 |
 |
- Search
| Neurospine > Volume 16(2); 2019 > Article |
|
|
Abstract
Objective
Magnetic resonance imaging (MRI)-verified neural axis abnormalities (NAAs) have been described in adolescent idiopathic scoliosis (AIS), and several risk factors have been associated with the presence of NAAs in AIS patients. However, the clinical significance of these findings is unclear. The purpose of the present study was to determine the prevalence of NAAs in a large consecutive cohort of AIS patients and to evaluate the clinical significance of previously proposed risk factors.
Methods
We prospectively included AIS patients referred to a tertiary facility for evaluation. Full-spine MRI scans were performed on all included patients irrespective of curve magnitude or proposed treatment modality. MRI scans were prospectively analyzed by a neuroradiologist and the pathologic findings were confirmed by a second independent radiologist.
Results
NAA was observed in 34 of the 381 patients (8.9%): 32 patients had a syrinx, 1 patient had an arachnoid cyst, and 1 patient had a Chiari malformation. Four patients were referred for a neurosurgical evaluation but none received any neurosurgical treatment. No statistically significant difference was observed between the NAA and non-NAA groups in terms of sex, major curve size, thoracic kyphosis, left thoracic curve, curve convexity, curve progression, or level of pain (p>0.05).
Conclusion
In this prospective study examining the risk factors for NAA in AIS patients, we found that previously proposed risk factors could not predict the MRI outcomes. The finding of an NAA had no clinical implications and we do not support MRI scans as a routine diagnostic modality in all AIS patients.
Adolescent idiopathic scoliosis (AIS) is a common condition in the adolescent population. The diagnosis is traditionally based on an uneventful medical history, a thorough clinical examination and radiographs showing a typical coronal deformity without congenital abnormalities. However, between 2%–12% of patients with a diagnosis of AIS have a neural axis abnormality (NAA) [1,2]. These range from small size syrinx to potentially more severe conditions like Chiari malformations, tethered cord or intramedullary tumors. The NAAs are inherently difficult to predict on clinical examination as the majority of patients present without neurological deficits. Several risk factors have been associated with the presence of NAA. These include juvenile onset of the deformity, male sex, neurologic deficit (i.e., asymmetric superficial abdominal reflexes), atypical curve patterns (left-sided thoracic curves), thoracic hyperkyphosis, rapid curve progression, severe curves, and the presence of pain [2-10]. The majority of studies have focused on NAAs in AIS patients requiring surgery. Since curve magnitude is not a reliable predictor for NAA there is a need for a large-scale study on all AIS patients irrespective of curve size to determine the actual prevalence and the clinical implications. Currently, there is no consensus regarding the role of routine MRI scans in AIS evaluation and the decision is based on local preferences. Additionally, there is a lack of consensus regarding the terminology of NAAs and whether NAAs without neurologic deficit have an increased risk of neurologic complications during deformity surgery [11-13].
The purpose of the present study was to determine the prevalence of NAA and the role of routine MRI in the clinical evaluation of AIS patients. Furthermore, we aimed to clarify whether clinical or radiographic features could predict the presence of NAAs.
The study population included all patients referred to a single tertiary spine unit from January 1, 2010 to December 31, 2015 with suspected or known AIS. We included all patients with verified idiopathic scoliosis diagnosed between 10–18 years of age. We excluded patients with congenital anomalies, neuromuscular disorders, pre-existing NAA or any syndrome known to be associated with skeletal deformities. Permission to review patients record without informed consent was obtained from the Danish Patient Safety Authority (No. 3-3013-2059/1) and The Danish Data Protection Agency (No. RH-2017-86).
All patients underwent a thorough medical history and physical examination by one of 5 experienced spine surgeons. Medical charts were reviewed for clinical and demographic variables. The presence of pain was noted as a binary variable.
Radiographic assessment was performed with standing posterior-anterior and lateral radiographs as well as total spine MRI. Radiographs were retrieved from PACS in DICOM format and uploaded to an online imaging software KEOPS (S.M.A.I.O, Lyon, France). Measurements included major curve magnitude, right-sided or left-sided main curve, curve type (thoracic, thoraco-lumbar, lumbar or double major), length of curve and thoracic kyphosis. Annual progression rate (APR) was calculated as the difference between major curve size at the last obtained radiograph and the major curve at the first radiograph multiplied with the difference in time between the 2 radiographs; APR=(major curve t2–major curve t1)×12 months/(t2–t1) with t1 and t2 spaced a minimum of 6 months apart [14]. A progression above 5° was considered significant. Thoracic kyphosis was measured as the Cobb angle between Th5–Th12 and a curvature of more than 30° was considered hyperkyphotic. We defined atypical curves as main thoracic curves with a left-sided convexity.
All patients included in the study underwent MRI with 1.5 or 3.0 Tesla Scanner. In cases where the initial examination was considered pathologic, gadolinium was injected as contrast. All MRI’s were analyzed by a neuroradiologist for any NAA. If MRI was conducted outside our hospital the external report and images were acquired. We found inconsistencies between the terminology used to describe syrinx as both “hydromyelia,” “dilated central canal,” “syringomyelia” and “hydrosyringomyelia” were used interchangeably. We chose to use the term “syrinx” to describe all fluid-filled cavities in the spinal cord. Syrinx diameter was measured in the axial plane on T2 images. Since it is unclear whether a central canal with a diameter of 1–3 mm is pathologic or an anatomic variant, syrinx was classified in 2 groups: insignificant syrinx ≤3 mm and significant syrinx >3 mm [11]. The diagnostic criterion of Chiari I malformation was herniation of the tonsils below the foramen magnum by 5 mm or more [15]. All MRI scans with NAA were analyzed by a second independent radiologist to confirm the diagnosis.
Patients were categorized based on MRI findings (NAA group and non-NAA group). Continuous data were tested for normal distribution using histograms and are reported as mean±standard deviation. For normal distributed data, group comparison between NAA and non-NAA were performed using unpaired t-test. Nonnormally distributed data are reported with median (interquartile range [IQR]). Differences in distribution for categorical data were assessed with Pearson chi-square test of independence or Fischer exact test where appropriate. Multiple logistic regression was used to examine the association between the 5 most commonly proposed risk factors and MRI results. A p-value<0.05 was considered statistically significant. All statistical analyses were performed using R ver. 3.2.3 (R Core Team, 2015, Vienna, Austria).
During the study period a total of 563 AIS patients were identified as eligible for inclusion and 381 patients underwent MRI scan corresponding to a 68% inclusion rate (Fig. 1). The mean age at inclusion was 14±2.05 years and 80% were women. Mean major Cobb angle was 39°±15.91° and 58% of the curves were thoracic. NAA was observed in 34 of 381 patients (8.9%). Thirty-two patients had a syrinx, 1 patient had an arachnoid cyst and 1 patient had a Chiari 1 Malformation without syrinx. Four patients with syrinx were referred to a neurosurgeon and 1 patient was consulted with the Neurosurgical Department. The patient with an arachnoid cyst was seen by our Orthopedic Tumor Department and is being followed with MRI every year. The patient with a Chiari malformation was referred to the Neurosurgical Department and a neuropediatrician due to headache. No hindbrain decompression was indicated. None of the NAA patients received any neurosurgical intervention and none of the patients presented with major clinical neurological abnormalities. Six patients with syrinx obtained serial imaging studies with MRI and none of the MRI scans showed signs of progression. The 6 patients referred or consulted with the Neurosurgical Department had a maximal syrinx diameter of 3.5 mm (IQR, 2–4) and the median span was 4.5 (IQR, 3–8) vertebral length. Furthermore, two patients had a follow-up MRI with contrast to rule out tumor pathology.
Including all the patients with syrinx, the median syrinx maximal diameter was 2 mm (IQR, 1–4). The median span of syrinx was 4.5 vertebral levels (IQR, 2–7.25) and 84% were in the thoracic spine (Fig. 2). Nine patients (2.4%) presented with a significant syrinx >3 mm at the largest axial diameter. In this group the median syrinx size was 4 mm (IQR, 4–5) and median length was 6 vertebral levels (IQR, 3–6). Difference of other measurements between the 2 syrinx groups was without significance (p>0.05) (Table 1).
There was no statistically significant difference between the NAA and non-NAA groups in terms of sex, major curve size, thoracic kyphosis, curve type, left thoracic curve, curve progression or level of pain (p≥0.085) (Table 2). Median age was 14.2±2.0 and 13.5±2.0 years in the non-NAA and NAA groups, respectively (p=0.043).
To explore the applicability of clinical and radiographic predictors, patients were categorized as previously described (Fig. 3). We found no difference between NAA and non-NAA groups in terms of the presence of pain, thoracic kyphosis >30°, major curve >50°, APR >5° and left thoracic curve (p≥0.277). The multiple logistic regression showed no significant change in the risk of NAA for either decreased age (odds ratio [OR], 0.83; 95% confidence interval [CI], 0.69–1.01), male sex (OR, 1.05; 95% CI, 0.40–2.79), increased major curve (OR, 1.01; 95% CI 0.98–1.03), thoracic kyphosis>30° (OR, 1.01; 95% CI, 0.48–2.13), or atypical curve (OR, 1.69; 95% CI, 0.35–8.19).
A total of 188 underwent deformity surgery in the follow-up period and 183 had a preoperative MRI scan. In this group we found similar results with no difference between the NAA and non-NAA group in terms of major curve size, thoracic kyphosis, left thoracic curve, length of curve, annual curve progression or pain (p>0.086). Seventeen of the patients with NAA underwent deformity surgery and 12 patients had confirmed intraoperative monitoring (IOM) including motor evoked potential and somatosensory evoked potential. Neuromonitoring changes occurred in 2 patients in the insignificant syrinx group and none in the group with significant syrinx. The amplitude drop in one of the patients improved with elevated blood pressure. The other patient lost signal to both legs, which in part came back when the blood pressure was elevated. Furthermore, a wake-up call at the end of surgery ensured that no neurologic complication had occurred. None of the patients experienced any peri- or postoperative neurological complications. One patient experienced severe pain both pre- and postoperatively. The patient had a persistent ventriculus terminalis in the conus medullaris with an axial diameter of 4 mm and a vertebral span of 2 and was referred to our Pain Management Unit without improvement during the follow-up period.
A total of 182 patients did not have an MRI according to protocol. Compared to the MRI-group these patients had a significantly smaller curve at first visit, older age and less likely to receive brace or surgical treatment. There was not statistically significant difference in terms of sex and atypical curve type between the 2 groups (Table 3).
The need for routine preoperative MRI to detect subclinical NAA in patients with AIS has been widely investigated, but data are lacking on AIS patients irrespective of treatment modality. Frequently proposed indications of NAAs are early age of onset, male sex, thoracic hyperkyphosis, atypical curve patterns, rapid curve progression and the presence of pain. We aimed to assess whether these clinical and radiographic factors could predict NAA. Additionally, we aimed to determine the prevalence of NAA in a wide cohort of AIS patients irrespective of treatment modality. The overall prevalence of NAA in pediatric spine deformity ranges from 2%–54% depending on study design, inclusion criteria and the definition of NAA [1,8]. Studies on AIS patients report a lower prevalence between 2%–12% [1,2] which is in line with the prevalence in the current study of 8.9%. To our knowledge, this is the largest MRI study on AIS patients irrespective of major curve size, treatment strategy, curve progression, sex or convexity. We could not reproduce the findings of previous studies proposing clinical and radiographic predictors of NAA and question the clinical applicability of these. We assessed a series of predictive parameters, which leads to a risk of type 2 error due to lack of sample size. However, these predictors were originally suggested in studies with considerably smaller sample sizes and larger patient heterogeneity. Therefore, we conclude that the current level of evidence suggests that they have limited clinical applicability and should not be applied in daily clinical practice until further substantiated.
The prevalence of NAAs is often found to be appreciably higher in both congenital, infantile and juvenile scoliosis [16,17]. Benli et al. [3] included 104 surgically treated patients over a period of 12 years. None of the adolescent patients (n=55) had NAAs but in the juvenile group they found a prevalence of 14.3% [3]. The authors found early onset of scoliosis and pain, to be predictive of NAA. In our study, we found a modest difference between the NAA and non-NAA groups in terms of age, but the difference was small and we do not consider it clinically relevant. Importantly, this association was not present when adjusted for additional proposed risk factors. Diab et al. [6] included 2,206 surgically treated patients from the Prospective Pediatric Scoliosis Study and found significant risk factors for NAA to be thoracic hyperkyphosis>40° and juvenile onset. Nevertheless, there was a marked difference between patients with and without MRI in terms of these 2 risk factors, indicating a preselected group of patients. Former studies evaluating risk factors for NAAs in scoliosis conclude conspicuously different results [2,6,18]. This might be explained by different study methods, patient populations and lack of identical terminology.
There is inconsistency regarding the terminology of fluid-filled cavities in the spinal cord as the terms syringomyelia, hydromyelia, dilated central canal, and the hybrid term syringohydromyelia are used interchangeably [19]. Also, the debate is ongoing about the true etiology and prevalence. Despite the general disagreement on the terminology and etiology, numerous cavities are found with the increasing use of MRI. When applying a broad definition of syrinx, one study found a prevalence of 1.5% of accidental findings [12]. The term idiopathic syrinx is used when there is no association between the syrinx and any known lesion such as neoplasm, traumatic injury, infection or congenital malformation [20]. Most studies [11,12] suggest that idiopathic syrinx are rarely symptomatic, remains stable or decrease in size and could be considered an normal variation, which is in accordance with the syrinx found in our cohort. Magge et al. [20] evaluated idiopathic syrinx in a pediatric population and concluded that syrinx size did not correlate with the major curve or the outcome in patients with idiopathic scoliosis. Our findings are akin with these results in terms of syrinx size, curve size and syrinx progression. Jones [11] suggest that in the absence of underlying pathology, there is no justification in labeling a central canal with a diameter of 1–3 mm, pathologic. Applying these guidelines, we found a prevalence of only 2.9% with “true” pathology. These findings are in line with several studies evaluating only surgically treated AIS patients [1,21] and give weight to the hypothesis of the relatively high prevalence we found is due accidental findings of idiopathic syrinx.
Syrinx and other NAAs present challenges in the surgical correction of spinal deformity. At present, it is difficult to deduct whether a surgical treatment of the syrinx can stop or reverse the scoliosis in some cases. Sengupta et al. [22] reported on 16 patients with syrinx and scoliosis with absence of major neurological deficit. All patients underwent hindbrain decompression and 6 patients experience improvement or arrest in their major curve. Similarly, studies on patients with scoliosis and Arnold Chiari Malformation type 1 undergoing posterior decompression found older children and adolescent with larger curves, kyphosis and double curves more likely to progress. In younger patients without these features the curve often stabilizes and occasionally resolves completely [23].
Another surgical aspect is the risk of neurologic complications in patients with NAAs and scoliosis undergoing deformity surgery. The finding of a preexisting NAA could alter the surgical approach. In the current study, however, there were no cases of preoperative MRI’s changing the planning of surgery. Some studies suggest increase risk of neurologic damage due to spinal cord distraction and instrumentation of the spine [13,24]. Nevertheless, newer studies report no increase in complication rate when surgically addressing only the scoliosis and not the NAAs. Wang et al. [25] reported it safe to leave syrinx untreated when patients were without neurologic symptoms and the correction rate was kept according to the bending film. Samdani et al. [26] reported on the outcome of patients with syringomyelia undergoing spine deformity surgery and found that syrinx size had an impact on outcome. Large syrinx >4 mm were fused longer had a higher estimated blood loos and less correction. Moreover, they found less reliable IOM but no increase in neurologic sequela. We were not able to reproduce these findings and found no increased risk of labile IOM in patients with increased syrinx size. More importantly, we found no increased risk of neurologic sequela in patients with NAAs undergoing deformity surgery. Thus, we find it safe to operate AIS patients with moderate NAAs and lack of major neurologic findings prior to surgery.
Our study has several limitations. Approximately one third of the patient population was eligible for inclusion but did not have an MRI. The non-MRI group had smaller curves, where older and generally did not receive any treatment. As these patients were low-risk it is likely that the treating physician neglected to do MRI as protocolled. Hence, we may have overestimated the prevalence of NAAs in the current study given that age was the only positive predictor of NAAs in this study cohort. Moreover, the markedly lower age in the MRI group compared with the non-MRI group supports this theory and could indicate a potential age effect. We found a relatively large curve size at referral. We have previously showed that in a public health care system without school screening, patients referred from general practitioners have larger curves (median, 35°) compared to systems with school screening [27].
Due to the retrospective collection of the clinical data, the precision of some of the clinical variables is limited. The medical reports showed inconsistency in evaluating the superficial abdominal reflexes as well as a systematic description of pain. This is a weakness since a number of studies have shown a correlation between asymmetrical superficial abdominal reflexes and NAA [2,5,9]. The presence of pain was not systematically registered and our relatively high prevalence of reported pain could be an overestimation of “real pain” (in contrast to conditions such as muscle fatigue).
Essentially, our findings provide a representative prevalence of NAA in AIS patients due to the broad inclusion criteria irrespective of proposed treatment modality, sex, curve size, or convexity. Our results have broad applicability to all physicians managing AIS patients and underline that routine MRI scans in AIS patients may not be indicated and have limited clinical consequence.
In this study, a large AIS cohort underwent MRI examination irrespective of treatment modality. We found NAA in 8.9% of patients but found no clinical implications for the patients. Applying more conservative definitions of NAA, we found a prevalence of only 2.9%. The results of our study do not support the hypothesis of previously proposed radiographic and clinical parameters as predictors for NAAs. Furthermore, we found no increased neurologic complications in AIS patients with modest NAA undergoing deformity surgery. As a result, MRI scans does not appear to be crucial as a routine diagnostic modality in all AIS patients. We suggest that MRI should be reserved for patients with neurological symptoms, relevant comorbidities or atypical symptoms (e.g., severe pain).
CONFLICT OF INTEREST
Benny Dahl received institutional grants from K2M and MEDTRONIC outside the submitted work. Martin Gehrchen received institutional grants from K2M and MEDTRONIC outside the submitted work. Sidsel Fruergaard received institutional grants from MEDTRONIC. Søren Ohrt-Nissen received institutional grants from K2M outside the submitted work. The other authors have nothing to disclose.
ACKNOWLEDGEMENTS
Portions of this work were presented in poster form at the annual Danish Orthopedic Conference, Copenhagen, Denmark, October 25, 2017.
Fig. 1.
Inclusion process. AIS, adolescent idiopathic scoliosis; MRI, magnetic resonance imaging; NAA, neural axis abnormality.
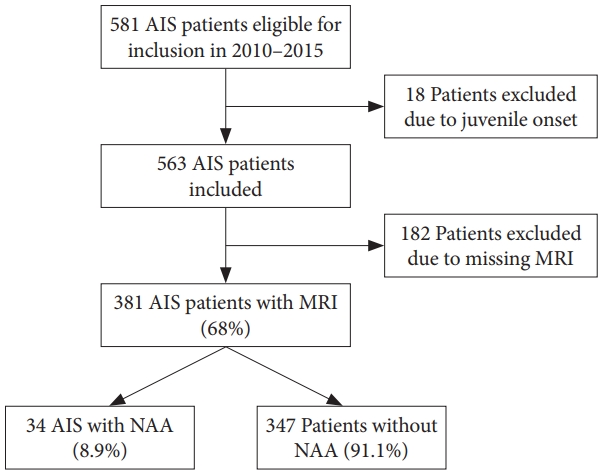
Fig. 2.
Magnetic resonance imaging with 1.5 Tesla Scanner, sagittal T2-weighted from a patient with a thoracic syrinx (arrow).
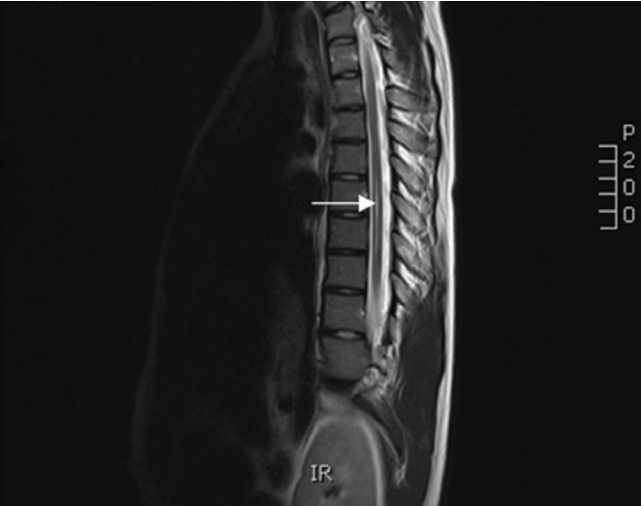
Fig. 3.
Proposed risk factors for neural axis abnormalities (NAAs) in adolescent idiopathic scoliosis. TK, thoracic kyphosis; APR, annual progression rate; Atypical, left side thoracic curve.

Table 1.
Comparison between the significant syrinx and the insignificant syrinx
Table 2.
Comparison between proposed risk factors and magnetic resonance imaging results
| Variable | Non-NAA (n = 347) | NAA (n = 34) | p-value |
|---|---|---|---|
| Age (yr) | 14.2 ± 2.0 (10–19) | 13.5 ± 2.0 (10–17) | 0.043 |
| Sex | 0.868 | ||
| Female | 276 (79.5) | 28 (82.4) | |
| Male | 71 (20.5) | 6 (17.6) | |
| Major curve at referral (°) | 39.0 ± 15.8 (10.0–97.0) | 40.5 ± 16.9 (10.0–75.0) | 0.614 |
| Major curve at follow-up (°) | 48.4 ± 18.8 (2.0–99.0) | 49.2 ± 21.1 (10.0–93.0) | 0.818 |
| Annual curve progression (°) | 4.4 ± 7.0 (-15.3 to 57.7) | 4.8 ± 5.3 (-4.3 to 21.0) | 0.686 |
| Left thoracic curve | 0.644 | ||
| Yes | 14 (4) | 2 (5.9) | |
| No | 333 (96) | 32 (94.1) | |
| Curve length (No. vertebrae) | 6.3 ± 1.4 (3–13) | 6.9 ± 1.5 (4–11) | 0.035 |
| Thoracic kyphosis (°) | 27.6 ± 13.0 (0–73) | 24.9 ± 13.3 (1–50) | 0.262 |
| Follow-up time (mo) | 28.2 ± 17.7 (1–90) | 23.7 ± 13.6 (6–53) | 0.085 |
| Paina) | 0.697 | ||
| No | 101 (32.3) | 9 (27.3) | |
| Yes | 212 (67.7) | 24 (72.7) | |
| Surgery during follow-up | 1.000 | ||
| No | 176 (50.7) | 17 (50) | |
| Yes | 171 (49.3) | 17 (50) | |
| Brace treatment during follow-up | 1.000 | ||
| No | 179 (51.6) | 18 (52.9) | |
| Yes | 168 (48.4) | 16 (47.1) |
Table 3.
Comparison between AIS patients without MRI and AIS patients with MRI
REFERENCES
1. Do T, Fras C, Burke S, et al. Clinical value of routine preoperative magnetic resonance imaging in adolescent idiopathic scoliosis. A prospective study of three hundred and twenty-seven patients. J Bone Joint Surg Am 2001 83-A:577-9.

2. Karami M, Sagheb S, Mazda K. Evaluation of coronal shift as an indicator of neuroaxial abnormalities in adolescent idiopathic scoliosis: a prospective study. Scoliosis 2014 9:9.




3. Benli IT, Uzümcügil O, Aydin E, et al. Magnetic resonance imaging abnormalities of neural axis in Lenke type 1 idiopathic scoliosis. Spine (Phila Pa 1976) 2006 31:1828-33.


4. Inoue M, Minami S, Nakata Y, et al. Preoperative MRI analysis of patients with idiopathic scoliosis: a prospective study. Spine (Phila Pa 1976) 2005 30:108-14.


5. Nakahara D, Yonezawa I, Kobanawa K, et al. Magnetic resonance imaging evaluation of patients with idiopathic scoliosis: a prospective study of four hundred seventy-two outpatients. Spine (Phila Pa 1976) 2011 36:E482-5.


6. Diab M, Landman Z, Lubicky J, et al. Use and outcome of MRI in the surgical treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2011 36:667-71.


7. Emery E, Redondo A, Rey A. Syringomyelia and Arnold Chiari in scoliosis initially classified as idiopathic: experience with 25 patients. Eur Spine J 1997 6:158-62.




8. Wu L, Qiu Y, Wang B, et al. The left thoracic curve pattern: a strong predictor for neural axis abnormalities in patients with “idiopathic” scoliosis. Spine (Phila Pa 1976) 2010 35:182-5.


9. Saifuddin A, Tucker S, Taylor BA, et al. Prevalence and clinical significance of superficial abdominal reflex abnormalities in idiopathic scoliosis. Eur Spine J 2005 14:849-53.



10. Morcuende JA, Dolan LA, Vazquez JD, et al. A prognostic model for the presence of neurogenic lesions in atypical idiopathic scoliosis. Spine (Phila Pa 1976) 2004 29:51-8.


11. Jones BV. Cord cystic cavities: syringomyelia and prominent central canal. Semin Ultrasound CT MR 2017 38:98-104.


12. Petit-Lacour MC, Lasjaunias P, Iffenecker C, et al. Visibility of the central canal on MRI. Neuroradiology 2000 42:756-61.




13. Leung YL, Grevitt M, Henderson L, et al. Cord monitoring changes and segmental vessel ligation in the “at risk” cord during anterior spinal deformity surgery. Spine (Phila Pa 1976) 2005 30:1870-4.


14. Williams BA, Matsumoto H, McCalla DJ, et al. Development and initial validation of the Classification of Early-Onset Scoliosis (C-EOS). J Bone Joint Surg Am 2014 96:1359-67.


15. George TM, Higginbotham NH. Defining the signs and symptoms of Chiari malformation type I with and without syringomyelia. Neurol Res 2011 33:240-6.


16. Dobbs MB, Lenke LG, Szymanski DA, et al. Prevalence of neural axis abnormalities in patients with infantile idiopathic scoliosis. J Bone Joint Surg Am 2002 84-A:2230-4.

17. Gupta P, Lenke LG, Bridwell KH. Incidence of neural axis abnormalities in infantile and juvenile patients with spinal deformity. Is a magnetic resonance image screening necessary? Spine (Phila Pa 1976) 1998 23:206-10.


18. Richards BS, Sucato DJ, Johnston CE, et al. Right thoracic curves in presumed adolescent idiopathic scoliosis: which clinical and radiographic findings correlate with a preoperative abnormal magnetic resonance image? Spine (Phila Pa 1976) 2010 35:1855-60.


19. Blegvad C, Grotenhuis JA, Juhler M. Syringomyelia: a practical, clinical concept for classification. Acta Neurochir (Wien) 2014 156:2127-38.



20. Magge SN, Smyth MD, Governale LS, et al. Idiopathic syrinx in the pediatric population: a combined center experience. J Neurosurg Pediatr 2011 7:30-6.


21. Hausmann ON, Böni T, Pfirrmann CW, et al. Preoperative radiological and electrophysiological evaluation in 100 adolescent idiopathic scoliosis patients. Eur Spine J 2003 12:501-6.




22. Sengupta DK, Dorgan J, Findlay GF. Can hindbrain decompression for syringomyelia lead to regression of scoliosis? Eur Spine J 2000 9:198-201.




23. Flynn JM, Sodha S, Lou JE, et al. Predictors of progression of scoliosis after decompression of an Arnold Chiari I malformation. Spine (Phila Pa 1976) 2004 29:286-92.


24. Wilson-Holden TJ, Padberg AM, Lenke LG, et al. Efficacy of intraoperative monitoring for pediatric patients with spinal cord pathology undergoing spinal deformity surgery. Spine (Phila Pa 1976) 1999 24:1685-92.


25. Wang G, Sun J, Jiang Z, et al. One-stage correction surgery of scoliosis associated with syringomyelia: is it safe to leave untreated a syrinx without neurological symptom? J Spinal Disord Tech 2015 28:E260-4.



- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























