outcome.
Results
The neurological states at last follow-up were improved in 16 patients (23.2%), unchanged in 47 (68.1%), aggravated in 6 (8.7%). In all patients, the functional outcomes were good in 52 patients (75.4%), fair in 10 (14.5%), poor in 7 (10.1%). Preoperative good neurological state was the strongest positive predictor of good functional outcome (p<0.05). In tumor location, functional outcomes of thoracic tumors were poor than those in cervical and conus medullaris region (p=0.011). High-grade tumor shows poor outcome compare to low-grade tumor (p=0.03).
Intramedullary spinal cord tumors (IMSCTs) compromise about 2-4% of all central nervous system neoplasm and about 20-25% of all spinal tumors2,12,35). Surgery of IMSCTs is very challenging to neurosurgeons, which may result of devastating neurologic deficit. Recently, surgical outcomes of patients with IMSCTs have been improved due to advances of diagnostic imaging, microsurgical technique, surgical equipment, and neurophysiologic monitoring. The purposes of our study were to evaluate the recent surgical outcomes of patients with IMSCT considering recent advances of diagnostic imaging and surgical technologies. In addition, we analyzed the prognostic factors affecting neurological outcome after surgical resection of IMSCTs.
Between 1998 and 2013, 69 patients with an IMSCT were admitted and underwent surgical treatment at one institution. There were 41 males (59%) and 28 females (41%). Mean age was 39.7 years, ranging from one to 78 years. The post-operative follow up periods were ranged from 6 to 171 month (mean 34.8 months). Basic demographic data, clinical presentation and radiologic exams were retrospectively reviewed in each patient.
The neurological state was classified according to American spinal injury association (ASIA) grade in order to achieve a grading of functional disturbance of daily life activities and gait disturbances. The neurologic outcome was classified as poor (A+B), fair (C) and good (D+E) according to the ASIA grade.
Patient's age at diagnosis, sex, histological origin and grade, tumor location, level of tumor extension, pre-operative neurologic state, initial presenting symptoms and extend of tumor resection were reviewed to analyzed prognostic factors. The histological origins of IMSCTs were classified into neuroepithelial and non-neuroepitheilial tumor. Neuroepithelial tumors were classified as low-grade tumors (GradeI+II) and high-grade tumors (Grade III+IV) by World Health Organization (WHO) classification. Tumor localizations were divided into cervical, thoracic and conus medullaris. Level of tumor extension was classified into less than four involved segments and four or more segments. Initial presenting symptoms were divided into pain, sensory change and motor weakness. Extend of tumor removals were classified as total resection (TR), subtotal resection (STR) or biopsy. The TR was defined as complete removal of tumor by microscopic surgical finding and postoperative MRI finding. All patients had taken magnetic resonance imaging (MRI) pre- and post-operatively. The patients have undergone standard microscopic operations using posterior approach. Few patients were monitored intraoperatively with somatosensory evoked potential (SSEP) and motor evoked potential (MEP). The steroid was prescribed preoperatively in cases with acute neurological deterioration or edematous signs of the surrounding spinal cord tissue in MRI.
Statistical analyses were performed using SPSS (version 12.0). Žć2 test was performed to assess the effect of different variable on outcome. All p values less than 0.05 were considered statistically significant.
The most common tumor localization was thoracic lesion (n=32, 46.4%). Conus medullaris (n=19, 27.5%) and cervical tumors (n=18, 26.1%) were followed. In 38 patients (55.1%), less than 4 segments were involved and in 31 cases (44.9%) four or more segments were involved.
There were forty cases of neuroepithelial tumors (58%) and 29 cases of non-neuroepithelial tumors (42%). Seven patients were diagnosed as a high-grade tumor (10.1%) whereas 62 patients (89.9%) showed low-grade histology. The most common histological origins of IMSCTs was ependymoma (n=26, 37.7%) and astrocytoma (n=41, 20.3%) was followed. The hemangioblastoma (n=7, 10.1%) was most common histological type in non-neuroepithelial tumors (Table 1).
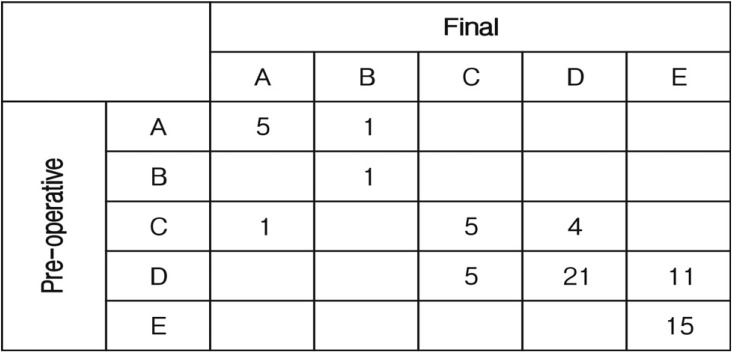
In all patients, the neurological outcomes were good in 52 patients (75.4%), fair in 10 (14.5%), and poor in 7 (10.1%). Forty seven patients (68.1%) showed the same final neurological states at last follow-up compared to the preoperative neurological state. Sixteen patients (23.2%) showed neurological improvement, whereas six patient's (8.7%) neurological states were aggravated. One patient showed neurological improvement of ASIA grade A to B, ASIA grade C to D in four patients and ASIA grade D to E in 11 patients. However, one patient's neurological state was aggravated ASIA grade C to A and 5 patients showed neurological worsening ASIA grade D to C (Fig. 1).
Postoperative complications were postoperative hematoma (2), CSF leakage (1), laminoplasty impingement (1), and aspiration pneumonia (1). In 8 patients, tumor recurred during follow-up.
Most strong predictive factor for good neurologic outcome after surgery for IMSCTs is a patient's preoperative neurologic state. Patients who can gait dependently or independently (ASIA grade D and E) preoperatively resulted 90.4% of good outcome and 9.6% of fair and poor outcomes. However, patients who cannot gait (ASIA grade A, B and C) resulted only 29.4% of good outcome and 70.6% of fair and poor outcomes (p=0.000). Similarly, patient's with motor weakness showed poor neurological outcome than other symptoms (p=0.029). Relations between pre-operative clinical predictive factors and post-operative neurological outcomes are summarized in Table 2.
Other predictive factors that affect the neurological outcome are location of tumor (p=0.011) and high grade tumor (p=0.03). Tumors in cervical (94.4%) and conus medullaris (88.9%) lesion result better neurological outcomes than in thoracic (59.4%) lesion. In contrast, poor outcome in higher in thoracic (18.8%) than in cervical (0%) and conus medullaris (5.3%). High grade tumor shows 42.9% of good outcome and 28.6% of poor outcome, whereas 79% of patients showed good outcome and 8.1% showed poor outcome in low grade tumor.
Patient's sex, age (less or more than 40 years), histological origin (neuroepithelial versus non-neuroepithelial), level of tumor extension, and extend of tumor resection (total resection, subtotal resection or biopsy) were not associated with postoperative neurological outcome.
In the past, some physicians recommended conservative surgery followed by irradiation for IMSCTs because total removal of tumor may injure normal spinal cord around the tumor34). However, improving medical instruments such as high field MRI, surgical tools including microscope with high-definition technology, ultrasonic aspirator and intraoperative monitoring, surgery of IMSCT has become much safer1). Recently, a microsurgical resection is considered as gold standard in the treatment of IMSCT with the aim of complete tumor removal3). Radiotherapy and chemotherapy should only be preserved for high grade lesions or some cases of low grade tumor with incomplete resection and clinical worsening28). Recently, stereotactic radiosurgery for intramedullary spinal lesion has been reported26). Stereotactic radiosurgery was done on metastasis, vascular malformation and benign tumor. Although very limited number of cases was done, they suggested that stereotactic radiosurgery is an effective and safe alternative option to conventional radiotherapy.
This study shows better outcomes than previous study (unpublished) of our institution on IMSCTs. Previous study analyzed 43 patients who received surgery for IMSCTs between 1984 and 1995. Neurological statues were improved in 11 (25.6%) patients postoperatively. Twenty-two patients (51.2 %) showed unchanged function outcome. However, 10 (23.3 %) patients showed worsening of functional outcome whereas only 6 patients out of 69 (8.7%) showed neurological worsening in this study. Even though improving medical techniques may result better clinical outcome after surgical removal of IMSCTs, surgical treatment of IMSCTs is very challenging. Various clinical factors were proposed as predictive factors of functional outcome after IMSCTs surgery. In this study, we also analyzed to identify predictors of postoperative functional outcome. Preoperative inability to walk, thoracic location and high-grade tumor were independently associated with poor post-operative functional outcome. Patient's sex, age, histological origin, level of tumor extension and extend of tumor resection were not significantly associated with postoperative functional outcomes in this study.
This study also supports the findings of previous reports about predictors about IMSCT that preoperative ability to walk is the strongest predictive factor of postoperative outcome5,6,9,10,11,13,15,32,33). These results not only emphasize the importance of early recognition of symptoms and evaluations of IMSCT but also recommend early surgical treatment outcome10,11,13,20,24,25). The operation should be immediately performed not to wait the onset of clinical deterioration to maximize a chance of preserving an ability of walk.
Furthermore, in our study the tumors in thoracic lesion were associated with poor prognosis (Fig. 2). Various studies also showed that tumors arising at thoracic cord were related to poor neurological prognosis1,13,24,33). These could be occurred irreversible damages caused by poor microcirculation and the narrow spinal canal than other spinal lesions during intraoperative maneuver. Early surgical interventions should be important in cases with thoracic tumors, even if the neurologic defect is mild. Because prolonged compression of IMSCT, amount of blood flow was increasingly reduced. The possibility of postoperative poor prognosis increased when neurological defect was happened. Additionally, thoracic spinal cord had been reported to be more liable to radiation damage18,27), which may be a cause of neurologic defects in survivors with malignancy tumors. On the other hands, thoracic IMSCT was associated with increased survival when compared to other locations22,25). Tumors located in the thoracic spinal cord take longer time to invade respiratory center leading to respiratory failure (common cause of death among patients with intramedullary malignancy tumors) than tumors initially growing from the cervical spinal cord25).
This report showed that high-grade tumors had poor neurological outcome. This is maybe due to poor margin between tumor and normal spinal cord. High grade tumor tends to infiltrate to normal spinal cord which results obscure surgical plane. Removal of high grade tumor may also injured normal spinal cord resulting poor neurological outcome. Additionally, high-grade tumors require pre- or post-operative radiotherapy, which could result poor functional outcome38,39). Preoperative radiotherapy may cause radiation-induced myelopahty and/or myelitis19) and compromise the spinal cord microvasculature which lead to spinal cord ischemia. Beside of neurological outcome, high grade tumors also show poor survival outcome. There are some reports that the patients with glioblastoma had a higher mortality and poor prognosis in malignancy spinal cord tumors21,29,36). These results were caused by the difficulty of total resection of high grade tumors. The rate of complete resection was approximately 90% for ependymoma and hemangioblastoma and 50-76% in low grade astrocytoma11). However, a complete resection was possible only in 16.6% of malignancy tumors37). But adult patients with malignant intramedullary tumors treated with gross total resection had a significantly lower mortality and prognosis than patients treated with subtotal resection, biopsy, or non-surgical measures36). The total resection of malignancy tumor was difficulty but may improve survival.
Recently, advanced microsurgical skills and intraoperative neurophysiological monitoring have made more aggressive chances for total and nearly total resection of IMSCT8,17,30). In this study, the extent of tumor resection was not associated with postoperative neurological outcome. But several authors reported a series with complete tumor removal and good postoperative functional outcome9,10,22). We didn't found statistical results between extent of tumor resection and neurological outcome, thought that total resection should be tried in low-grade tumors. Only in high-grade tumors and the tumors where total removal had not been possible, we suggested partial resection or biopsy with radiotherapies and chemotherapies. Neurosurgical advances such as improving neuroimaging, intraoperative neurophysiological monitoring, microsurgical technique, and operative instruments made it possible to achieve total resection of IMSCTs. So neurosurgeons should be having a challenging attitude to total removal of low-grade tumors.
The intraoperative neurophysiologic monitoring (IONM), combined recording of SSEPs and transcranial MEPs, has become a best material for intraopertative testing of spinal cord function in intramedullary spinal cord tumor surgery7). In many reports showed that IMSCT surgery with IONM was appeared aggressive removal of IMSCT and good state of neurologic performance16,23,31). These reports used a combined D-wave (epidural MEPs) and muscular MEPs (mMEPs). Hyun et al.14) reported that IONM combined SSEPs and mMEPs was good method to prevent irreversible pyramidal tract damage. However, Choi et al.4) reported that the groups performed operation under SSEPs and mMEPs didn't significantly associate with total excision of tumor and postoperative well neurologic outcomes. In our study, only 7 patients' operations were performed with IONM combined SSEPs and mMEPs and one patient showed decreased MEP during tumor removal. For this reason, it is hard to explain the relationship between the usage of IONM and the neurological outcome in this study. It was necessary that prospective and randomized controlled research to confirm an association between IONM(SSEPs and mMEPs) and postoperative neurologic outcomes.
The recent surgical result of patients with IMSCTs was relatively good with minimal deterioration and complication rate. The most reliable predicting factor of postoperative neurological outcome is the preoperative neurological state. Another predictive factor is the histological grades of the IMSCTs. High-grade tumors result poor neurological outcome. In addition, IMSCTs in thoracic region showed relatively bad outcome and had a risk of postoperative morbidity compared to that in other regions.
References
1. Ardeshiri A, Chen B, H├╝tter BO, Oezkan N, Wanke I, Sure U, et al. Intramedullary spinal cord astrocytomas: the influence of localization and tumor extension on resectability and functional outcome. Acta Neurochir (Wien) 2013 155:1203-1207. PMID: 23700256.


2. Barker DJ, Weller RO, Garfield JS. Epidemiology of primary tumors of the brain and spinal cord: a regional survey in southern England. J Neurol Neurosurg Psychiatry 1976 39:290-296. PMID: 932744.



3. Brotchi J, Bruneau M, Lefranc F, Baleriaux D. Surgery of intraspinal cord tumors. Clin Neurosurg 2006 53:209-216. PMID: 17380754.

4. Choi I, Hyun SJ, Kang JK, Rhim SC. Combined muscle motor and Somatosensory evoked potentials for intramedullary spinal cord tumour surgery. Yonsei Med J 2014 55(4):1063-1071. PMID: 24954338.



5. Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: Surgical morbidity and long term follow-up evaluation in 164 children and young adults. J Neurosurg 2000 93:183-193. PMID: 11012047.

6. Cooper PR. Outcome after operative treatment of intramedullary spinal cord tumors in adults: Intermediate and long-term results in 51 patients. Neurosurgery 1989 25:855-859. PMID: 2601814.


7. Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol 2008 119:248-264. PMID: 18053764.


8. Epstein FJ, Farmer JP, Freed D. Adult intramedullary astrocytomas of the spinal cord. J Neurosurg 1992 77:355-359. PMID: 1506881.


9. Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: The result of surgery in 38 patients. J Neurosurg 1993 79:204-209. PMID: 8331401.


10. Fornari M, Pluchino F, Solero CL, Giombini S, Luccarelli G, Oliveri G, et al. Microsurgical treatment of intramedullary spinal cord tumours. Acta Neurochir Suppl 1988 43:3-8. PMID: 3213654.


11. Han IH, Kuh SU, Chin DK, Kim KS, Jin BH, Cho YE. Surgical Treatment of Primary Spinal Tumors in the Conus Medullaris. J Korean Neurosurg Soc 2008 44:72-77. PMID: 19096696.



12. Helseth A, Mork SJ. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg 1989 71:842-845. PMID: 2585075.


13. Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H. Results of microsurgical treatment for intramedullary spinal cord ependymomas: Analysis of 36 cases. Neurosurgery 1999 44:264-269. PMID: 9932879.


14. Hyun SJ, Rhim SC. Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in 17 consecutive procedures. Br J Neurosurg 2009 23(4):393-400. PMID: 19637010.


15. Jallo GI, Danish S, Velasquez L, Epstein F. Intramedullary low-grade astrocytomas : Long-term outcome following radical surgery. J Neurooncol 2001 53:61-66. PMID: 11678433.


16. Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus 1998 4(5.

17. Kothbauer KF. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin 2007 37:407-414. PMID: 18083496.


18. Lambert PM. Radiation myelopathy of the thoracic spinal cord in long term survivors treated with radical radiotherapy using conventional fractionation. Cancer 1978 41:1751-1760. PMID: 417797.


19. Marcus RB Jr, Million RR. The incidence of myelitis after irradiation of the cervical spinal cord. Int J Radiat Oncol Biol Phys 1990 19:3-8. PMID: 2380091.


20. McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg 1990 72:523-532. PMID: 2319309.


21. McGirt MJ, Goldstein IM, Chaichana KL, Tobias ME, Kothbauer KF, Jallo GI. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery 2008 63(1):55-60. PMID: 18728568.


22. Minehan KJ, Shaw EG, Scheithauer BW, Davis DL, Onofrio BM. Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg 1995 83:590-595. PMID: 7674006.


23. Morota N, Deletis V, Constantini S, Kofler M, Cohen H, Epstein FJ. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery 1997 41:1327-1336. PMID: 9402584.


24. Nakamura M, Ishii K, Watanabe K, Tsuji T, Takaishi H, Matsumoto M, et al. Surgical treatment of intramedullary spinal cord tumors: prognosis and complications. Spinal Cord 2008 46:282-286. PMID: 17909556.


25. Nakamura M, Chiba K, Ishii K, Ogawa Y, Takaishi H, Matsumoto M, et al. Surgical outcomes of spinal cord astrocytomas. Spinal Cord 2006 44:740-745. PMID: 16670687.


26. Park HK, Chang JC. Review of stereotactic radiosurgery for intramedullary spinal lesion. Korean J Spine 2013 10(1):1-6. PMID: 24757449.



27. Phillips TL, Buschke F. Radiation tolerance of the thoracic spinal cord. Am J Roentgenol Radium Ther Nucl Med 1969 105:659-665.


28. Raco A, Piccirilli M, Landi A, Lenzi J, Delfini R, Cantore G. High-grade Intramedullary astrocytomas: 30 years' experience at the Neurosurgery Department of the University of Rome "Sapienza". J Neurosurg Spine 2010 12:144-153. PMID: 20121348.


29. Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery 2005 56(5):972-981. PMID: 15854245.

30. Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: The role of intraoperative (neurophysiological) monitoring. Eur Spine J 2007 16:S130-S139. PMID: 17653776.


31. Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery 2006 58:1129-1143. PMID: 16723892.


32. Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery 1994 35:865-873. PMID: 7838335.


33. Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, Egelhof T, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: Experience with 78 patients. Spinal Cord 2005 43:34-41. PMID: 15326473.


34. Schwade JG, Wara WM, Sheline GE, Sorgen S, Wilson CB. Management of primary spinal cord tumor. Int J Radiat Oncol Biol Phys 1978 4:389-393. PMID: 99397.


35. Stein BM, McCormick PC. Intramedullary neoplasms and vascular malformations. Clin Neurosurg 1992 39:361-387. PMID: 1458751.

36. Wong AP, Dahdaleh NS, Fessler RG, Melkonian SC, Lin Y, Smith ZA, et al. Risk factors and long-term survival in adult patients with primary malignant spinal cord astrocytomas. J Neurooncol 2013 115:493-503. PMID: 24158670.


37. Yang S, Yang X, Hong G. Surgical Treatment of One Hundred Seventy-Four Intramedullary Spinal Cord Tumors. Spine 2009 34:2705-2710. PMID: 19910775.


38. Woodworth GF, Chaichana KL, McGirt MJ, Sciubba DM, Jallo GI, Gokaslan Z. Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery 2007 61(1):99-105. PMID: 17621024.

39. Xu QW, Bao WM, Mao RL, Yang GY. Aggressive surgery for intramedullary tumor of cervical spinal cord. Surg Neurol 1996 46:322-328. PMID: 8876712.


Fig.┬Ā2
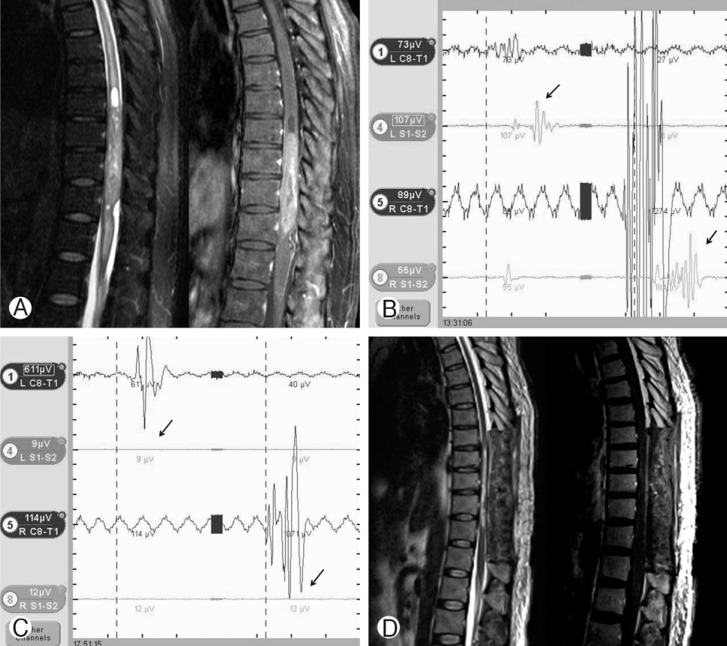
This 55 years old male patient visit hospital with paraparesis (ASIA grade C). Pre-operative spinal MRI shows intramedullary ependymoma from T9 to T12 with cystic component and syrinx and heterogeneous enhancement (A). Intra-operative neurophysiologic monitoring before tumor removal shows weak motor evoked potential (MEP) (black arrow) (B). MEP is diminished (black arrow) after tumor removal (C). Post-operative spinal MRI shows that tumor is totally removed (D). However, the patient's neurological state is aggravated to ASIA grade C to A.
Table┬Ā1.
Histological origin of IMSCTs
Table┬Ā2.
Statistic result of predictive factor for post-operative neurological outcome

- TOOLS
-
METRICS

-
- 25 Crossref
- Scopus
- 6,405 View
- 71 Download
- Related articles in NS
-
Recent Molecular and Genetic Findings in Intramedullary Spinal Cord Tumors2022 June;19(2)
Emerging Technologies in the Treatment of Adult Spinal Deformity2021 September;18(3)
Results of Surgical Treatment for Metastatic Cervical Spine Tumor.2008 June;5(2)
-
Journal Impact Factor 3.2