Surgical Outcomes of Anterior Cervical Fusion Using Deminaralized Bone Matrix as Stand-Alone Graft Material: Single Arm, Pilot Study
Article information
Abstract
Objective
To investigate the safety and efficacy of demineralized bone matrix (DBM) as a bone graft substitute for anterior cervical discectomy and fusion (ACDF) surgery.
Methods
Twenty consecutive patients treated with ACDF using stand-alone polyestheretherketone (PEEK) cages (Zero-P) with DBM(CGDBM100) were prospectively evaluated with a minimum of 6 months of follow-up. Radiologic efficacy was evaluated with a 6-point scoring method for osseous fusion using plain radiograph and computed tomogrpahy scans. Clinical efficacy was evaluated using the visual analogue scale (VAS), Owestry disability index (ODI), and short-form health questionnaire-36. The safety of the bone graft substitute was assessed with vital sign monitoring and a survey measuring complications at each follow-up visit.
Results
There were significant improvements in VAS and ODI scores at a mean 6-month follow-up. Six months after surgery, solid fusion was achieved in all patients. Mean score on the 6-point scoring system was 5.1, and bony formation was found to score at least 4 points in all patients. There was no case with implant-related complications such as cage failure or migration, and no complications associated with the use of CGDBM100.
Conclusion
ACDF using CGDBM100 demonstrated good clinical and radiologic outcomes. The fusion rate was comparable with the published results of traditional ACDF. Therefore, the results of this study suggest that the use of a PEEK cage packed with DBM for ACDF is a safe and effective alternative to the gold standard of autologous iliac bone graft.
INTRODUCTION
Historically, autologous bone harvested from a patient's iliac crest has been used as a cervical interbody fusion material1942). Although high fusion rates have been reported with autogenous bone grafts, frequently reported donor-site morbidity associated with iliac crest harvesting, such as chronic donor site pain, infection, wound hematoma, and numbness due to nerve injury, affects patient satisfaction39). In addition, the quality of the autologous graft material can be a concern in patients with osteoporosis, metabolic disorders, neoplasia, chronic inflammatory diseases, or infections, and, therefore, surgeons have attempted to identify substitute bone graft materials54).
Demineralized bone matrix (DBM) is made of bone that has been acid-treated to remove the mineralized portion while maintaining the organic matrix and various growth factors46). DBM consists of collagen and other growth factors, such as bone morphogenic protein (BMP), which provides osteoinductivity. Unlike allograft bone, DBM produces no immunological rejection as the surface antigenic structures are destroyed during demineralization. However, the osteogenic capacity of the bone is also lost during processing50). Previously published studies have supported the use of DBM as a potential alternative option for bone grafting133251), but there is no clinical evidence to support its usage as a stand-alone graft material.
The purpose of this study was to investigate the safety and efficacy of a DBM alone as a bone graft substitute for cervical interbody fusion surgery.
MATERIALS AND METHODS
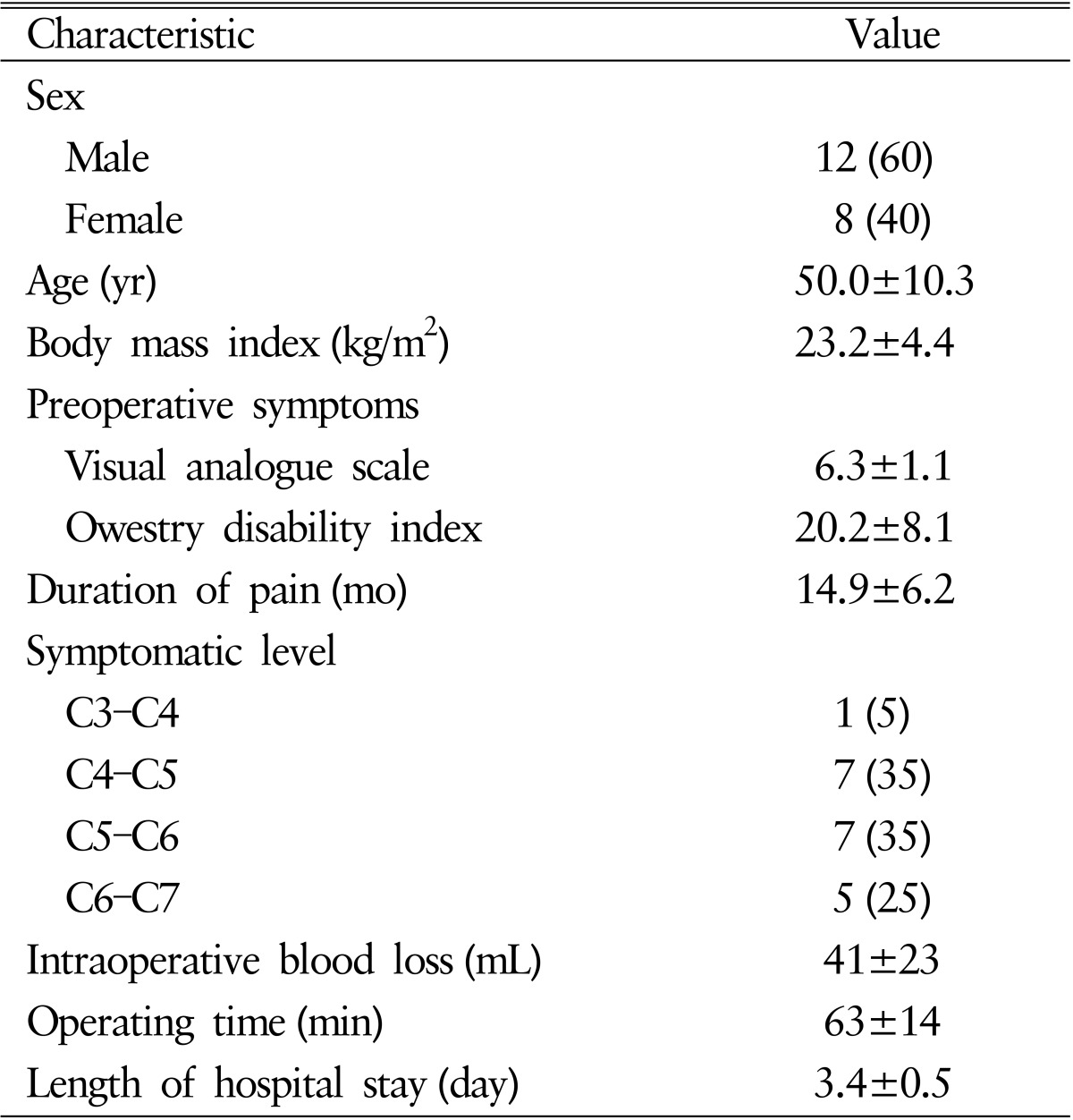
This pilot study was conducted from September 2012 to November 2013 in Seoul St. Mary's Hospital, The Catholic University of Korea. Twenty consecutive patients treated with ACDF using stand-alone polyestheretherketone (PEEK) cages (Zero-P, Depuy-Synthes Spine Inc., Raynham, MA, USA) with DBM(CGDBM100, CGBio Inc., Seongnam, Korea) at the single level were prospectively evaluated with a minimum of 6 months of follow-up (mean, 12.2 months). All patients included in the study were between 20 and 70 years of age, had single-level cervical degenerative disease, and were scheduled for ACDF surgery. Patients were excluded if acute fracture, infection, neoplastic disease, or systemic diseases such as Alzheimer disease or cerebrovascular stroke which can affect surgical outcomes were identified during preoperative evaluation. We received informed consent form all participants before surgery and permission for this study. The patients ranged in age from 29 to 68 years, with a mean age of 50 years, and the male-to-female ratio was 3:2. The affected level was C3-4 in 1 patient, C4-5 in 7, C5-6 in 7, and C6-7 in 5. Patients' demographic data are shown in Table 1.
The surgical procedure was identical in all patients. A standard anterior cervical discectomy was performed using a right-sided approach by a single surgeon. After the completion of discectomy and decompression of neural structures under a microscope, end-plate preparation was done with curettage. An appropriately sized trial implant was then placed into the disk space to confirm the size, position, and height of the implant to be used. In patients with foraminal narrowing, the posterior half of the uncovertebral joint was removed with a high-speed burr under the microscope. A Zero-P cage was filled with CGDBM100 in putty form and inserted into the disc space under fluoroscopic guidance (Fig. 1). The height of the cage (5, 6, or 7mm) was determined after considering the stability of overdistraction. No additional bone graft was inserted anterior or lateral to the cage. After removing the Caspar screw, screws in the stand-alone cage were inserted into the vertebral body in an oblique upward and downward fashion. All patients were reviewed at 1, 3, and 6 months postoperatively in order to evaluate the efficacy and safety of the procedure.
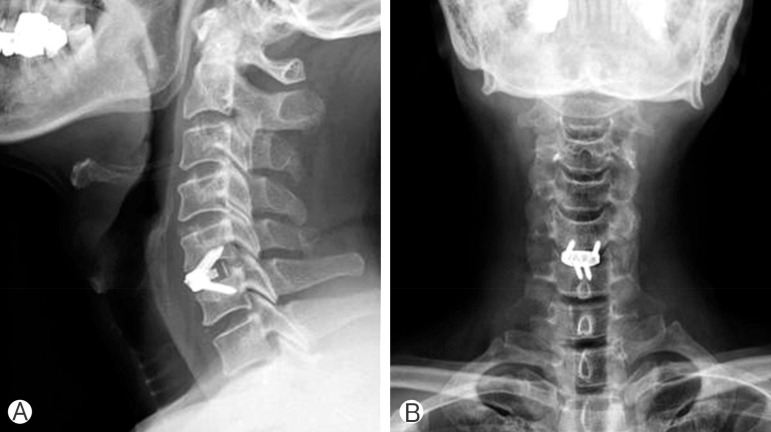
Zero-P cage (Depuy-Synthes Spine Inc., Raynham, MA, USA) was ipacted with putty foam of CGDMB100 and inserted into the disc space under fluoroscopic guidance. (A) Lateral view of postoperative plain radiograph. (B) Anteropsterior view of postoperative plain radiograph.
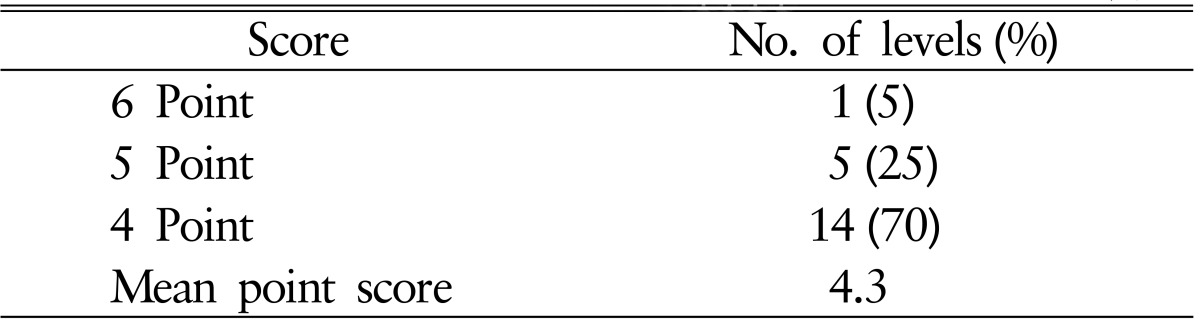
Radiologic efficacy was evaluated with a 6-point scoring method for osseous fusion at 1, 3, and 6 months postoperatively using plain radiograph and computed tomography (CT) scans26) (Fig. 2). We considered a bridging bone between the cage and the adjacent endplate of the vertebral body on each of six surfaces (anterior, posterior, superior, inferior, and both lateral sides) as one indicator point. Bridging bone formation on more than three surfaces was considered to be solid fusion with stability. Formation of bridging bone was examined on 6 surfaces around the graft using axial, coronal, and sagittal CT images. Additionally, stability on dynamic X-ray (motion between the adjacent spinous process <2mm) was assessed for fusion status. The intervertebral disk space height (DSH) was calculated as the mean value of the anterior and posterior intervertebral disk heights as measured on plain lateral radiography. The measurements were performed by a single independent observer who was not involved in the surgery or care of the patients.
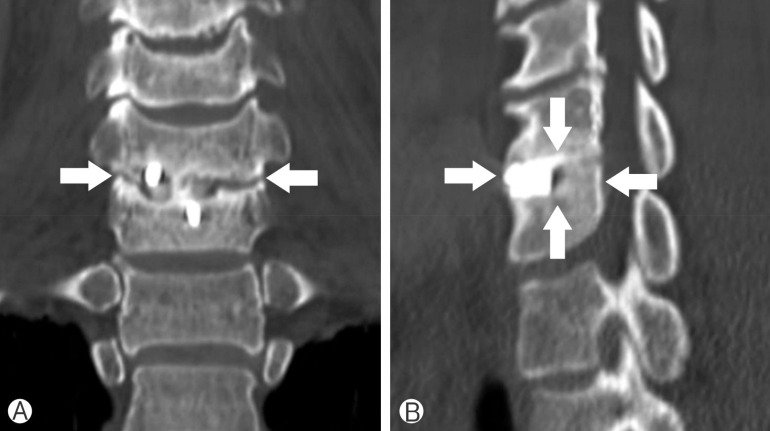
Evaluation of bone fusion by "6-point scoring" system. Imaging studies of a patient with C6-7 ACDF with Zero-P stand-alone cage with CGDBM100 (solid fusion was achieved with 4-point by the system). Bone fusion check points (white arrow) represent bridging bone of 6 surfaces (anterior, posterior, superior, inferior and both lateral sides) around the graft. (A) Coronal reconstruction image from postoperative computed tomography (CT) scans representing bridging bone of both lateral surfaces. (B) Sagittal reconstruction images form the postoperative CT scans representing bridging bone of anterior, posterior, superior, and inferior surfaces.
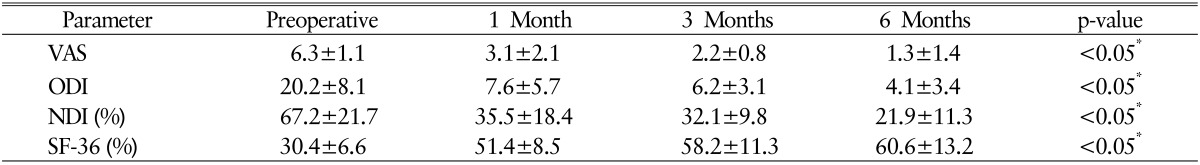
Clinical efficacy was evaluated using visual analogue scale (VAS) score, Owestry disability index (ODI), neck disability index, and short-form health questionnaire-36 (SF-36) at 1, 3, and 6-month follow-up visits. The safety of the bone graft substitute was assessed with vital sign monitoring and a survey regarding complications at each follow-up visit.
Data were analyzed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA); the paired Student t-test was used for the analyses. Data are presented as the mean with standard deviation. For all analyses, p<0.05 was considered statistically significant.
This study was approved by a Catholic Medical College Cinical Research Coordinating Center (CMC CRCC) (approval number: KIRB-00355_31-002)
RESULTS
The intraoperative data are shown in Table 1. The mean duration of the operation was 63±14 minutes and mean intraoperative blood loss was 41±23mL. There were no surgeryrelated complications such as hoarseness, dysphagia, or hematoma in any patients.
There were significant improvements in VAS and ODI scores after a mean 6 months of follow-up. The mean VAS score decreased from 6.3±1.1 to 1.3±1.4 and the mean ODI score decreased from 20.2±8.2 to 4.1±3.4 (p<0.05 preoperative vs. final follow-up). The mean neck disability index score decreased significantly at the first 3 months after surgery and remained steady until 6 months postoperatively. Quality of life was notably improved, as the mean SF-36 score went from 30.4% before surgery to 60.6% at the last follow-up. A summary of the clinical data is shown in Table 2. Neurologic deterioration related to the fusion segment was not observed in any patients.
In all patients, stability of the graft was confirmed by plain radiograph and CT scan at 1, 3, and 6 months after surgery. At 6 months after the surgery, solid fusion was achieved in all patients as evidenced by formation of bridging bone on the surface of the graft on CT scans. The mean score in the 6-point scoring system was 4.3 and bony formation achieved at least 4 point in all patients. Six of 20 fusion levels (30%) showed 5- to 6-point fusion (Table 3). In addition, no mobility was observed on the dynamic radiograph in any operated segment. When the clinical outcome parameters were compared between the point groups, there were no significant statistical differences.
Intervertebral DSH was significantly improved after surgery and well maintained over the next 6 months. The mean preoperative DSH was 3.5±1.2mm. The mean DSH at 1 month after surgery was 7.2±0.9mm; at the final follow-up it was 6.9±1.8mm(p<0.05 before surgery vs. after surgery, p<0.05 before surgery vs. final follow-up).
There was no case of implant-related complications such as dysphagia, cage failure, or migration, and there were no compli cations associated with the use of CGDBM100 during the follow-up period.
DISCUSSION
ACDF surgery is a well-established gold standard treatment for cervical degenerative disease. Solid bony fusion is essential for positive outcomes following ACDF surgery as it prevents foraminal stenosis and late angulation deformity2240). However, the choice of optimal fusion material is still controversial; various fusion materials have been claimed to promote superior outcomes27). In this study, we attempted to analyze the clinical and radiologic efficacy of DBM as a stand-alone fusion material in single-level ACDF surgery.
Although use of an autograft harvested from the iliac crest as an interbody fusion material provides satisfactory clinical results and fusion rates, the rate of donor-site morbidity has been reported to be as high as 20% to 30% and can often reduce patient satisfaction and quality of life 911142228). Various materials have been proposed as interbody grafts for ACDF surgery to avoid the problems associated with autologous bone grafts12204345). The characteristics of an ideal graft material include immediate structural biomechanical stability and the capacity for subsequent osteogenesis 152835). Titanium, carbon fiber, and PEEK are the most commonly used materials for cervical interbody cages. A titanium cage may lead to vertebral body collapse if the end plate is damaged during discectomy and has been associated with high degree of subsidence2937). Moreover, radiological metallic artifacts may complicate postoperative radiologic imaging. Transparent carbon fiber cages have been used widely, but a high rate of pseudoarthrosis, unexpected local connective tissue formation, and a risk of systemic uptake have frequently been reported 430).
In most prior studies on allograft fusion materials, a cage packed with allogeneic cancellous bone chips was used to avoid donor-site complications1741). We used DBM as a stand-alone graft material and packed the PEEK cage for maximal contact with a prepared endplate on either side of the cage. DBM has been demonstrated to have both osteoinductive and osteoconductive properties21). The principal components of DBM are BMPs, which are responsible for its osteoinductive activity, and the organic portions of bone, such as collagen, provide osteoconductive activity46). Recent studies advocate the use of DBM as a potential graft substitute or enhancer, but there was no prior clinical evidence to support its use as a stand-alone graft material. Moreover, DMB must be used in combination with other types of grafts because of its amorphous consistency; many spine surgeons prefer structural graft materials46).
There have been a few clinical trials on DBM as a fusion material in ACDF surgery 132332334749). One of the first reports, a 2-center prospective randomized controlled clinical trial comparing allograft mixed with DBM and iliac crest autograft, showed no significant difference in the rate of pseudoarthosis47). However, graft collapse was significantly more likely in the allograft-DBM group and the authors suggested the use of an autograft for better outcomes. In another level 3 study comparing the use of PEEK cages packed with morphogenic protein-2 (rhBMP-2) against allograft spacers with DBM, there was no significant difference in clinical outcomes or fusion rates between the two groups32). The DBM group demonstrated a significantly lower rate of postoperative swallowing difficulty, and the cost of implants was more than three times greater in the rhBMP-2 group. Those authors advocated the use of DBM over rhBMP-2 for anterior cervical fusion. Another four studies investigated the use of PEEK cages and DBM (Grafton, Medtronic Sofamor Danek USA, Inc., Memphis, MN, USA) in patients undergoing ACDF surgery13233349). The authors advocated the use of PEEK cages packed with DBM as interbody fusion materials for the treatment of degenerative cervical diseases, as satisfactory fusion rates and clinical results were achieved in long-term follow-ups.
In the present study, the fusion rate of ACDF surgery using DBM alone as a fusion material was comparable with that of published results of ACDF using an autologous bone graft. The PEEK cage provides immediate structural support and its hollow center allows a graft-host interface that facilitates adequate bone fusion28). We experienced excellent short-term clinical outcomes and high patient satisfaction with the elimination of donor-site morbidity and anterior plating. No cage- or graft material-related complications were encountered, and DSH was well-preserved during the follow-up period. Therefore, the results of present study suggest that use of a stand-alone cage packed with DBM in ACDF surgery is a safe and effective alter native to conventional autologous iliac bone grafts.
In the present study, stand-alone PEEK cages were used in all operations. PEEK is a semicrystalline polyaromatic linear polymer that provides a good combination of strength, stiffness, toughness, and environmental resistance with biocompatible, nonabsorbable, and corrosion-resistant abilities13255256). Furthermore, the cage structure, which consists of (1) 2 titanium spikes on the upper and lower frames anchoring the vertebral body, providing immediate solid fixation, and (2) 4 holes with screw treads for screw fixation, 2 inferior medial ones and 2 lateral ones, giving passages for cranial screws, offers a fixation mechanism similar to that of an anterior plate and screw system13). In addition, the PEEK cage is radiolucent and does not produce an imaging artifact, which enables convenient evaluation of fusion status1013).
Cervical interbody cages have been developed to provide immediate stability and high fusion rates with and without supplemental fixation. Augmentation with plate fixation may seem preferable owing to higher fusion and lower reoperation rates and better pain relief8172244). In spite of these benefits, anterior plating is associated with a morbidity rate of 2.2% to 24.0% according to previously published literature2436). Complications include screw pullout, screw breakage, injury to neurovascular structures, injury to the esophagus, prolonged dysphagia, and wound infection616384855). Additionally, the operative time is usually longer because of the need for additional retraction to apply the anterior plate and the asymmetry of the anterior cervical surface, which is related to the presence of osteophyte secondary to degenerative changes48). In contrast to plate fixation, stand-alone cages are recessed below the margin of the anterior verterbral body, providing no-profile internal fixation, which avoids such complications2). Moreover, stand-alone cages possess the advantage of a reduced risk of adjacent level degeneration and spondylotic changes313453).
Many studies on ACDF using stand-alone cages have demonstrated a high rate of cage subsidence resulting in sagittal imbalance and segmental height loss3518). However, using a zeroprofile PEEK cage with additional screw augmentation, we did not observe any cage relate-complications. Moreover, our study showed that the DSH of the index level was significantly improved postoperatively and well-preserved during the followup period.
This study has several limitations, including a small number of study subjects, nonrandomized case selection, and a relatively short follow-up duration. Although well-designed randomized controlled studies with comparison groups are required for confirmation, our results suggest that stand-alone PEEK cages packed with DBM are a promising alternative fusion material for patients undergoing ACDF surgery.
CONCLUSION
ACDF using DBM as a stand-alone graft material demonstrated good clinical and radiologic outcomes at a minimum 6-month follow-up. The fusion rate was comparable to that of published results on traditional ACDF surgery using tricortical iliac crest grafts. With the use of the stand-alone cage and DBM, donor-site morbidity is eliminated, resulting in reduced postoperative pain. Therefore, the results of this study suggest that the use of DBM alone in ACDF surgery is a safe and effec tive alternative to the gold standard of autologous iliac bone graft.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.