Retrospective Outcome Evaluation of Cervical Nucleoplasty Using Digital Infrared Thermographic Imaging
Article information
Abstract
Objective
Percutaneous cervical nucleoplasty (PCN) is used to treat cervical disc herniation. Radiological imaging studies, including plain radiography, computed tomography (CT), and magnetic resonance imaging (MRI), have been used to make early predictions of cervical spinal surgery outcomes. However, simple radiological studies do not provide sufficiently detailed information; moreover, CT and MRI are highly expensive. Herein, we aimed to elucidate the usefulness of digital infrared thermography imaging (DITI) as an outcome marker after cervical nucleoplasty by correlating the changes in thermal difference (ΔTD) with the changes in pain intensity after PCN expressed as visual analogue scale (ΔVAS) scores.
Methods
For this study, 255 patients treated with PCN at Thomas Hospital between March 2012 and August 2014 were included. For each patient, demographic and clinical data, including preoperative MRI results, ΔVAS, ΔTD at the disc level treated with PCN, subjective symptom improvement, procedure-related discomfort, overall satisfaction, and adverse effects, were collected and evaluated for up to 3 months retrospectively.
Results
Thermal difference (TD) and VAS scores improved after PCN (p<0.05), but ΔTD showed no significant correlation with ΔVAS. If the preoperative TD was larger, the postoperative VAS was worse and there was less pain relief (ΔVAS) after PCN (p<0.05). Only few adverse effects were noticeable after PCN.
Conclusion
In DITI, which was used to evaluate the outcomes after cervical nucleoplasty, the ΔTD did not seem to reflect the ΔVAS after PCN. However, preoperative DITI findings could be useful for predicting VAS reduction and clinical improvements after PCN.
INTRODUCTION
Cervical disc herniation (CDH) causes neck pain, radicular pain, and warm and cold sensations in the involved dermatomal area owing to compression of the nerve root by the herniated cervical disc. Percutaneous cervical nucleoplasty (PCN), which is now widely used to treat CDH, has been proven effective and safe in the treatment of contained CDH [1]. In addition, in a previously reported case, the patient’s symptoms began to improve within 1 day after PCN [2].
The evaluation of the clinical outcomes of PCN is not straightforward [3]. We generally rely on postoperative imaging studies such as computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate spinal surgical outcomes. However, they often require a long period to detect morphological changes and create a large financial burden [4]. Moreover, sometimes they do not match the PCN results and the patient’s subjective symptomatic improvement [3]. Therefore, there is an increasing need for a cheap, quick, reliable, and objective imaging tool to evaluate the outcomes of PCN. The ability to evaluate subjective symptomatic improvement after PCN objectively and earlier in the postoperative period will greatly help further management.
Digital infrared thermographic imaging (DITI) is a method that detects abnormalities by measuring skin temperature [5,6]. DITI has been widely used to complement the diagnostic process because it is noninvasive, user-friendly, and effective for visualizing subjective pain [5]. DITI revealed the painful arm in CDH as a hypothermic or hyperthermic change with a radiating pattern and was reportedly useful for diagnosing CDH [6]. Furthermore, DITI has proven usefulness in the outcome evaluation of cervical chemonucleolysis [4].
The purpose of this study was to evaluate and validate DITI as an outcome measure by correlating the change in thermal difference (ΔTD) with the change in pain intensity expressed as visual analogue scale (ΔVAS) scores and patient satisfaction after PCN.
MATERIALS AND METHODS
1. Study Population
This retrospective study was approved by the Korean National Institute for Bioethics Policy (KoNIBP, P01-201506-21-011) and registered with the Clinical Research Information Service (CRiS, KCT0001548). After written informed consent was obtained, the medical records and imaging studies of 256 patients who underwent elective PCN in Thomas Hospital between March 2012 and August 2014 were reviewed. Patients aged 20–70 years with an American Society of Anesthesiologists (ASA) physical status classification grade of ≤III were included. The exclusion criteria were refusal to participate, absence of MRI or DITI studies, psychotic disorders and history of cervical spine, neck and upper extremity surgery.
2. Procedures
The target discs for PCN were selected on the basis of the patient’s symptoms, physical examination, simple radiological studies, and MRI findings. Routine monitoring were applied and oxygen was administered throughout the procedures. No premedication was administered. After aseptic preparation of the skin, all surgical procedures were performed by 1 surgeon (DHK) according to the standardized protocol under local anesthesia with lidocaine [3]. No opioids were administered during the hospital stay.
3. Evaluation
The classification of disc pathology in this study was based on the preoperative MRI findings read by an independent board-certified radiologist (SSJ) as follows: bulging, protrusion (central, subarticular [paramedian], foraminal, lateral, and anterior), extrusion, (central, subarticular [paramedian], foraminal, lateral, and anterior), and stenosis (canal and foraminal). Patient symptoms were classified into 6 categories as follows: unilateral radicular (UR), bilateral radicular (BR), unilateral radicular with axial (URA), bilateral radicular with axial (BRA), unilateral axial (UA), and bilateral axial (BA).
4. DITI Procedures and Evaluation
DITI scans were taken before and 1 day after PCN. Pain severity, neurological function, and complications were evaluated before, and 1 day, 1 week, 1 month, and 3 months after PCN. Pre- and post-PCN thermal difference (TDs) between the affected and nonaffected limbs were evaluated using DITI. DITI was performed in accordance with the method established in a previous study [5-7]. Briefly, the patient was placed in a closed examination room at a constant temperature of 23°C and was sheltered from external light and heat. After a 20-minute adaptation to the room, the patient was positioned 1 m from the DITI equipment (IRIS, Medicore Co., Seoul, Korea) in an upright position. We then took infrared images of the trunk and both arms and hands. The TD in CDH at C3/C4 included the posterior upper back and shoulder and the anterior shoulder (C4). The TD in CDH at C4/C5 included the middle and lateral aspects of the triceps muscle, proximal radial region, and posterior medial aspect of the forearm and distal lateral forearm (C5). The TD in CDH at C5/C6 included the anterior aspects of the thenar, thumb, and second finger and the anterior aspects of the radial region and posterior aspects of the pararadial region (C6). TD in CDH of C6/7 included the posterior aspect of the ulnar and palmar region and the anterior aspects of the ulnar region and some fingers (C7). The TD in CDH at C7/T1 included the scapula and the posterior and anterior medial aspects of the arm (C8) [6]. Zhang et al. [6] reported that areas of TD caused by CDH were similar with the dermatome. To evaluate the DITI scans in the UR and URA groups, the lowest skin temperature of the patients’ painful dermatome and the symmetrical area on the opposite limb were measured and the TD was calculated. The TD was defined as follows:
TD=T1−T2
T1: skin temperature of the most painful area, the affected limb
T2: skin temperature of the symmetrical area of T1, the unaffected limb
TD: TD between the painful area and symmetrical area on the opposite limb
ΔTD=TDpost−TDpre
TDpre: largest TD between the areas with the most severe pain and the symmetrical area on the other limb before PCN
TDpost: TD between the reference areas of TDpre after PCN
ΔTD: change in TD after PCN
In the BR, BRA, UA, and BA groups, the areas with the largest TDpre or most relevant areas with the disc pathology on the basis of preoperative MRI were compared. We used the contralateral upper extremity and trunk as a control, and a blinded radiologist measured the ΔTD perioperatively.
The visual analogue scale (VAS; from 0 [no pain] to 10 [worst pain imaginable]) was used perioperatively to measure pain. The change in VAS score after PCN at each postoperative measuring point (ΔVAS) was calculated as follows:
ΔVAS=VASpre−VASpost
VASpre: VAS before PCN
VASpost: VAS after PCN at each postoperative measuring point (VAS1D, VAS1W, VAS1M, and VAS3M; VAS scores at postoperative day [POD] 1, POD7, POD30, and POD90, respectively)
ΔVAS: change in VAS score after PCN at each postoperative measurement point (ΔVAS1D, ΔVAS1W, ΔVAS1M, and ΔVAS3M; change in VAS score at POD1, POD7, POD30, and POD90, respectively)
5. Outcome Scales
Sensory deficit was assessed as the percentage of decrease on the affected side compared with the opposite side by using the prick test with a 23-G hypodermic needle and cold sensation with alcohol swabs at 1 day after operation. Motor weakness was evaluated at 1 day post PCN by a 5-point scale (0 [no contraction] to 5 [normal strength]). Adverse effects such as nausea/vomiting, dizziness, urinary retention, and Horner syndrome were recorded.
Satisfaction with PCN was assessed as excellent, good, fair, poor, or worse according to the Modified McNab criteria at 3 months post-PCN [3]. This study converted the Modified McNab criteria grading scale to 1–5 to simplify the median and standard deviation calculations.
The outcome measures at 1 week, 1 month, and 3 months post-PCN were collected through outpatient visits or telephone.
6. Statistical Analysis
We used an intent-to-treat strategy – that is, all participants were included in the analysis irrespective of whether they had completed the study. All participants were observed at baseline. However, the data for the outcome variables at 1 or 3 months after operation were missing for some patients, and the missing data were completed using a last observation carried forward analysis. The missing values were assumed to have normative behavior and the averaged values were assigned.
The Shapiro-Wilk test was used to perform a normality test of the continuous variables. Age, ΔVAS1D, ΔVAS1W, and ΔVAS1M passed the normality test. As height, weight, body mass index (BMI), surgery time, onset, TDpre, TDpost, ΔTD, VASpre, VAS1D, VAS1W, VAS1M, VAS3M, and ΔVAS3M did not pass the normality test, we additionally checked the Q-Q plot, which showed no marked deviation from linearity. Therefore, we considered all variables normally distributed.
The primary outcome was ΔTD and ΔVAS1D. The secondary outcome was the correlation between TD and VAS. The tertiary outcome was set as the correlation between the classified groups and ΔTD or ΔVAS.
A paired t-test was used to compare the preoperative and postoperative TD and VAS values. The correlations between TD and VAS were calculated using the Pearson correlation coefficient. Analysis of variance (ANOVA) followed by the Tukey B test was used to compare patient-related factors (age, height, weight, BMI, operative time, onset of symptom, and modified McNab score). Intergroup comparisons over time (TD and VAS) were achieved by repeated-measures ANOVA followed by Tukey honestly significant difference test. Moreover, ΔVAS was compared between the patients with ΔTD values of >0 and ≤0. Categorical data such as sex, ASA physical status, group classifications according to MRI or symptoms, symptom laterality, treated levels, and adverse events were compared using the chi-square test. Data are expressed as mean±standard deviation or number of patients (%). IBM SPSS Statistics version 23 (IBM, Armonk, NY, USA) was used for the statistical analyses. Values of p<0.05 were considered statistically significant.
RESULTS
In this study, 225 patients were included, whereas 31 were excluded, 4 owing to a history of prior cervical spine surgery, 3 owing to prior upper extremity surgery, 2 owing to psychotic disorders, and 22 owing to the absence of preoperative or postoperative DITI. All PCN procedures were performed successfully without complications. The mean number of treated discs with PCN was 1.7±0.7 (Table 1). The C5/C6 intervertebral disc was the most frequent target for PCN, followed by C6/C7 (Table 1). No significant differences in demographic data were observed between the groups (Table 1). The most common MRI findings of this study (in order) were protrusion, extrusion, and stenosis (Table 2). Patients most often complained of simultaneous radicular and axial pain (Table 3).
Compared with TDpre, TDpost improved significantly; that is, the difference was decreased or the temperature of the affected limb increased compared with the unaffected limb after PCN (p<0.05) (Table 1; Figs. 1, 2). Compared with VASpre, the postoperative VAS values (VAS1D, VAS1W, VAS1M, and VAS3M) decreased significantly (i.e., ΔVAS increased at every measuring point; p<0.05) (Table 1, Fig. 3). However, no significant difference in ΔTD, ΔVAS, or modified McNab score were found between the groups and subgroups according to MRI findings and subjective symptom classification (not significant). TDpre and VASpre values were similar in the groups, while ΔTD was not significantly correlated with ΔVAS. No significant differences in ΔVAS and modified McNab score were found when ΔTD was >0. When TDpre was larger, the postoperative pain severity increased and pain relief (ΔVAS) after PCN decreased significantly (p<0.05). The patients felt significantly greater satisfaction after PCN (excellent and good according to modified McNab score; p<0.05) (Table 1, Fig. 4). Seven patients underwent an additional surgery because of no improvement after PCN (anterior cervical discectomy and fusion 5 and PCN 2). In 1 patient, arrhythmia was observed during PCN but disappeared soon after PCN. One patient complained of hoarseness and another complained of difficulty swallowing, which normalized after 1 day. More than 6 months later, 2 patients revisited the clinic because of recurrence. Among them, one had a traffic accident. Two patients complained of pain in the contralateral arm that developed after PCN.
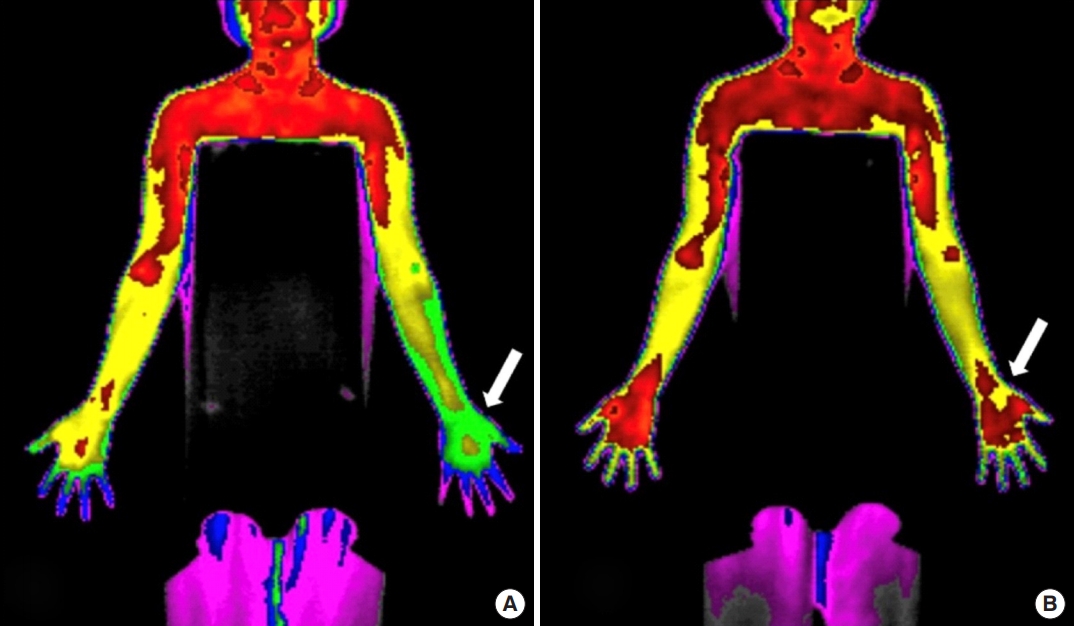
Thermographic images before (A) and after (B) cervical nucleoplasty. The figures shows the change in thermal difference (ΔTD). The arrow indicates the most painful area of the affected limb. The preoperative TD in panel A had decreased in panel B postoperatively.
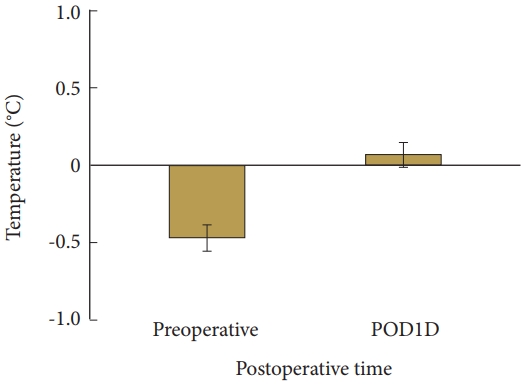
Change in TD after PCN. PCN, percutaneous cervical nucleoplasty; TD, thermal difference between the painful area in dermatome and the symmetrical area on the opposite limb; POD1D, postoperative day 1. Data are expressed as mean±95% confidence interval.
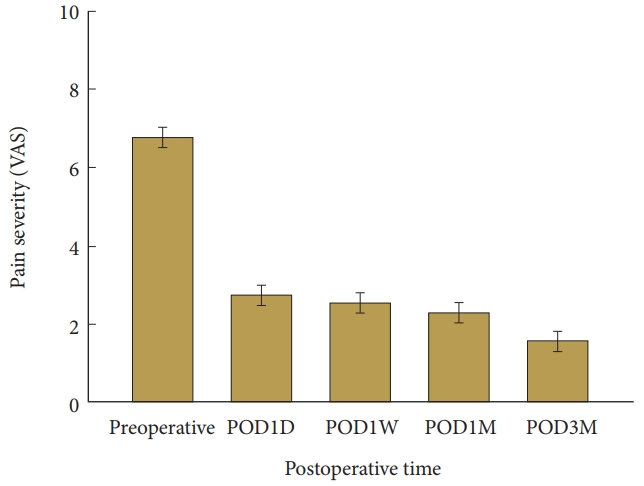
Change in pain severity after PCN. PCN, percutaneous cervical nucleoplasty; TD, thermal difference between the painful area in the dermatome and the symmetrical area in the opposite limb; VAS, visual analogue scale (from 0 [no pain] to 10 [worst pain imaginable]); POD1D, postoperative day 1; POD1W, postoperative week 1; POD1M, postoperative month 1; POD3M, postoperative month 3. Data are expressed as mean± 95% confidence interval.
DISCUSSION
The data obtained from this study, demonstrated that PCN improved TD and VAS score, and featured low complication and high satisfaction rates. However, ΔTD at 1-day post-PCN was not significantly correlated with ΔVAS (Fig. 5).
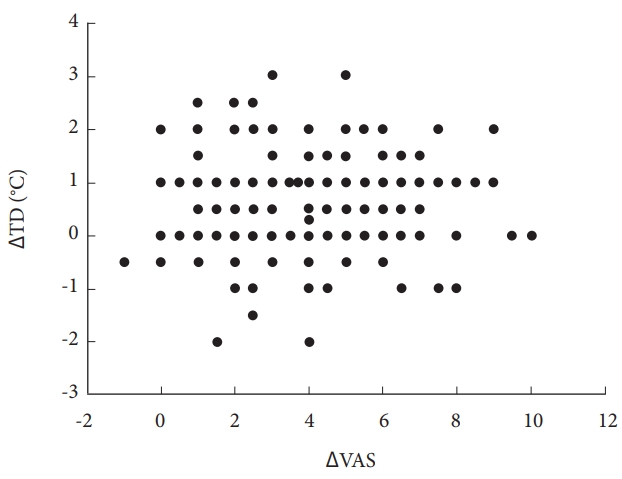
Scatter plot of ΔTD and ΔVAS. Pearson correlation coefficient (r)=0.027, p=0.540. PCN, percutaneous cervical nucleoplasty; TD, thermal difference between the painful area in the dermatome and the symmetrical area in the opposite limb; ΔTD, change in TD after PCN; VAS, visual analogue scale (from 0 [no pain] to 10 [worst pain imaginable]); ΔVAS, change in VAS after PCN on postoperative day 1.
PCN is an effective and safe procedure for the treatment of CDH [1,2]. Sharps and Isaac [8] performed PCN and achieved a 79% success rate for pain control in the patients. Nucleoplasty uses Coblation technology for disc decompression. Coblation technology allows simple efficient disc decompression because of controlled and highly localized ablation, resulting in minimal damage to the surrounding healthy tissue [3]. However, excessive tissue removal leads to loss of disc height and to disk regression [9]. Moreover, the disc may be damaged by heat [10]. Patients with a contained disc herniation and minimal disc degeneration confirmed using MRI, who are unresponsive to conservative treatment and have pain in their arm rather than the axial neck can be candidates for PCN.
Infrared thermography is a method that detects abnormalities by measuring skin temperature. Infrared thermography has been widely used to complement the diagnostic process, as it is noninvasive, user-friendly, and effective for visualizing subjective pain [5]. Various opinions exist on the relationship between VAS and TD in intervertebral disc disorder. Zhang et al. [11] reported that if the discogenic pain is more severe, then the disc herniation is more protruded, or if the symptom duration is shorter, then TD is more significantly prominent. The relationship between VAS and TD shows that the efferent output of vasoconstriction is determined by the amount of afferent input in the compressed spinal nerve root. When the spinal nerve root is compressed, irritation of the nerve root stimulates the recurrent meningeal nerve located in the lumbar region, which increases the temperature of lumbar area and stimulates the sympathetic nerve running with the ventral ramus of the nerve root, causing vasoconstriction and decreasing the temperature of the thermatome innervated by the nerve root of the lower extremity [11]. Moreover, Kim et al. [7] reported that preoperative TD in lumbar disc herniation decreased if the pain resolved postoperatively.
Subjective symptoms of a cool or warm sensation in the arm could be shown objectively using thermography with the detection of thermal changes in the case of radiculopathy, including CDH [6]. Zhang et al. [6] reported that the areas of thermal changes in each CDH included wider sensory and sympathetic dermatomes and a statistically significant change in temperature in those areas of thermal change in all CDH patients. Therefore, the areas of thermal change in CDH can be helpful for diagnosing the level of disc protrusion and detecting the symptomatic level in patients with multiple CDH [6]. Therefore, DITI is useful for objectively evaluating cervical discogenic pain and will help improve the doctor-patient relationship, treatment planning, and analyses of therapeutic effectiveness.
In general, the VAS, McNab criteria, and Odom criteria are used to evaluate outcomes of minimally invasive spinal surgery. However, these measures are too subjective. Therefore, the use of CT or MRI are considered to objectively assess minimally invasive spinal surgical outcomes, but only at 2 months after operation [4]. Moreover, they may not match the patient’s symptoms and signs [4]. However, patients who receive PCN will feel symptomatic relief within 1 day [2]. As the DITI reflects the physiological changes sooner than CT/MRI and offers direct visualization, it will be helpful in the evaluation of clinical symptomatic improvement.
In our study, TDpre had a direct proportional relationship with VASpost and an inverse relationship with ΔVAS after PCN. This means that if the preoperative TD is greater, the postoperative surgical outcome in terms of VAS score is poorer. We assume that the higher the TDpre, the longer the disease duration and the more severe the lesion and, therefore, lesser pain relief after surgery.
This study has limitations. First, infrared thermography is not operator-dependent and therefore not free from interobserver bias. Second, in our study, preoperative TD increased according to the preoperative pain severity. However, the pain had improved after PCN even if TD showed little or no improvement at 1 day after operation. This means that the symptomatic improvement preceded the improvement in TD. In previous studies, DITI was performed at 3 days (chemonucleolysis) or 7 days (laminectomy) postoperatively [7,12]. Therefore, DITI after PCN should be taken at 3 rather than 1 day postoperatively. Third, in our study, postoperative MRI was not used because of its high economical burden. If postoperative MRI was used, we could correlate the actual disc shrinkage after PCN with decreased pain severity or improved TD. Moreover, it would be important to determine why the evaluated patients did not respond to PCN.
In conclusion, the ΔTD at 1 day after operation did not significantly correlate with the ΔVAS. Therefore, thermography for the postoperative evaluation of PCN should be performed more than 1 week after operation. However, a preoperative thermographic evaluation can help clinicians predict the clinical outcome of PCN in CDH. If TDpre was large, then VASpost would be large and ΔVAS after PCN would be small.
CONCLUSION
DITI which was used to evaluate the outcomes after cervical nucleoplasty revealed that the ΔTD does not seem to reflect the ΔVAS after PCN. However, preoperative DITI findings could be useful for predicting the VAS reduction and clinical improvement after PCN. Longer-term follow-up is necessary for a more accurate identification of the correlation between ΔTD and ΔVAS after PCN.
Notes
The authors have nothing to disclose.
Acknowledgements
This research was supported by Chung-Ang University Research Grants in 2016.




