Posterior Surgical Techniques for Cervical Spondylotic Myelopathy: WFNS Spine Committee Recommendations
Article information
Abstract
Objective
This study was conducted to determine and recommend the most up-to-date information on the indications, complications, and outcomes of posterior surgical treatments for cervical spondylotic myelopathy (CSM) on the basis of a literature review.
Methods
A comprehensive literature search was performed, using the MEDLINE (PubMed), the Cochrane Register of Controlled Trials, and Web of Science databases, for peer-reviewed articles published in English during the last 10 years.
Results
Posterior techniques, which include laminectomy alone, laminectomy with fusion, and laminoplasty, are often used in patients with involvement of 3 or more levels. Posterior decompression for CSM is effective for improving patients’ neurological function. Complications resulting from posterior cervical spine surgery include injury to the spinal cord and nerve roots, complications related to posterior screw fixation or instrumentation, C5 palsy, spring-back closure of lamina, and postlaminectomy kyphosis.
Conclusion
It is necessary to consider multiple factors when deciding on the appropriate operation for a particular patient. Surgeons need to tailor preoperative discussions to ensure that patients are aware of these facts. Further research is needed on the cost-to-benefit analysis of various surgical approaches, the comparative efficacy of surgical approaches using various techniques, and long-term outcomes, as current knowledge is deficient in this regard.
INTRODUCTION
Cervical spondylotic myelopathy (CSM) is a degenerative disease that causes compression of the spinal cord structure, posing major problems and resulting in daily dysfunction [1]. The lesions can be complete or incomplete; therefore, the clinical symptoms can vary, but generally involve the onset of cervical radiculopathy or myelopathy.
The biomechanical dynamic factors involved in normal cervical vertebral motion can exacerbate spinal cord injuries triggered by direct static compression, especially if the patient has other related diseases such as poor bone density, tumors, or trauma [1]. In some definitions, degenerative cervical myelopathy includes CSM, ossification of the posterior longitudinal ligament (OPLL), ossification of ligamentum flavum, and degenerative disc disease (Fig. 1) [2-4].
Wu et al. [5] reported that the incidence of CSM was proportional to age and higher in men, with an overall prevalence of 4.04 per 100,000 annual hospitalizations. Boogaarts and Bartels6 stated that the prevalence of CSM was 1.60 per 100,000 in the Netherlands. Nouri et al. [2] reported that the prevalence of CSM was 4.1–60.5 per 100,000 per year in the North American region. Furthermore, the most common co-symptom was tetraparesis or paraparesis [7,8].
This study was performed by the World Federation of Neurosurgical Societies (WFNS) Spine Committee, with the goal of determining and recommending the most up-to-date information on the indications, complications, and success rate of posterior surgical treatments for CSM on the basis of a literature review.
METHODS
A comprehensive literature search and analysis was performed with the search terms “cervical spondylotic myelopathy,” “ossification of posterior longitudinal ligament,” “laminoplasty,” “laminectomy,” and “cervical laminectomy and fusion” in the MEDLINE (PubMed), the Cochrane Register of Controlled Trials, and Web of Science databases for peer-reviewed articles published in English during the last 10 years. Articles relevant for the purpose of this review were selected by the authors if they included 50 patients or more in the study and lacked heterogeneity in the pathology for which posterior surgery was done. Articles were then reviewed for scholarly integrity and then synthesized into a broader understanding. This review is not a systematic review or a meta-analysis, but an overview of the available relevant literature. The senior author’s experience with posterior techniques for CSM was also taken into account.
RATIONALE FOR SELECTION OF THE SURGICAL APPROACH
Operative action is chosen if the symptoms of CSM significantly disturb the patient. Surgery is the main choice for patients with increasingly severe myelopathy symptoms, including imbalance and loss of hand dexterity. Operative management in CSM patients aims to decompress the spinal cord, restore sagittal alignment, and stabilize the spine. The options for treatment available to a spine surgeon consist of both anterior and posterior approaches. Spine surgeons consider several factors, namely: (1) sagittal curvature, (2) location of the compressive pathology, (3) the number of levels involved, and (4) the patient’s comorbidities [9]. Additional factors affecting the choice of the anterior versus posterior approach include the extent of ventral compression (K-line +/-) [10,11] and the presence of radiculopathy pain, numbness, or weakness.
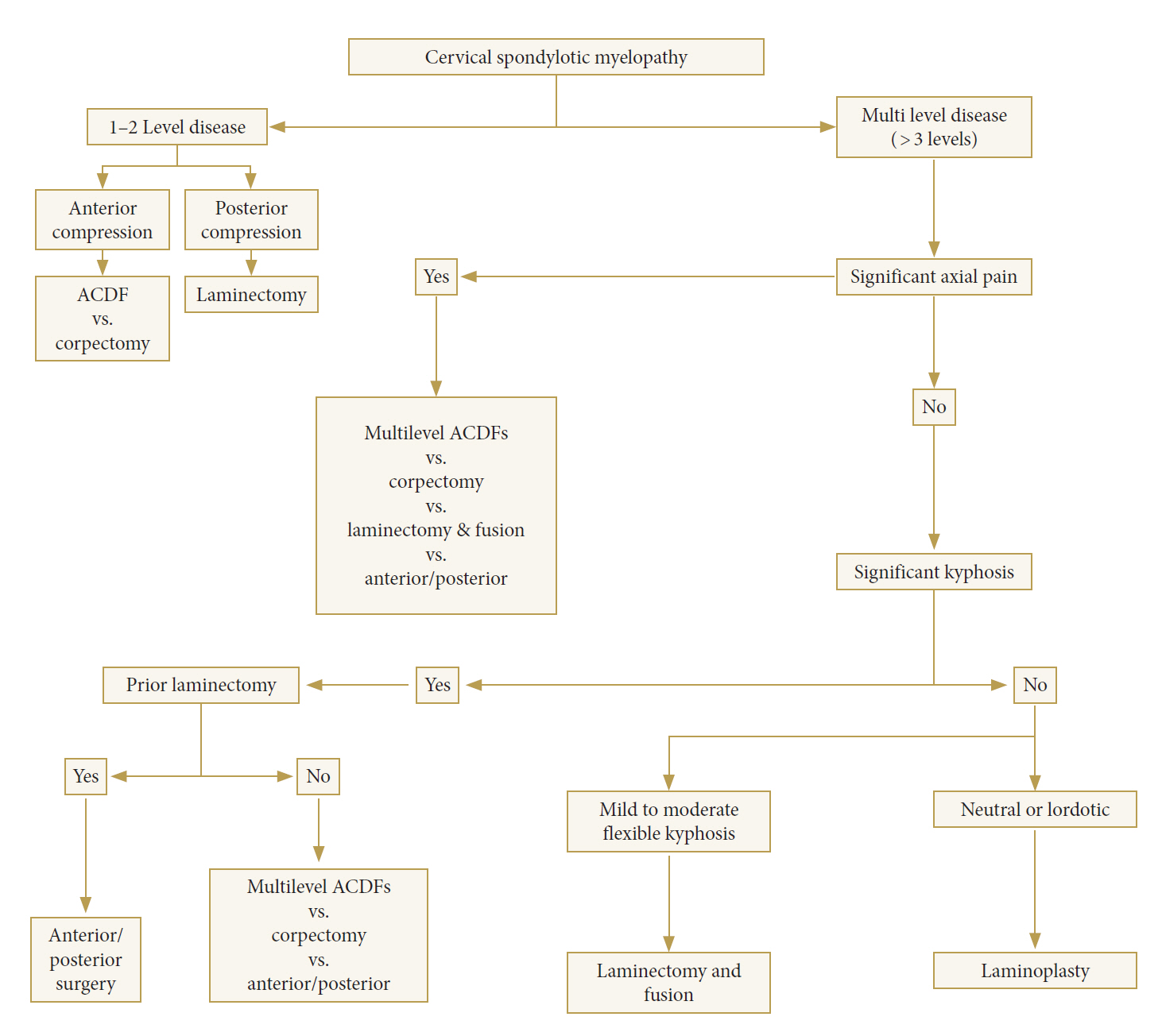
The anterior, posterior, and combined approaches have advantages and disadvantages specific to each case of CSM. Debates continue regarding the optimal approach for handling multilevel CSM. The anterior approach is considered to be advantageous in several contexts involving disc herniation, osteophytes, and OPLL; furthermore, it minimizes postoperative pain. The anterior approach is also commonly performed for CSM with kyphotic lesions. However, several studies have stated that the posterior approach can be considered for multilevel CSM. Rhee and Basra [12]. proposed such an algorithm, as shown below (Fig. 2).
Anterior decompression with graft placement and fusion is preferred in cases of CSM with a straightened spine or kyphotic cases with compression at fewer than 3 levels. This procedure is done to relieve pressure in the nerve and/or spinal cord and to relieve symptoms caused by the compressed nerves. The anterior approach is also considered to be effective in reducing the clinical presentation of concomitant cervical radiculopathy and anterior midline and/or paramedian compressive pathology. However, posterior decompression is preferred for the treatment of cervical lordosis involving more than 3 levels of compression, and when posterior ligamentous hypertrophy and ossification contribute most to the compressive pathology [13].
Posterior approach techniques consist of laminectomy alone, laminectomy with fusion, and laminoplasty, and are often used in patients with 3 or more levels involved.
ANTERIOR CERVICAL SURGICAL APPROACH
As shown in Table 1 [14] and Table 2 [14], the anterior approach is preferred in disorders involving one or 2 vertebral bodies. The anterior approach enables better correction of cervical lordosis than the posterior approach. This is hypothesized to be because the anterior approach allows adjustment of the cervical angle with graft placement. The anterior approach also yielded a better postoperative Japanese Orthopedic Association (JOA) score, indicating that the anterior approach was able to provide better postoperative neural function in the first 5 years [15]. However, additional similar studies with longer follow-up periods are needed. Some of the advantages of the anterior approach are direct decompression, stabilization in the arthrodesis, and the ability to provide axial lengthening of the spinal column. Some effects that may arise in the anterior approach include graft complications such as dislodgement, the need for postoperative bracing limitation, and loss of motion [16-20].
POSTERIOR CERVICAL SURGICAL APPROACH
Surgeons have a choice of standard laminectomy alone or skip laminectomy, which involves the preservation of key posterior muscle attachments and the selective removal of partial laminae; laminoplasty and its multiple variations; and laminectomy with fusion and stabilization, for example by subaxial lateral mass screws. The comparative efficacy of the most common approaches—posterior laminoplasty versus laminectomy with posterior spinal fusion—remains unclear.
1. Laminectomy Alone
Laminectomy involves surgical removal and removal of the spinal lamina. Laminectomy is the most common surgical procedure for managing CSM. Several recent studies have reported that laminectomy can provide an improvement of postoperative JOA scores and a cure rate of more than 50%. Jain et al. [21] stated that younger age (<57 years), early onset of symptoms (<4 months), and high preoperative JOA score (>10) predict better neurological outcomes for CSM. We must stress that laminectomy tends to reduce the cervical lordotic angle [21]. Laminectomy alone is not recommended for children or for people who already have a kyphotic curve in the cervical spine. Generally, laminectomy has been recommended for patients with cervical canal stenosis, multilevel CSM (>2), compression due to ligamentum flavum, and multilevel OPLL. However, in the last decade standalone laminectomy has been abandoned due to several complications such as postoperative kyphosis, indirect decompression, late instability, nerve root damage, and bowel disorders [21]. An alternative method, myoarchitectonic spinolaminoplasty, which preserves all of the nuchal muscles and reconstitutes all of the musculoskeletal couplings to the posterior elements of the vertebrae, was proposed as an effective way to preserve the volume and function of the nuchal musculature while minimizing postoperative musculoskeletal complaints [22].
2. Laminectomy With Fusion
Laminectomy with fusion is performed to stabilize the residual structure in order to prevent complications of standalone laminectomy, such as kyphosis. Fusion can be done between vertebral bodies (known as interbody or anterior fusion), to the bone lamina (posterior fusion), to the transverse process (posterolateral fusion), or using a combination of these fusion techniques [23]. Some popular techniques for posterior cervical fixation, such as lay grafts, spinous process wiring, facet wiring, and Halifax interlaminar clamps, cannot be used after laminectomy. Lateral mass fusion techniques using polyaxial screw-rod constructs have been the treatment of choice after laminectomy for many years.
Although there is a cervical pedicle screw fixation technique, it is still not widely applied because it requires preoperative axial computed tomography (CT) evaluation and navigation guidance to achieve safe screw placement [23]. Indications for laminectomy with fusion are multilevel cervical stenotic myelopathy (≥3-level disease) with preserved cervical lordosis or cases with signs of instability [14]. Patients with flexible kyphosis may also undergo laminectomy and fusion surgery.
Fusion has several advantages; for instance, it can prevent the occurrence of postlaminectomy kyphosis and can improve instability if the patient has undergone a previous laminectomy. Fusion can also reduce repetitive microtrauma effects. Some studies have also reported good results of laminectomy with fusion in patients with lower axial pain. In multilevel disease with neutral cervical alignment or patients with reducible cervical kyphosis, this procedure is an attractive alternative to standalone laminectomy or laminoplasty [12,23].
3. Laminoplasty
Laminoplasty procedures were first introduced by Japanese spine surgeons in the 1970s, and continue to be developed [24]. Some indications for laminoplasty are OPLL, multilevel (>2 vertebrae) CSM, or congenital spinal stenosis with posterior compression and syringomyelia. Laminoplasty also helps reach the spinal cord in cases of tumors, vascular problems, and functional surgery, in which procedures such as laminectomy are difficult [25]. However, laminoplasty should be avoided in patients with cervical kyphosis and significant preoperative axial neck pain [25]. Neck pain itself could be a common complication of laminoplasty [25]. Nonetheless, Stephens et al. [26], reported that laminoplasty did not lead to worsening axial neck pain in a carefully selected group of myelopathic patients without significant diffuse axial pain preoperatively and appropriate sagittal alignment.
Some of the benefits of laminoplasty include being able to expand the spinal canal so that it can maintain motion, stability, and spinal cord protection. Laminoplasty also helps to avoid the occurrence of postlaminectomy membrane, which is often caused by laminectomy. The number of dural tear events can also be reduced. Laminoplasty can decrease the risk of degeneration of adjacent segments. If necessary, laminoplasty can be done together with fusion [27].
Many laminoplasty techniques and variations have been described, but they all share the principle of expanding the cervical canal and protecting part or all of the posterior elements. Modifications have been made to the cutting site of the lamina or spinous process. More recently proposed techniques include the use of ceramic spacers and titanium miniplates, which can reduce surgical time and improve the safety of the procedure [28,29]. These newly developed procedures are also intended to maintain the musculature attachments in the paraspinal muscles and the posterior tension band. This approach results in a good range of motion and maintains spinal curvature, reducing the risk of kyphosis. Some of the most recent techniques for laminoplasty are open-door laminoplasty, the sagittal spinous process-splitting technique, and the expansive midline threadwire saw (T-saw) technique [30]. Two of the most common methods for implementing laminoplasty are the open-door and French door techniques. Although various surgical modifications have been suggested, the basic concepts of most procedures are similar to 1 of these 2 techniques.
In the open-door technique, the hinge is created unilaterally; in the French door modification, the hinge is created bilaterally. In the open-door procedure, the opening is made on the opposite lateral mass-laminar junction, while the French door is performed at the midline, providing sufficient space for the spinal cord [30].
ANTERIOR VERSUS POSTERIOR APPROACH
The optimal surgical approach in CSM cases is still debated. Obtaining a better understanding of the indications of each procedure will lead to improvements in management options and outcomes. The advantage of the anterior approach is the ability to provide direct decompression of the cervical spine so that the damaged disc can be accessed without disturbing the spinal cord; the incision can be made on the neck, and the neck muscles, trachea, and esophagus can be moved to the side to the access spine, enabling stabilization with arthrodesis and the provision of good axial neck pain relief [3]. If compression is mainly anterior and involves only 1 to 2 levels, the anterior approach should be chosen. When more than 2 levels are anteriorly compressed, the complication rate of the anterior approach increases, so that the posterior approach should be considered. Complications of the anterior approach include graft dislocation, pseudarthrosis, the need for postoperative bracing, and loss of motion. A combined approach can also be considered if kyphosis is present. The posterior approach has the advantage of allowing wider decompression [3].
Shamji et al. [31], in their study of 124 patients with CSM, examined JOA scores, the Nurick scale, and the Neck Disability Index. They concluded that there were no significant differences in the outcome of lordotic patients treated with either the anterior approach or the posterior approach. Kyphotic patients experienced better results when handled with an anterior or combined approach, which was hypothesized as being related to the C2–7 sagittal vertical axis (SVA), calculated as the deviation between the C2 plumb line and the posterior superior end plate of C7 [31]. Another study conducted by Tang et al. [32] analyzed 113 patients with cervical stenosis, myelopathy, and kyphosis who had undergone multilevel posterior cervical fusion. They found that disability was most strongly correlated with cervical SVA at a threshold of 40 mm, which can help spine surgeons choose the procedure most likely to yield optimal results in terms of cervical sagittal alignment. Furthermore, Hardacker et al. [33] explained that cervical plumb lines from the odontoid to C7 for all 100 volunteers were distributed in a narrow range (16.8±11.2 mm) anterior to the center of C7.
Hirai et al. [34] concluded that the anterior approach with fusion and laminoplasty gave the same outcomes at a 10-year followup. The anterior approach provided better sagittal alignment at a middle-term follow-up at 10 years. However, the anterior approach group had a higher reoperation incidence. In a meta-analysis, Luo et al. [35] reviewed 10 studies comparing anterior and posterior approaches for multilevel (>2 levels) CSM. The study concluded that the anterior approach was associated with a higher 24-month postoperative JOA score and a better recovery rate. Complications such as the reoperation rate, intraoperative blood loss, and operation time and the length of stay were more severe with the anterior approach. In terms of postoperative neurological clinical status, there were no significant differences between the 2 approaches [35].
COMBINED APPROACH
In some studies, especially those investigating multilevel CSM, a combined anterior-posterior approach has been proposed as the best solution, either in a single stage or in multiple stages [36]. Indications for combined approach include osteotomy for releasing the spine, a high risk of hardware failure (as in elderly people with osteoporosis), and complications of previous procedures [37]. The current literature states that same-day surgery is superior in terms of blood loss and length of stay. Determining whether to use a combined approach should include due consideration of decompression, stability, and good cervical alignment. Of note, patients undergoing 2-stage surgery are more prone to complications [38].
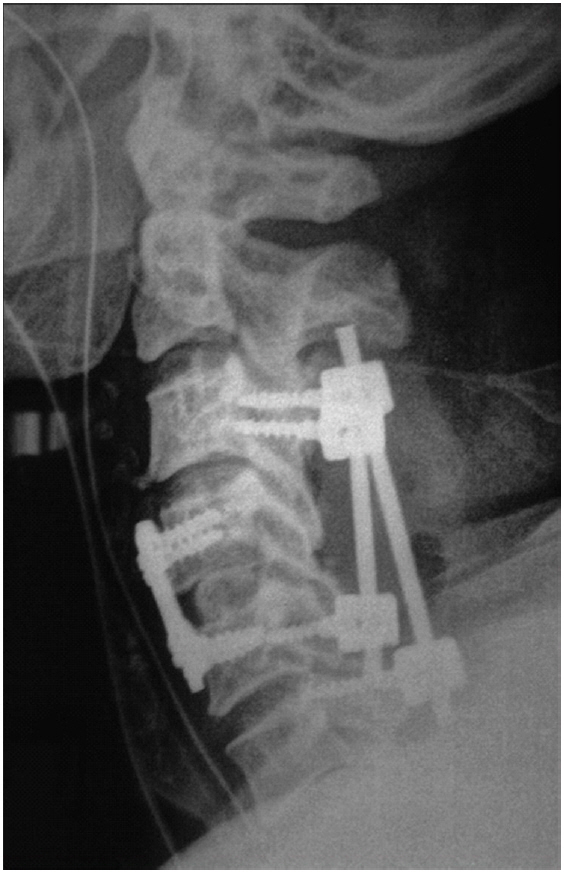
The following distribution of approaches in CSM procedures has been reported: 51%–60% for the anterior approach, 35% for the posterior approach, and a combined approach in the remaining cases [39]. A combined approach is generally used in cases with both ventral and dorsal compression of the thecal sac (Fig. 3) or if a patient with multilevel disease presents with kyphosis [40]. Fig. 3 depicts the postoperative radiograph of a patient with cervical canal stenosis from C3 to C6 with focal ventral compression at C4–5. This patient underwent anterior cervical discectomy with fusion at C4–5, followed by lateral mass screw fixation and laminectomy from C3 to C6 in the same sitting (one lower screw is in C5 and another at C6 because of fracture of one lateral mass during placement). A combined approach can be chosen in patients with significant ventral and dorsal osteophytic compression that cannot be handled holistically with single anterior or posterior surgery. In patients with severe osteoporosis, those with poor bone quality due to renal disease, or heavy smokers in whom poor bone fusion is anticipated, a combined approach can also be an option. A combined approach can also be considered in patients with significant focal kyphosis, posterior pathology, and multilevel decompression instability [14,40].
Song et al. [41] compared a combined approach group and an anterior fusion–alone group, and concluded that the combined approach led to better maintenance of sagittal alignment, a higher rate of fusion, a lower incidence of complications, and better clinical outcomes in cases of cervical kyphosis, despite a longer operating time. In patients with an inability to tolerate the prone position, active posterior infection, or previous irradiation to the posterior neck, a combined approach must at least be considered [14].
COMPLICATIONS OF POSTERIOR SURGERY FOR CSM
Yonenobu et al. [42] reported that patients who underwent laminoplasty demonstrated a significantly lower complication rate than those who underwent corpectomy (7% vs. 29%). As expected, the majority of complications in the corpectomy group were graft-related, including pseudarthrosis, graft displacement, and graft fracture. In the laminoplasty group, the only complications were 3 cases of C5 root paresis, all of which resolved [42]. Edwards et al. [43] also found similar neurological outcomes between the 2 procedures, but again found a much lower complication rate in the laminoplasty group. In their series, the majority of the complications in patients who underwent corpectomy were related to the anterior surgical approach (4 cases of persistent dysphagia, 2 cases of persistent dysphonia) [43].
Reported complications resulting from posterior surgery of the cervical spine include injury to the spinal cord and nerve roots, complications related to posterior screw fixation or instrumentation, C5 palsy, spring-back closure of lamina, and postlaminectomy kyphosis [44].
The overall incidence of neurological complications has been reported to be 0.18% [45], with a higher rate in cases of severe cervical kyphosis correction (2.6%) [46]. Subaxial lateral mass screws carry a risk of nerve root injury (1.3%) and lateral mass fracture [47].
Several studies have reviewed the incidence of C5 palsy in relation to posterior cervical surgery. Its incidence is highly variable, but it is important to consider, because it causes significant morbidity for the patient and can lead to dissatisfaction with the outcome of surgery. Factors thought to affect C5 palsy include drift of the cord with stretching of the nerve rootlets, preoperative factors, and aspects of the operative technique such as direct pressure on the nerve roots in the foramina at time of the operation. Yonenobu et al. [48] reported a 3.4% incidence of early postoperative C5 nerve root deterioration. These injuries are usually motor-dominant, but may also involve sensory and radicular pain. C5 dysfunction can occur immediately to 20 days postoperatively [49]. Recovery usually takes weeks, months, or as long as 6 years [50]. According to Pan et al. [51], foraminotomy and intraoperative neuromonitoring are the 2 main methods used to prevent C5 palsy.
Spring-back closure of the elevated lamina has a reported rate of 40% after laminoplasty [52]. However, this complication has only been reported with suture fixation, and has yet to be observed in modern screw or plate fixation [45].
Progressive postoperative kyphosis is one of the main arguments against traditional laminectomy. The incidence of kyphosis deformity after multilevel laminectomy is 20% [53]. Laminectomy includes removal of the spinous processes, interspinous and supraspinous ligaments, laminae, and ligamentum flavum and loss of the capsules of the facet joints that comprise the posterior stabilizers. Continuing normal flexion forces result in kyphosis, which develops gradually; for this reason, patients are usually well in the early postoperative period [45]. Sagittal malalignment and axial neck pain are the main issues regarding postlaminectomy kyphosis, while neurological deficits are rarely encountered. Laminectomy should be avoided in young patients without cervical lordosis, because the posterior facet joints should not be disrupted intraoperatively; instead, fusion should be considered for these patients in the same procedure [45].
Other general complications include wound infection, dural tear, and cerebrospinal fluid leak [44]. The risk of durotomy during laminectomy is 0.3%–13%, and can increase to 18% with revision surgery [54].
Careful analysis of the bony and vascular anatomy should be done preoperatively, especially when internal fixation is contemplated. In order to prevent postoperative neck pain and delayed kyphosis, posterior muscles and their attachments should be preserved.
When carefully and properly executed, posterior techniques for CSM can be effective with an acceptable rate of complications, and most complications are manageable with adequate preparation [44]. Current trends include the use of intraoperative CT and computer-assisted navigation systems to improve screw trajectory and reduce screw perforation.
SUCCESS RATES OF POSTERIOR TECHNIQUES FOR CSM
1. Neurological Outcomes
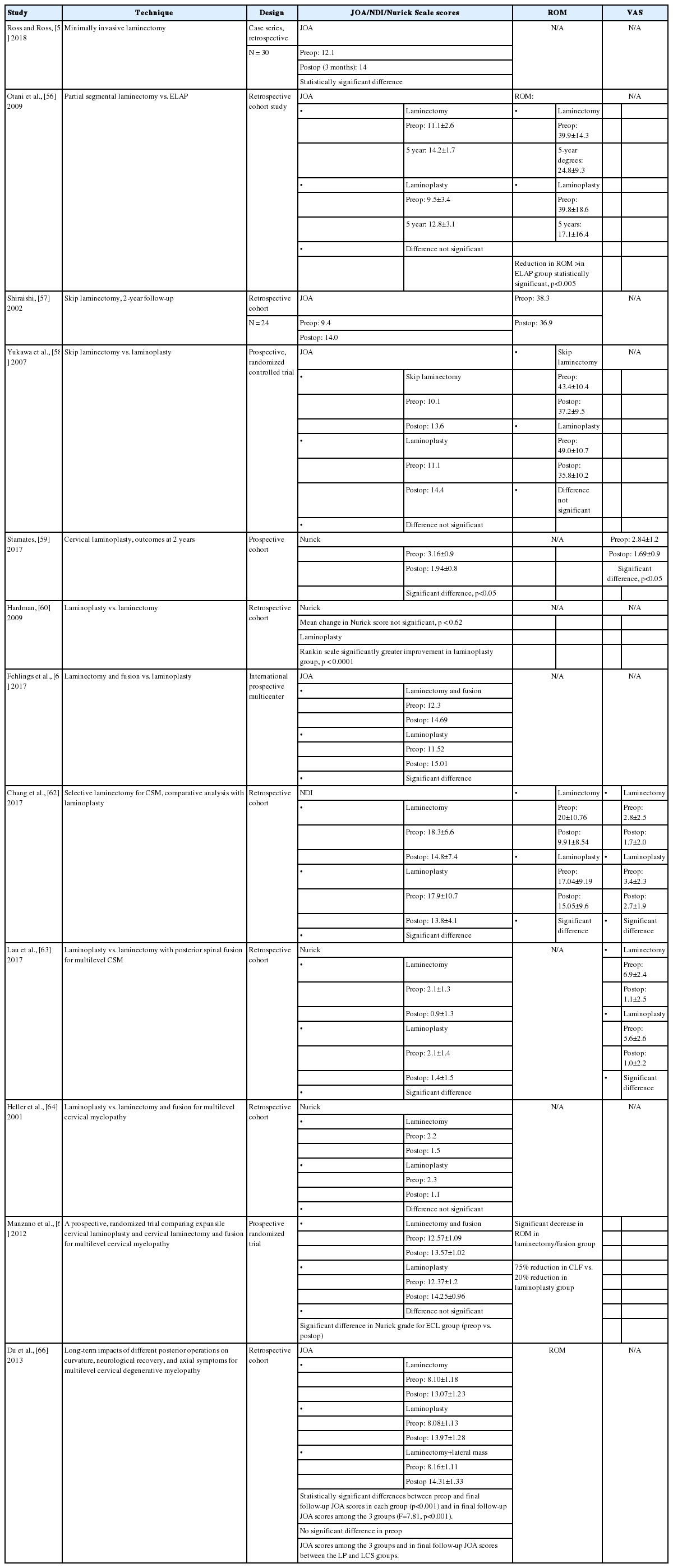
Most of the studies selected reported good improvement in neurological function for all posterior cervical surgical procedures. A comparison of the literature regarding different techniques is presented in Table 3 [55-66]. It is not possible to state at this time that one technique is superior to others due to variation in the scoring systems been used for these studies, such as the JOA score and the Nurick scale. Study designs have often been disparate. A meta-analysis by Yoon et al. found low-quality evidence for the equivalence of laminectomy and fusion with laminoplasty for this reason [67]. Heller et al. [64], however, found that the improvement tended to be slightly better in the laminoplasty group and that the higher complication rate of laminectomy and fusion favored laminoplasty. Lau et al. [63] found that fusion seemed to yield improvements in neurological function, as measured by the Nurick scale, but Lee et al. [68] reported that they led to equal results in both loss of lordosis and neurological improvement. Comparing laminectomy to laminoplasty, there is a trend for laminoplasty to be better than traditional laminectomy, but largely equivalent to newer techniques of minimally invasive skip laminectomy.
2. Range of Motion
Chang et al. [62] demonstrated a significant decrease in ROM in patients who underwent selective laminectomy compared to laminoplasty. Manzano et al. [65] showed clear evidence that standard laminoplasty resulted in greater preservation of motion than was the case for fusion. However, Otani et al. [56] reported a statistically significant greater reduction in ROM postoperatively in patients who underwent expansive laminoplasty than in those who underwent selective laminectomy. Yukawa et al. [58] found no difference in the postoperative loss of ROM between both groups.
Interestingly, other studies have reported conflicting evidence regarding the effect of laminoplasty on ROM. For example, the long-term follow-up study of Hyun et al. [69] demonstrated evidence of time-dependent loss of ROM at a 5-year follow-up. We speculate that variations in operative technique and patient populations may account for discrepancies in reports regarding loss of neck mobility after laminoplasty. For example, placement of a large amount of graft material on the hinge side in open-door laminoplasty may promote excessive fusion.
LONG-TERM OUTCOMES OF POSTERIOR SURGERY FOR CSM
Outcomes research following posterior cervical decompression has primarily focused on short-term results, with scarce data regarding long-term clinical and radiographical results. The evidence points to maintained improvements in clinical outcome measurements, with trends towards worsening radiographic outcomes. Hyun et al. [69] reported outcomes following unilateral open-door laminoplasty with a minimum follow-up of 5 years. They demonstrated a time-dependent continued loss of ROM after laminoplasty, along with maintained improvement in JOA scores at the final follow-up. Motosuneya et al. [70] demonstrated a similar time-dependent loss of ROM after laminoplasty, along with maintained clinical outcome scores at a 10-year follow-up. They noted greater loss in the ROM of patients with OPLL than in those with CSM, which is in keeping with the findings of Hyun et al., who also reported a statistically significantly greater loss of ROM in patients with OPLL at the final follow-up.
WFNS SPINE COMMITTEE RECOMMENDATIONS
1. Rationale for Selection of the Surgical Approach
• Several factors should be considered for selection of the surgical approach in patients with CSM: sagittal curvature, the location of the compressive pathology, the number of levels involved, and the patient’s comorbidities.
• Posterior surgical decompression is an effective technique for improving the neurological function of patients with CSM.
• Posterior surgical techniques for CSM include laminectomy alone, laminectomy with fusion, and laminoplasty. These techniques are often used if there are 3 or more levels of anterior compression. However, in cases with significant posterior compression at 1 or 2 levels, posterior decompressive surgery is mandatory.
• The relative merit of various posterior decompression techniques has not been clearly determined. In cases of kyphosis—especially flexible kyphosis—laminectomy and posterior fixation with fusion should be chosen. However, in rigid kyphosis, anterior surgery combined with posterior decompression is preferred. In cases with preserved lordosis, laminoplasty is a good option. Patients with severe axial neck pain should not be candidates for laminoplasty. However, for some cases that fall into a gray zone, such as straightened cervical spine, we are not certain which approach is better.
• A combined approach should be chosen in patients with significant ventral and dorsal osteophytic compression that cannot be handled holistically with single anterior or posterior surgery.
• Multiple factors must be taken into account when deciding on the appropriate operation for a particular patient. Surgeons need to tailor their preoperative discussion to ensure that patients are aware of these facts.
2. Complications of Posterior Surgery for CSM
• Complications resulting from posterior surgery for CSM include injury to the spinal cord and nerve roots, implantrelated complications, C5 palsy, spring-back closure of lamina after laminoplasty, and postlaminectomy kyphosis.
3. Success Rate of Posterior Surgery for CSM
• Comparing laminectomy to laminoplasty, laminoplasty tends to be better than traditional laminectomy, but largely equivalent to newer techniques of minimally invasive skip laminectomy.
4. Future Directions
• Further research is needed on the cost-to-benefit analysis of various surgical approaches, the comparative efficacy of surgical approaches using various techniques, and long-term outcomes, as current knowledge is deficient in this regard. Therefore, continued research into the outcomes of cervical spine surgery is essential.
• Since randomized controlled studies are very difficult to conduct in the field of spine surgery, prospective registries with long-term follow-up will be important for future decisions.
CONCLUSION
The posterior approach to cervical spine decompression for CSM is an effective technique for improving patients’ neurological function. The relative merits of techniques such as laminectomy, laminectomy with fusion, laminoplasty, or less invasive skip laminectomy have not been determined. In cases of kyphosis, laminectomy and posterior fixation with fusion should be chosen. In cases with preserved lordosis, laminoplasty should be performed. However, in cases that fall into a gray zone, such as straightened cervical spine, we are not certain which approach is better. A combined approach (posterior and anterior approach) should be chosen for significant ventral and dorsal osteophytic compressions that cannot be handled holistically with single anterior or posterior surgery. Multiple factors must be taken into account when deciding on the appropriate operation for a particular patient. Surgeons need to tailor their preoperative discussion to ensure that patients are aware of these facts.
Notes
The authors have nothing to disclose.