Clinical Outcomes and Prognostic Factors in Patients With Myelopathy Caused by Thoracic Ossification of the Ligamentum Flavum
Article information
Abstract
Objective
The objective of this study was to investigate the surgical outcomes and prognostic factors for thoracic ossification of the ligamentum flavum (OLF) after decompressive laminectomy, focusing on the quantitative signal intensity ratio (SIR) of preoperative magnetic resonance imaging (MRI) and its prognostic significance.
Methods
We retrospectively reviewed 24 patients who previously underwent total laminectomy to remove OLF from 2010 to 2015. MRI and computed tomography were performed to detect OLF. The SIR between the regions of interest of high signal intensity lesions and the normal cord at the T1–2 disc levels was calculated. We divided patients into 2 groups based on the extent of the modified Japanese Orthopaedic Association (JOA) recovery rate (RR): good (RR ≥ 50%) and poor (RR < 50%).
Results
The mean preoperative and postoperative modified JOA scores for thoracic myelopathy were 6.67 ± 1.73 and 8.63 ± 1.81, respectively (p < 0.001). The preoperative JOA score (7.5 vs. 5.83, p = 0.028), postoperative JOA score (9.83 vs. 7.42, p = 0.000), and SIR (1.16 vs. 1.41, p = 0.009) were significantly different between the good and poor RR groups. A higher preoperative JOA score and lower SIR were associated with a good RR according to the JOA criteria.
Conclusion
The clinical outcomes for thoracic OLF after decompressive laminectomy were favorable. A higher RR was correlated with a lower SIR and higher preoperative modified JOA score. Therefore, a relatively low SIR on MRI and a relatively high preoperative JOA score could be positive prognostic indicators for the JOA RR in patients with thoracic OLF.
INTRODUCTION
Ossification of the ligamentum flavum (OLF) is a rare disease that is characterized by replacement of the ligamentum flavum with mature and hypertrophic lamina bone [1]. OLF is increasingly recognized as a major cause of acquired thoracic spinal canal stenosis, which frequently results in thoracic myelopathy [2,3].
The prevalence of OLF has been reported to vary from 3.8% to 26% in East Asia, particularly in countries such as China, Japan, and Korea [4-6]. Recently in Korea, the prevalence of thoracic OLF based on magnetic resonance imaging (MRI) has been reported to be 16.9% [7]. OLF frequently affects the lower thoracic spine, T9–12, and often leads to slowly progressive thoracic radiculomyelopathy [8]. Patients eventually visit clinics when their myelopathic symptoms become aggravated owing to severe spinal cord compression. Decompressive surgery is considered the best treatment option for patients with OLF with radiculomyelopathy [9,10]. Patients with thoracic OLF have various symptoms, such as sensory abnormality of the trunk or lower extremities, gait disturbance, and urinary dysfunction [8]. Although decompressive surgery is an available treatment option for this disease, the surgical outcome is not always satisfactory [3,11]. Furthermore, prognostic factors are still controversial and it is very difficult for the surgeon to predict postoperative recovery.
Signal intensity (SI) changes of the spinal cord on MRI reflect pathological changes in the spinal cord and are indicative of prognosis in cervical compressive myelopathy [12]. Many researchers have reported a correlation between clinical outcomes and SI changes in MRI [13,14]. Furthermore, a previous study evaluated the correlation between surgical outcomes and signal changes on MRI by measuring the signal intensity ratio (SIR) of the spinal cord and comparing the signal change at the lesion and the normal area [15]. Therefore, in this study, we evaluated clinical outcomes for the thoracic OLF after decompressive laminectomy and focused on the quantitative SIR of preoperative MRI and their prognostic significance.
MATERIALS AND METHODS
1. Patients and Surgical Procedure
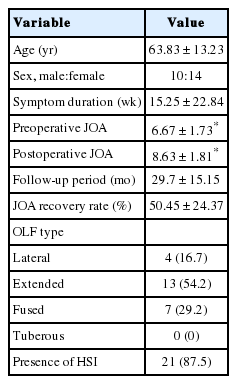
Approval for the current study was granted by the Institutional Review Board of Chonnam National University Hospital (approval number: 2016-127). Informed consents were waived by our IRB because of retrospective study. All patients were diagnosed using thoracic MRI and computed tomography (CT) scans to detect thoracic OLF. A total of 49 patients (21 men and 28 women), who underwent decompressive total laminectomy and OLF removal at index level from January 2010 to December 2015, were included initially in this study; however, we excluded 14 patients who had simultaneous cervical or lumbar decompressive surgery owing to coexisting cord compression or thecal sac compression at another spinal segment. Of the remaining of 35 patients, we excluded 11 patients because of previous spinal surgery, experience of trauma, and spinal tumor. Therefore, a total of 24 consecutive patients with pure thoracic OLF were enrolled in this study. Distributions and types of thoracic OLF, the type of initial symptom, and preoperative symptom duration were evaluated.
2. Radiologic Assessment: SIR
All patients underwent high-resolution MRI using the MAGENTOM Trio 3.0T (Siemens, Erlangen, Germany) or Discovery MR750 3.0T (GE, Little Chalfont, England), T2-weighted sagittal and axial imaging of the thoracic spine. The SI values of the spinal cord on the sagittal view of T2-weighted image (T2WI) were obtained, and the regions of interest (ROI) at high SI lesion by OLF were taken as 4.98 mm2. The normal cord SI, as reference SI, at the T1–2 disc levels was obtained and the ROIs were taken as 4.98 mm2. The SIR between ROIs of high SI lesion and normal cord at the T1–2 disc levels was calculated (Fig. 1). If no high SI change was noted on T2WI, the ROIs were taken as 4.98 mm2 in the area of most severe compression of the cord. The SIR on T2WI was calculated by the following equation [16]:
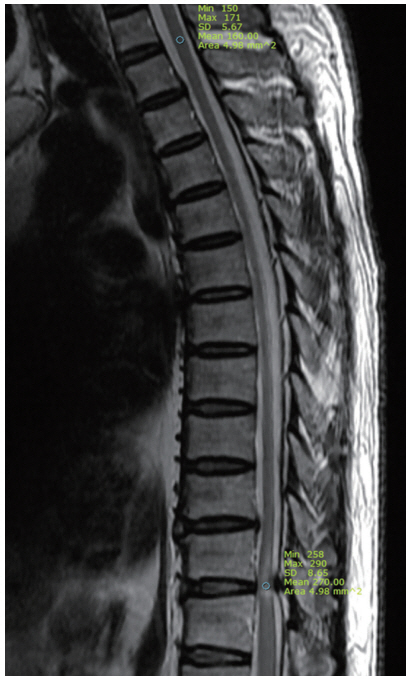
Radiologic measurement of signal intensity ratio on T2-wighted sagittal image of thoracic magnetic resonance imaging. Mean regions of interests were measured as 4.98 mm2 at the spinal cord of T1–2 disc level (upper circle) and intramedullary high signal change area (lower circle) of pathologic level.
SIR=(ROI of high SI lesion [4.98 mm2])/(reference ROI of the sagittal normal cord between T1–2 disc levels [4.98 mm2])
The types of the axial configuration of thoracic OLF by lateral, extended, and fused type were also evaluated [7,17].
3. Clinical Outcome Assessment
The modified Japanese Orthopaedic Association (JOA) scoring system was used to evaluate the neurological status of patients before and after surgical decompression, which excluded the sections regarding upper extremity function [10]. The maximum score of 11 indicates normal function, total score ≤3 was considered severe neurological impairment, 4–6 was moderate, and ≥7 was mild [18]. Postoperative improvement of symptoms was estimated on the basis of the recovery rate (RR)=(postoperative JOA score−preoperative JOA score)/(11−preoperative JOA score)×100%. A score of 75 to 100% was designated as excellent, 50% to 74% as good, 25% to 49% as fair and 0% to 24% as poor [18-20]. Therefore, the extent of recovery was defined by RR, with good (RR≥50%), poor (RR<50%).
4. Statistical Analysis
Since the number of subjects were less than 30, we used the nonparametric analysis methods. Wilcoxon signed rank test was used to figure out the significant pairwise change between preoperative and postoperative JOA scores. Mann-Whitney Utest and Fisher exact test were used to compare the difference between good and poor RR groups. Spearman correlation analysis was used to examine the relationship between age, symptom duration, pre- and postoperative JOA scores, SIR on T2WI, and JOA RR. We performed the multiple linear regression analysis using the input method.
RESULTS
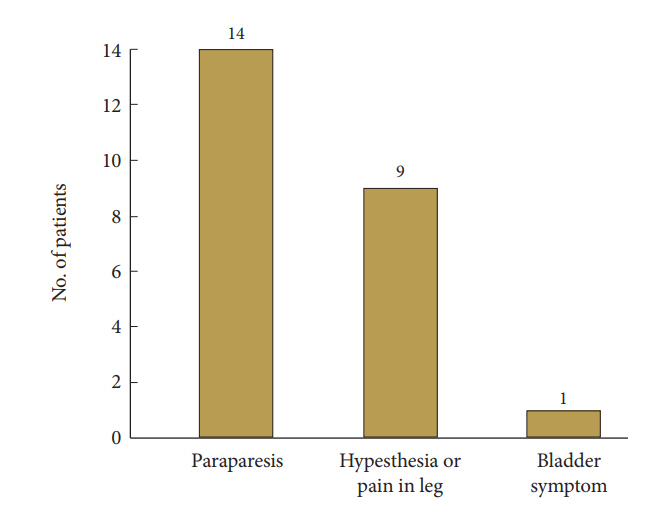
A summary of patients’ demographics, clinical characteristics, and neurologic outcome score are shown in Table 1. All 24 participants (10 men and 14 women; mean age, 63.83 years; age range, 34–83 years) underwent 1- or 2-level total laminectomy and OLF removal. The duration of preoperative symptoms ranged from 1 to 84 weeks (mean, 15.25±22.84 weeks). After the surgical operation, the mean follow-up period was 29.7±15.15 months (range, 12–56 months). The overall mean preoperative and postoperative modified JOA scores for thoracic myelopathy were 6.67±1.73 and 8.63±1.81, respectively (p<0.001) in Fig. 2. Neurological outcomes for all patients with thoracic OLF improved to a mean of 50.45%±24.37%, based on the JOA RR. The initial symptoms were paraparesis (14 patients), hypesthesia or leg pain (9 patients), and bladder dysfunction (1 patient) in Fig. 3. There were 35 affected levels of OLF in total, and the primarily involved segments were T11–12 (n=11, 31.4%), T9–10 (n=10, 28.5%), and T10–11 (n=8, 22.8%) in Fig. 4. Of 24 patients assessed, 8 (33.3%) patients had multilevel OLF.

The preoperative and postoperative modified Japanese Orthopaedics Association (JOA) score in total patients with thoracic ossification of the ligamentum flavum after decompressive laminectomy.
1. Comparison of Clinical and Radiological Data Between “Good” and “Poor” Groups Based on JOA RR
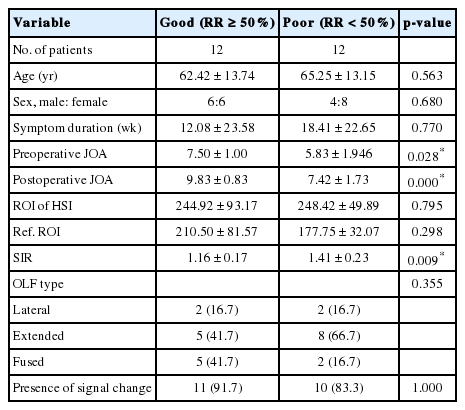
As above mentioned in the methods part, we divided patients into 2 groups based on the extent of JOA RR: good (RR ≥50%) and poor (RR<50%). Between good and poor groups, the preoperative JOA score (7.5 vs. 5.83, p=0.028), postoperative JOA score (9.83 vs. 7.42, p=0.000), and SIR (1.16 vs. 1.41, p=0.009) had significant differences (Table 2). There were no significant differences between groups in age (p=0.563), sex (p=0.680), symptom duration (p=0.770), ROI of high SI (p=0.795), and reference of ROI (p=0.298).
2. Relationship Among Variables Affecting JOA RRs
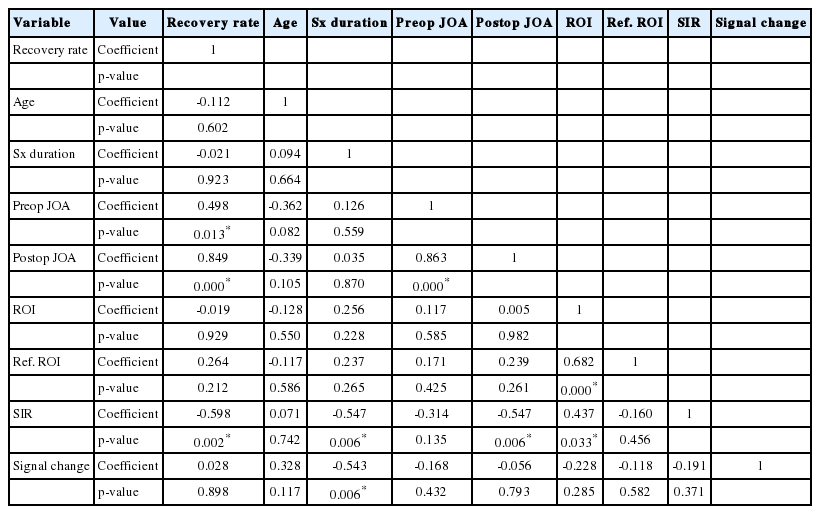
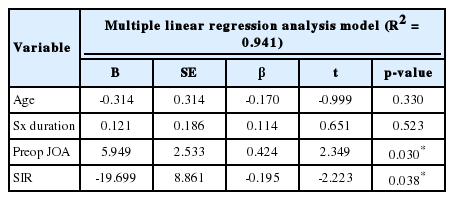
Table 3 shows the correlation among the independent variables, which included age, symptom duration, pre- and postoperative JOA score, SIR, ROI, reference ROI, and JOA RR. We found significant correlations between pre- and postoperative JOA score, SIR, and JOA RR. Furthermore, multiple linear regression analysis using input method showed that preoperative JOA, and SIR were the significant contributors for predicting the JOA RR (Table 4). Higher preoperative JOA score (B=5.949, β=0.424, p=0.03) and lower SIR (B=-9.699, β=-0.195, p=0.038) were affecting factors of increased JOA RR.
DISCUSSION
OLF is generally observed in the lower thoracic spine and frequently results in thoracic myelopathy. The lower thoracic region (T9–12) was involved in 82.7% of patients in this study. Surgical intervention is generally necessary for patients with symptomatic thoracic OLF. Decompressive surgery is recommended and should be performed if paralysis has developed. Posterior decompressive laminectomy and removal of the OLF is commonly supported because thoracic OLF compresses the dorsal spinal cord, and the surgical procedure provides wide decompression of the spinal canal [3]. In the present study, the JOA scores of all patients with thoracic OLF after decompressive resection improved from 6.67±1.73 to 8.63±1.81 and the mean JOA RR improved to 50.45%±24.37%. Therefore, posterior decompressive laminectomy provided satisfactory neurological improvement in patients with thoracic OLF, similar to other studies [3,8].
There are various clinical and radiological factors such as age, gender, duration of symptoms, preoperative JOA score, SI changes, and OLF type in predicting the postsurgical prognosis of patients with thoracic OLF [1,3,8,10,13,20]. However, it is also uncertain whether these factors are predictive of surgical outcome, as our findings are inconsistent with the results of previous studies.
OLF located in multiple segments and tandem ossification is common [7]. In our series, 33.3% of total patients have also multiple segments of OLF. Some reports have mentioned that their surgical outcomes were not always satisfactory after laminectomy if tandem ossification exists [7,20]. Therefore, we exclude the tandem ossification that might have any impact on recovery. No significant difference was observed in the correlation between other factors (age, gender, and duration of symptoms), similar to the other results [1,20]. However, a shorter duration of preoperative symptoms, a thoracic canal less affected by stenosis and the absence of proximal stenosis are correlated with a better postoperative neurological recovery [13,21]. In this study, duration of symptoms did not affect the JOA RR. This might be due to the insidious onset of clinical symptoms and hypesthesia in leg which might have confused the patient’s recognition and progression of symptoms associated with thoracic OLF [8].
Preoperative severity of myelopathy was considered as the most important predictor of the postoperative outcome [11,20]. In our results, the preoperative JOA scores correlated with RR in the correlation analysis. Multiple linear analysis of RR revealed that higher preoperative JOA score correlated with increased overall RR. These results are in agreement with those of previous studies evaluating the prognostic factors of thoracic OLF [11,20,21].
The SI changes on T2WI indicates local pathologic changes in the spinal cord, and patients with thoracic OLF and high-SI on T2WI usually have a poor prognosis even after surgical intervention [13,14,22]. The SI changes on T2WI were presumed to be myelomalacia or cord gliosis secondary to a long-standing compressive effect of the spinal cord [22]. However, the presence and grading of the SI change did not always correlate with prognosis [8,23]. Only SI change on MRI as a poor prognostic factor is controversial. In this study, we attempted to use disinterested quantitative data to investigate the relationship between signal intensities, clinical manifestations, and prognosis. With recent developments and advances in MRI techniques and software, we can quantify various signal intensities of the spinal cord using ROIs in these patients. Zhang et al. [18] reported that preoperative SIR on T2WI of MRI could be potentially useful for the prediction of surgical outcomes in patients with thoracic OLF. Results of the present study corresponded with the results of an earlier study, which reported low SIR as a prognostic factor correlated with high JOA RR. Furthermore, 2 previous studies reported that patients with preoperative SIR greater than or equal to 1.54 or 1.55 are likely to experience poor postoperative recovery [12,18]. In accordance with previous studies [12,18], mean SIR (1.41±0.23) was higher in the poor RR group than (1.16±0.17) in the good RR group (p=0.009) (Table 2). The SIR value in the poor RR group differs because the area of the ROIs and the levels at which they are measured are different in this study than in previous studies. It is difficult to constantly obtain 3.00 mm2 of ROIs, measured in previous studies, in picture archiving and communication system. Therefore, it is possible to increase the reliability of SIR by uniformly measuring 4.98 mm2 of ROIs, which is relatively easy to measure in the thoracic spinal cord. Thus, the normal cord SI as reference SI at the disc level of T1–2 was obtained, and the ROIs were taken as 4.98 mm2. The SIR between ROIs of high SI lesion and normal cord at the T1–2 disc level was calculated.
OLF morphology might affect recovery or preoperative neurological status. Kuh et al. [24] introduced the beak and round type classification system and considered intramedullary cord signal change with a beak type lesion to be a poor prognostic factor in 2 patients. However, Kang et al. [4] observed that localized compression of the spinal cord could be better corrected than compression of a round type OLF. In our study, we did not find any statistical correlation between RR and OLF type in accordance with previous studies [8,17,25].
This study has some limitations. This study is retrospective and nonrandomized. Additionally, the number of patients in this study were relatively small. Thoracic OLF is often associated with coexisting spinal disorders, such as ossification of posterior longitudinal ligament, disc herniation, canal stenosis or OLF at other spinal levels. Hence, patients with these coexisting conditions were excluded because they were reported to make the surgical decision more complicated and the surgical outcome more unpredictable. Further investigation on prognostic factors utilizing a larger sample size will be performed in the future.
CONCLUSION
Clinical outcomes for thoracic OLF after decompressive laminectomy is favorable. The increase in RR was correlated with lower SIR and higher preoperative modified JOA score. Therefore, lower SIR on MRI and higher preoperative JOA score could be a good predictable factor of increasing JOA recovery rate in thoracic OLF.