 |
 |
- Search
| Neurospine > Volume 16(4); 2019 > Article |
|
|
Abstract
An estimated 60% of the world’s population lives in Asia, where the incidence of neural tube defects is high. Aware that tethered cord syndrome (TCS) is an important comorbidity, the purpose of this systematic review was to explore the treatment of TCS among individuals living with spina bifida (SB) in Asia. MEDLINE and Embase databases were searched for relevant studies published from January 2000 to June 2018. Search terms such as ‘spinal dysraphism,’ ‘spinabifida,’ ‘diastematomyelia,’ ‘lipomeningocele,’ ‘lypomyelomeningocele,’ ‘meningomyelocele,’ and ‘tethered cord syndrome’ were used in diverse combinations. Of the 1,290 articles that were identified in accordance with PRISMA (Preferred Items for Systematic Reviews and Meta-Analyses) guidelines, 15 Asia-based studies met the inclusion criteria. Significant differences in the diagnostic criteria and management of TCS were documented. As the surgical techniques for prenatal closure of the spinal defect continue to evolve, their adoption internationally is likely to continue. In this setting, a clear and evidence-based approach to the definition and management of TCS is essential. The recent publication by the Spina Bifida Association of America of their updated care guidelines may serve as a tool used to promote a systematized approach to diagnosing and treating TCS among individuals with SB in the region, as well as globally.
An estimated 60% of the world’s population lives in Asia [1]. As with many other parts of the globe, disparities in medical care exist among countries in this region. The Lancet Commission on Global Surgery reports that more than 70% of the world’s population does not have access to timely, safe, or affordable surgical care. Access to proper surgical care is even less prevalent in South Asia, where up to 97% of the population lives without access to surgical care; this is in sharp contrast to the reality lived among higher-income regions, where only 3.6% of the population has a similar experience [2]. Additionally, although mandatory folic acid fortification has resulted in a lower prevalence of neural tube defects (NTDs) worldwide, rates of new folic acid preventable NTD cases remain calcitrant to reduction, and divergent prevalence rates exist within nations [3]. In Asia specifically, mandatory legislation enforcing fortification has lagged behind the evidence supporting it [4]. Given the size of the population across Asia and the presence of this surgical disparity, attention as to how to best allocate resources and provide greater access to avant-garde surgical techniques is mounting within the global health field.
Worldwide NTDs, comprising anencephaly and spinal dysraphism, are estimated to occur in 15–23 per 10,000 live births, with a higher prevalence reported in Southern Asia, at an estimated 22–43 per 10,000 live births [5]. The most common form of spinal dysraphism occurs along the dorsal spine, referred to as open spina bifida (SB). Myelomeningocele (MMC), the most common form of open SB, has long been associated with several comorbidities such as hydrocephalus, Chiari II malformation, tethered cord, scoliotic and kyphotic spinal deformities, skin injuries, as well as, neurogenic bowel and bladder [6,7]. Additionally, correction of a complex spinal deformities can produce severe pressure wound complications during treatment, after spinal instrumentation, or ambulation [8]. Differences in the related health outcomes vary widely contingent upon access to medical care, financial resources, and cultural/educational barriers that are specific to each region. Given the significant phenotypic variation in NTDs, the critical need for research-driven approach to this condition, as well as the multidisciplinary perspectives in its management, have long been recognized [9,10]. In response, the Spina Bifida Association of America (SBA) has developed evidence-based guidelines for the provision of care to individuals living with SB. Concurrently, global prevention efforts to reduce congenital disability have focused on the nutritional fortification of grains with folic acid and its prophylactic use by women in the childbearing years [11-13]. However, care is still needed to address the many related comorbidities and improve the quality of life among those living with NTDs. This is centered on the provision of appropriate medical and surgical services. Historically, surgical interventions have primarily focused on comorbidity mitigation. However, in the recent past, closure of the spinal defect prenatally has given rise to the possibility of preventing further damage secondary to ongoing exposure of the neural elements to amniotic fluid. These prenatal interventions have evolved over time from an open access hysterotomy approach to endoscopic coverage of the spinal defect [14-16]. Moreover, during the 2nd Asia Pacific Conference on Fetal Therapy in Singapore, a round table discussion was held to create a candidate model which can be applied in Asia to offer fetal surgery for MMC [17].
Presenting with 2 etiologies, tethered cord syndrome (TCS) is a common SB comorbidity, present as part of the congenital syndrome (primary) or as secondary to the open MMC closure, which has an incidence of 14%–32% [18-21]. When individuals with NTDs begin to display early symptoms of neurological deterioration such as impaired motor function, lumbosciatica, scoliotic and kyphotic spinal deformities, bowel/bladder incontinence, or foot deformities, then surgical untethering is paramount. The untethering procedure leads to an improvement of neurological function in an estimated 42%–75% of individuals with TCS [19,22,23]. However, it should be noted that the benefit of surgical intervention is most optimal among symptomatic individuals, versus those who have asymptomatic tethering [24]. In an era of expanding global surgical care [25], a common understanding of the clinical indications, time of intervention, and surgical technique in the management of TCS is fundamental. However, as with the surgical technique for endoscopic closure of the spinal defect, the definition of TCS as a condition that merits surgical intervention has also evolved over time. Another literature review defined TCS as “a diverse clinical entity characterized by symptoms and signs which are caused by excessive tension on the spinal cord” [26]. For this review, the same definition was used. We hypothesized that there would be variation in the approach to TCS throughout Asia. Therefore, the purpose of this systematic review was to explore the treatment of TCS among individuals living with SB in Asia.
This review was conducted and reported in line with the Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA) [27]. The MEDLINE, PubMed, and Embase databases were searched for English language studies published from January 2000 to July 2018. A maximally expanded search was applied using the following terms ‘spinal dysraphism,’ ‘spina bifida,’ ‘diastematomyelia,’ ‘lipomeningocele,’ ‘lypomyelomeningocele,’ ‘meningomyelocele,’ and ‘tethered cord syndrome’ in diverse combination following search strategy described by McKibbon et al. [28] The inclusion criteria were: (1) children and adolescents (0–18 years old) with NTDs and TCS, (2) quantitative studies, and (3) Asian region-based studies as defined by the United Nations [29]. Reviews, animal studies, case reports, conference abstracts, editorials, and comments were excluded. Studies that included less than 6 individuals with NTDs as a subset of a population were also excluded. Also we included a search of the gray literature (Google Scholar), personal communications, as well as a hand search of high-impact journals in the field using the reference lists of identified articles. Title and abstracts were screened before analyzing the full texts to determine their eligibility. Two reviewers independently assessed relevant studies to be included based on eligibility criteria. Any disagreements were resolved by discussion with a third reviewer. Methods of the analysis and inclusion criteria were specified in advance and documented in a protocol; the review protocol is available upon request.
Tethered cord and NTD-related information were extracted by one trained researcher using a standardized extraction form and checked by a second trained researcher. Data were obtained for the following study variables: authors, publication date, geographic location, experimental design, level of evidence, sample size, patients’ diagnoses, patients’ age, time of surgical intervention, duration of follow-up, and health outcomes related to the surgical intervention.
The systematic review was conducted on June 11, 2018. One thousand two hundred eighty-eight articles were identified in accordance with PRISMA guidelines, of which 15 Asia-based studies met the inclusion criteria for the systematic review (Fig. 1). Study characteristics and results of individual studies are presented in Table 1.
Studies meeting inclusion criteria were published from Turkey (4), Iran (3), China (2), South Korea (1), Japan (1), India (1), Pakistan (1), Saudi Arabia (1), as well as, Turkey and Iran (1) as a collaborative study. The most frequent study designs were case series (6 of 15 studies) and cross-sectional studies (3 of 15 studies). Seven of the 15 studies had less than 50 participants with TCS in their cohort. The spectrum of articles was representative of children of various ages: 3 studies had an average age for their participants above 1 year, and 8 studies had age ranges between 2 and 5 years.
The primary focus for the included studies was an analysis of surgical results and complications (n= 7), description of epidemiology, and initial clinical TCS presentation (n= 3), as well as development and comparison of diagnostics (n= 3). There was wide variability in the description of neurological status and also a divergence in the reasoning for conducting untethering surgery in asymptomatic cases. For example, Khoshhal et al. [30] reported progressive neurologic deficit in only 13/35 (37%) of cases, while Balkan et al. [31] reported that 18 of 20 of individuals (90%) had progressive neurologic deficit before surgery. Geyik et al. [32] reported that 97 of 161 of the cases (60%) were asymptomatic, and Kumar et al. [33] reported that 108/160 (68%) of individuals did not have motor weakness. Moreover, 56 of 69 (81%) were asymptomatic in the study by Yun-Hai et al. [34] Cha et al. [35] used intraoperative neurophysiological monitoring to predict bladder function after surgical untethering. However only 27 individuals (25.5%) had preoperative voiding difficulties, and 22 (20.8%) had electromyographical abnormalities. Shang et al. [36] had at least 38 individuals (11.6%) with no neurological deficit. The presence of neurologic symptoms was not a prerequisite for untethering in the study by Shahjouei et al. [37] Lastly, only 3 studies used urodynamic testing as part of their patients’ examination [31,35,38].
Three studies did not include surgical treatment but focused on nonsurgical evaluation and analysis of individuals with TCS [39-41]. Among surgical studies (n= 12), there was no clear or standardized description in the untethering technique utilized [30-34,37,38,42-44]. Among these surgical studies, only 6 described their complications in the results section of the respective article. Additionally, 5 out of the 13 studies did not provide information on the length of follow-up after surgical intervention. Concerning the potential source of bias, the most frequent risk for bias was the lack of clear diagnostic criteria, variability in study design, as well as the lack of homogeneity in sample sizes, indications for interventions, and outcome measures.
This literature review is the first to summarize published studies from Asia relating to surgical management of TCS among individuals with NTDs. In our systematic review, we observed differences in the diagnostic criteria of TCS and widely variable health outcomes following surgery in Asian countries [31,38-40]. Our findings suggest that among studies conducted in this region, the management of TCS was aggressive in the surgical approach leading to untethering and often did not rely on the presence of progressive neurologic deficits as a criterion for initial surgical treatment of spinal cord tethering [30,33,34,42].
To address the observable variation in the diagnostic criteria for TCS, Lew and Kothbauer [26] defined TCS as “a diverse clinical entity which presents with symptoms and signs resulting from abnormal spinal cord tension.” They advocated that surgical untethering was only necessary in cases with progressive or new-onset symptomatology attributable to TCS and raised questions regarding the benefits of surgical untethering among asymptomatic individuals. Furthermore, Yamada and Won [45] proposed that the terms “tethered cord syndrome” and “tethered spinal cord” be used exclusively to describe the presence of a functional disorder. Based on these parameters, individuals described as having TCS should exhibit symptoms attributable to a tethered cord.
To detect symptoms related to spinal cord tethering, noninvasive imaging techniques have been employed. Dias [46] has demonstrated that magnetic resonance images (MRIs) of the spine exhibit signs of tethering in most individuals with MMC. However, clinically significant symptoms are only present in about 30% of these cases. Additionally, Bowman et al. [21] reported that all children born with MMC have a low-lying cord when examined on MRI, even after initial repair and untethering. These findings are consistent with tethering and scarring resulting from prior MMC closure. However, less than one-third of these children will ever display signs of neurological, orthopedic, or urological impairment, despite the probability of being anatomically tethered.
As the surgical technique of prenatal MMC closure continues to evolve, its adoption in different regions of the world will continue to increase [17]. However, a trend towards an increased rate of secondary tethering following the use of this new prenatal approach has been documented [47,48]. Danzer et al. [49] reported that 14 out of 42 children (33%) developed spinal cord tethering, despite the prenatal surgical repair. This issue requires particular attention as children undergoing prenatal closure of the spinal defect may not receive longitudinal follow-up if the fragmentation of care is not explicitly addressed after fetal surgery, or as they transition from pediatric-centered to adult-centered healthcare. Chiefly as The Singapore Consensus demonstrates [17], centers across Asia are adopting emerging fetal surgical management techniques; therefore the potential risk for observing an increase in the incidence of TCS is ongoing. Therefore, a clear and evidence-based approach to the definition and management of TCS is crucial to provide the best quality of care possible across the globe.
In the United States (US), Yamada and Won [45] has defined TCS as a “stretch-induced functional disorder of the spinal cord” and has recommended close observation for asymptomatic individuals instead of surgical intervention as a primary approach. In our review, 3 out of the 15 included studies focused on diagnostic interventions for TCS [39-41]. For instance, Alamdaran et al. [41] compared the efficacy of MRI scans to ultrasonography for the detection of spinal abnormalities. Although ultrasound has a much lower resolution quality than MRI, it was described as a useful screening tool for TCS with best results under a 2 months of age window [50,51]. However, it is important to note that the average age in the study by Alamdaran et al. [41] was more than 2 years of age, which may have influenced their published results.
There were 12 studies that focused on surgical management, of which only 4 described their complications [32,37,38,44]. Overall, the mortality rate after spinal cord untethering was relatively low and usually resulted from infectious complications such as meningitis [52]. Given the morbidities (e.g., infectious complications, pseudomeningocele) affecting 10%–35% of cases [53-57], it should be noted that individuals with MMC are at an even higher risk than those with occult spinal dysraphism due to increased incidence of multiple recurrences, scarring, and poorly vascularized covering tissues. In light of our findings and the growing body of evidence in other regions of the globe, prophylactic surgical intervention among asymptomatic cases in Asia is called into question [58].
Currently, prophylactic untethering in asymptomatic individuals is not widely practiced in the US, as there are no controlled, prospective studies that have shown the benefits of the intervention in light of a high incidence of recurrent tethering [59]. In our review, we did not find a consistent evidence-based standard approach applied throughout the treatment centers. In most instances, the authors seemed to rely on anecdotal experience or previously established institutional practice. Concurrently, Buekens et al. [60] argue for a move to international collaboration to catalyze the use of tested interventions upfield. Buekens and colleagues argue that evidence-based global health necessitates the implementation of scientifically sound methods as the basis to interventions as they are adopted internationally. On October 25th 2018, the SBA released its updated “Guidelines for the Care of People with Spina Bifida” (available from: https://www.spinabifidaassociation.org/resource/guidelines/). The guidelines provide expert consensus and evidence-based guidance to the management of individuals living with SB; a summary of the TCS recommended approach is outlined in Table 2. Centers desiring to utilize an evidence-based approach to TCS management can implement these published recommendations ushering in the international opportunity for a cohesive approach to the diagnosis and management of TCS.
Evidence-based medicine refers to a set of practice standards that are based on scientific evidence, clinical expertise, and individual patient needs [61]. The development and later use of evidence-based medicine has improved clinical healthcare [62] and significantly influenced the comparative effectiveness of research [63], decreased over or under diagnoses and treatment [64], enhanced measures of quality of care [65], improved publishing standards [66], ensured that trials are registered [67], and curtailed the use of misguided interventions that have previously become part of established practice [68].
The research-based evidence and consensus of neurosurgeons who contributed to the aforementioned guidelines frame a standardized approach to individuals with NTDs and TCS. The focus according to these experts is to preserve function. Monitoring individuals, especially those who are still undergoing vertical growth is fundamental. It is suggested that preforming regular and ongoing assessments of neurological function is paramount, as well as teaching families the signs of TCS for which to monitor (e.g., back pain or declining lower extremity sensorimotor function). In addition to a careful physical examination, collaboration with urological colleagues and interpretation of urodynamic studies is crucial. If there has been a worsening of the neurogenic bladder function, according to the urodynamic study, then this is additional evidence that a surgery may be indicated for TCS. Only with sufficient evidence is TCS diagnosed and then timely release is performed with the goal of preserving spinal cord function and minimizing the recurrence of spinal cord tethering.
The recent publication of the SBA’s updated guidelines may serve as a useful tool for the evaluation and management of TCS among individuals with SB internationally. As such, a standardized approach to the diagnosis and management of TCS could also be employed throughout Asia to allow for proper analysis and comparison of surgical outcomes among different treatment centers [17]. Moreover, we contend that the use of a standardized criteria will play a fundamental role in the global initiative to standardize the care and treatment of individuals with TCS across the globe.
There are several limitations common to literature review studies. Our search was limited to studies published in English, peerreviewed, and indexed in PubMed (MEDLINE) or Embase databases. Therefore, it is possible that some eligible publications were excluded due to not having an English translation of the article. However, the use of broad search terms, additional examination of study reference lists, and the employment of a broad date range support confidence in our review. Factors that limited the synthesis of some of the findings included various sample sizes, study designs, indications, and outcomes measures. Another limitation of this study is the broad and often divergent definition of TCS used in the articles identified and included. It is acknowledged, however, that this is the nature of the current status of this body of literature throughout the region.
In summary, this study is the first to systematically review the literature from Asia relating to surgical management of TCS among individuals with NTDs. Significant differences in the diagnostic criteria and management of TCS were documented. As the surgical techniques for prenatal MMC closure continue to evolve, their adoption internationally is likely to continue. However, a documented potential increased rate of tethering following fetal surgery is reason for caution. In this setting, a clear and evidence-based approach to the definition and management of TCS is crucial. Therefore, the recent publication of the SBA’s updated care guidelines may serve as a useful tool for a systematized approach to TCS among individuals with SB in the region, as well as globally.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the search strategy and selection criteria. Adapted from Moher et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. https://doi.org/10.1371/journal.pmed1000097 [27].
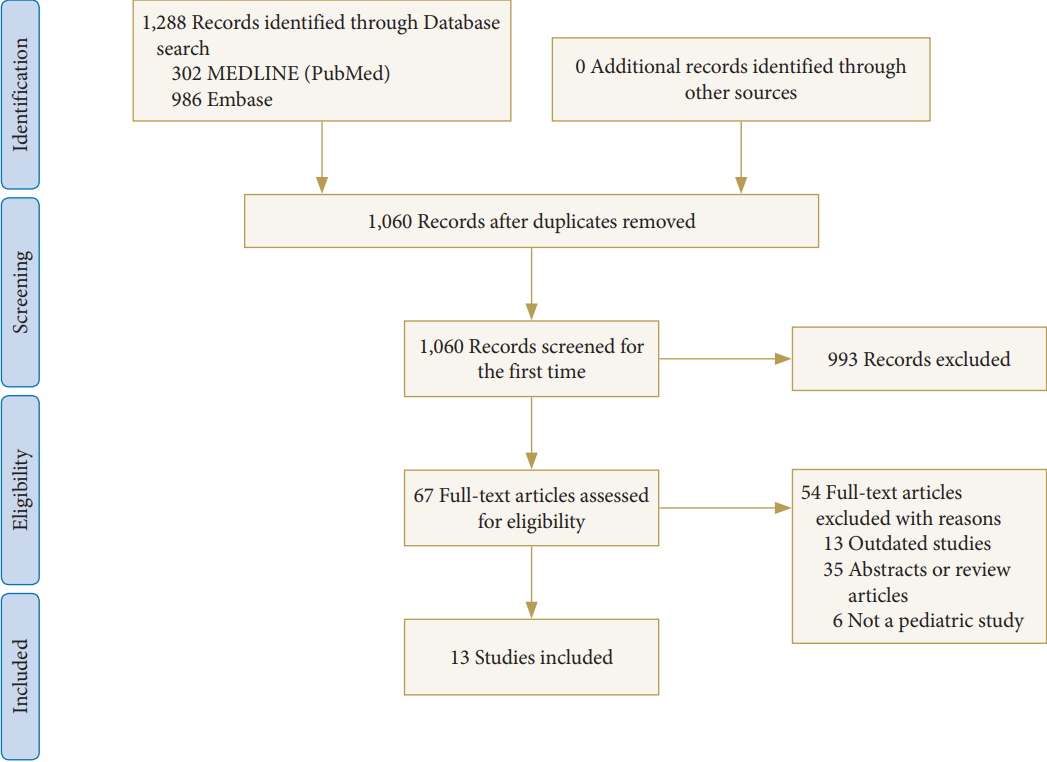
Table 1.
Literature review of the management of tethered cord syndrome (TCS) among individuals living with spina bifida in Asia
| Study/country | No. of patients | Type of study/level of Evidence* | Studied population age | Purpose | Initial presentation | Diagnostic or surgical intervention | Follow-up | Results/conclusions |
|---|---|---|---|---|---|---|---|---|
| Balkan et al.,[31] 2001/Turkey | 20 (all SD with TCS) | Case series/4 | 5 mo–13 yr (no mean was provided) | To evaluate the effect of division of the tethered spinal cord urodynamically in spinal dysraphic cases. | 17 Patients (85%) had unstable detrusor contractions, 19 patients (54%) had neurogenic bladder dysfunction, 18 (51%) had progressive neurological deficits of the lower extremities. | Surgical untethering, filum terminale cut (laminectomy+flavectomy). | N/A | Lower urinary tract dysfunctions secondary to tethered cord syndrome are very common in spinal dysraphic cases and significant improvements can be achieved with a judiciously timed division of the spinal tethered cord. |
| Diastematomyelic spur excision or shunting for syringomyelia. | ||||||||
| Sasani et al.,[38] 2008/Turkey, Iran | 10,000 (109 with TCS) | Cross-sectional study (retrospective cohort study)/2 | 6–19 mo (mean age for untethering 1.2 yr) | To examine the importance of cutaneous lesions and their correlation with clinical presentation, radiological examination, and urodynamic assessment. | All 119 neonates (100%) with low-lying conus and tethered cords had syndrome symptoms. | Surgical untethering, release of the spinal cord. | 12.8 mo for retethering | The correlation between urodynamic assessment and cutaneous lesions with a tethered cord found by MRI examination allows for early diagnosis and the possibility of prompt treatment. |
| Bademci et al.,[39] 2006/Turkey | 5,499 (all suspected occult SD) 5 SB, 2 with TCS | Cross-sectional study (prospective cohort study)/2 | 6–15 yr (mean 10.65±2.34 yr) | To investigate the prevalence and associated factors of primary tether cord syndrome (PTCS). | 6 Patients (100%) with TCS had neurologic symptoms. | Nonsurgical evaluation of frequency and presentation of neglected spinal dysraphism cases. | N/A | The general prevalence of PTCS was found to be 0.1% of 5,499 analyzed children and 1.4% of enuretic children. A good outcome after untethering was found in 83.0% in this series. |
| Early surgical intervention may halt the progression of the neurologic deficit and stabilize or reverse symptoms. | ||||||||
| Nejat et al.,[40] 2008/Iran | 176 (SBO=88, controls=88) (5 TCS/12 spine abnormalities) | Prospective cohort study/2 | 5–14 yr (mean 0.68±0.51 yr) | To evaluate the importance of SB occulta in radiographs of children with lower urinary tract or bowel dysfunction. | All 2 operated patients had urinary or bowel symptoms. | Nonsurgical analysis of MRIs and radiographs in children with lower urinary tract/ bowel dysfunction. | N/A | The findings of this study do not support the use of spinal MRI in patients with radiographic SBO and functional lower urinary tract/ bowel disorders in the absence of additional indications for neuroimaging. Spina bifida occulta was not shown to be a reliable indicator of spinal cord structural abnormalities. |
| Kumar et al.,[33] 2010/India | 160 (all with TCS) | Retrospective cohort study/3 | <18 yr (mean 3.2 yr) | To evaluate the significance of multiple tethering in patients with spinal dysraphism. | 108 Patients (68%) had motor weakness. Single level tethering group - 74 (62%), multilevel tethering group - 34 (83%). | Surgical untethering, release of the spinal cord. | >6 mo | The mere presence or absence of tethering is not sufficient documentation to predict its effect on the cases of spinal dysraphism. Tethering needs to be further classified in terms of the number of tethering lesions, which significantly affect the pre- and postoperative status of the patients. |
| Resection of lipomas, epidermoids, bony spurs, fibrous septums. | ||||||||
| Khoshhal et al.,[30] 2012/Saudi Arabia | 35 (all with TCS) | Case series/4 | 2 mo–11 yr (mean 2.96 yr) | To determine the presentations in patients and to study the natural history of untreated late presenting cases. | 13 Patients (37%) had progressive neurological deficit or 68% of >2 yr of age. | Surgical filum terminale cut (laminectomy). | N/A | Patients suspected of having TCS must be referred and treated by the age of 2 yr, or soon after diagnosis. Normal radiology in the presence of clinical features of cord tethering should not exclude the diagnosis of TCS. |
| Yun-Hai et al.,[34] 2015/China | 69 (all with TCS) | Case series/4 | 3 days–8 yr (mean 9.5 mo) | To investigate the relationship between meningocele and TCS using an MRI-based approach. To determine the best surgical procedure and when to perform surgery. | 13 Patients (19%) had symptoms, 56 patients (81%) were asymptomatic. | Surgical untethering, release of the spinal cord. Repair of the protruded meninges, removal of the spinal lesions. | 45.2 mo (8 mo–6 yr) | The rate of meningocele is highly correlated with TCS, for the diagnosis MRI is necessary. Surgical treatment is recommended immediately after definite diagnosis. The protruding meninges repair, revision of the spinal canal, and release of the tethered cord are necessary. |
| Geyik et al.,[32] 2015/Turkey | 162 (primary TCS=43, secondary TCS=119) | Case series/4 | 2 mo–17 yr (mean 5.2 yr) | To document experiences on the surgical treatment of TCS in childhood. | 86 Patients (53%) had back pain, 29 (19%) leg weakness and 26 (16%) had urinary problems. 97 (60%) were asymptomatic. | Surgical release of the spinal cord, filium terminale cut and correction of the associated malformation. | 47 months (2–120 mo) | Hypertrichosis was the most common physical finding while back pain was the most common complaint. Lipoma, split cord malformation, dermal sinus tract, and MMC were associated with malformations for secondary TCS. |
| Shahjouei et al.,[37] 2016/Iran | 161 (all with TCS) | Randomized control trial/1 | 1 day–7 yr (mean 3.14±3.80 yr) | To evaluate the effectiveness of prone positioning and acetazolamide administration on complication rates following spinal cord untethering surgeries. | The presence of neurological symptoms was not mandatory, all clinically asymptomatic patients had at least 1 pathological finding in urological studies (radiological or urodynamic). | Surgical untethering and postoperative management evaluation. | 10 days as per RCT protocol | Prone positioning after untethering surgeries was related to a significantly lower rate of complications. Acetazolamide (isolated or in combination) was ineffective at lowering complication rates and added the burden of side effects. |
| Seki et al.,[42] 2016/Japan | 31 (all with TCS) | Retrospective cohort study/3 | 1 day–18 yr (mean 34 mo) | To compare long-term results of surgery with the outcomes of symptomatic and asymptomatic TCS in children and adolescents. | 19 Patients (61%) had symptoms, 12 (39%) were asymptomatic. | Surgical untethering, filium terminale cut. | 116 mo (7–223 mo) | Prophylactic surgery for TCS should be conducted in those aged <34 months, or as soon as possible. |
| Iqbal et al.,[43] 2016/Pakistan | 50 (all with TCS) | Case series/4 | 0–15 yr (mean 4 yr) | To assess the common presentations of TCS and the surgical outcomes of different presentations. | 45 Patients (90%) had urinary sphincter problem, 16 (32%) presented with progressive back or leg pain, 7 (14%) with scissoring of feet, and 6 (12%) patients were paraplegic. | Surgical filium terminale cut. | 12–48 mo | Following surgery, the most common complaint was urinary sphincter problems (90%). The majority of patients had four distinct pathologies: thickened filum terminale, diastematomyelia, lipoma, and MMC. The outcome of patients with TCS varies according to pathology and severity of symptoms. Surgery outcomes were best for individuals with diastematomyelia and thickened filum. |
| Kural et al.,[44] 2015/Turkey | 36 (all with MMC, 10 had secondary TCS) | Case series/4 | 0–24 mo (mean 4 mo) | To report experiences on the management of lumbosacral MMC in children. | 27 Patients (75%) had paraparesis, 6 (17%) had paraplegia, 3 (8%) ankle weakness and foot deformity in 7 patients (19%). Therefore, neurological deficits were observed in all patients. | Surgical MMC closure in a watertight fashion, release of the spinal cord in case of retethering. | 36 mo | Surgical treatment using appropriate microsurgical techniques are crucial for lumbosacral myelomeningoceles in children. Early surgical intervention with close follow-up will improve the neurological condition of the patients. |
| Alamdaran et al.,[41] 2017/Iran | 40 (14 with TCS) | Cross-sectional study (prospective cohort study)/2 | 5 mo–45 mo (mean 25.73±19.15 mo) | To evaluate the diagnostic value of ultrasonography in detection of spinal abnormalities in children with neurogenic bladder. | 40 Patients (100%) had a neurogenic bladder. | Nonsurgical comparison of MRI scans with ultrasonography for the detection of spinal abnormalities. | N/A | Ultrasonography has an acceptable and desirable sensitivity and specificity in the diagnosis of most of the spinal cord abnormalities except for dural ectasia, hydromyelia and syringomyelia, diastematomyelia, and the spinal cord masses in children with a neurogenic bladder. |
| Cha et al.,[35] 2018/South Korea | 106 (all with TCS, 16 with LMMC) | Retrospective cohort study/3 | <18 yr (mean 3.3±1.0 yr) | To investigate the predictive value of intraoperative bulbocavernosus reflex in TCS patients in predicting post-operative voiding function. | 27 Patients (25.5%) had preoperative voiding difficulty, 22 (20.8%) had electromyographical abnormality. | Surgical untethering, intraoperative bulbocavernosus reflex monitoring. | 6 mo | Intraoperative bulbocavernosus reflex monitoring can predict bladder function 6 mo postoperatively with high specificity (88.5%), particularly in patients with diagnosis other than LMMC (93.4%), indicating that voiding function deterioration will not occur if intraoperative bulbocavernosus reflex is preserved. |
| Shang et al.,[36] 2019/China | 326 (all with TCS) | Retrospective cohort study/3 | <15 yr (mean 8.50±3.94 yr) | To determine the effect of surgical untethering, to identify differences between various types of TCS. | Surgical untethering | 3–36 mo | Therapeutic effect of surgical untethering is markedly different in patients with different types of tethered cord syndrome. |
Table 2.
Recommendations for screening and care of individuals with spina bifida with tethered cord syndrome
| 0–11 Months | ||
| ^a. | Surgically reapproximate the pial edges of the neural placode (surgical neurulation) and close the wound in sequential layers. | |
| ^b. | Follow infants younger than 12 months in the clinic, at 3- to 4-month intervals. | |
| *c. | Orthopedic evaluations are recommended every 3 months in the first year of life. | |
| 1–2 Years 11 months | ||
| ^a. | Follow children at 6-month intervals for routine care in the Spina Bifida clinic and remain available in the event of clinical change. | |
| ^b. | Teach families the signs of TCS (back pain, declining lower extremity sensorimotor function). Follow the child clinically to observe for these signs. Relevant for all subsequent ages | |
| ^c. | Use adjunctive studies judiciously (imaging such as MRI/CT, urodynamics) during routine well-child visits, according to experience, preference, and best clinical judgment, to augment clinical decision-making. Relevant for all subsequent ages | |
| *d. | Monitor the spine for the development or progression of a deformity that may be due to a tethered cord or syrinx. Obtain anteroposterior and lateral scoliosis radiographs if a deformity is suspected on clinical exam. Perform radiographs in a sitting position if the patient is able to sit but not able to stand or in a standing position if the patient can stand. Repeat radiographs every 1 to 2 years if the deformity is present, depending on rate of progression. | |
| *e. | Evaluate for neurologic changes or progression of scoliosis and discuss with neurosurgery specialists. | |
| 3–5 Years 11 months | ||
| ^a. | Follow children at intervals of 6–12 months in the Spina Bifida clinic. | |
| *b. | Evaluate the spine clinically and obtain scoliosis radiographs every one to two years if a progressive spinal deformity is suspected. | |
| Perform radiographs in a sitting position in children who can sit but not stand and in a standing position in children who can stand. | ||
| *c. | Work with neurosurgery specialists to determine whether a neurogenic cause of scoliosis progression is present. | |
| *d. | It is recommended that surgical treatment of scoliosis be reserved for a progressive deformity that is unresponsive to nonoperative management. For example, when there is a progression of scoliosis in spite of bracing and after a neurosurgical cause, such as a tethered cord, it has been ruled out. It is also recommended that management with growing rod surgery and fusionless technique should include spinal cord monitoring in patients with distal neurologic function. | |
| 6–12 Years 11 months | ||
| ^a. | Follow children aged 6–12 years 11 months at 12-month intervals in the Spina Bifida clinic. | |
| *b. | It is recommended that surgical treatment of scoliosis be reserved for a progressive deformity that is unresponsive to non-operative management. An example is when scoliosis has progressed in spite of bracing and after a neurosurgical cause, such as a tethered cord, has been ruled out. It is also recommended that management with growing rod surgery and fusionless technique should include spinal cord monitoring in patients with distal neurologic function. Growing rod surgery with sacral-pelvic fixation is effective in correcting the deformity and achieving growth. | |
| 13–17 Years 11 months | ||
| ^a. | Follow children ages 13–17 years 11 months at 12-month intervals in a Spina Bifida clinic. | |
| *b. | Monitor for the development or progression of scoliosis clinically, with radiographs as necessary, if indicated by the physical exam. | |
| Perform radiographs in a sitting in a position in those who can sit but not stand and in a standing if position in those who can stand. If the curve has progressed to an operative magnitude (50°), discuss the risks and benefits of surgical treatment with the family. | ||
REFERENCES
1. Statista. Distribution of the global population 2018, by continent [Internet]. New York, Statista, Inc. 2018 [cited 2018 Sep 6]. Available from: https://www.statista.com/statistics/237584/distribution-of-the-world-population-by-continent/.
2. Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 2015 386:569-624.


3. Castillo J, Castillo H. Global health and chronic non-communicable conditions: spina bifida care across a worldwide community. J Pediatr Rehabil Med 2017 10:167-8.


4. Atta CA, Fiest KM, Frolkis AD, et al. Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta-analysis. Am J Public Health 2016 106:e24-34.



5. Blencowe H, Kancherla V, Moorthie S, et al. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann N Y Acad Sci 2018 1414:31-46.


6. Kaufman BA, Terbrock A, Winters N, et al. Disbanding a multidisciplinary clinic: effects on the health care of myelomeningocele patients. Pediatr Neurosurg 1994 21:36-44.


7. Sawin KJ, Liu T, Ward E, et al. The National Spina Bifida Patient Registry: profile of a large cohort of participants from the first 10 clinics. J Pediatr 2015 166:444-50.


8. Bradko V, Hill J, Castillo H, et al. Team approach: guidelinebased management of skin injury in individuals with myelomeningocele. JBJS Rev 2019 7:e1.

9. Liptak GS. Evidence-based practice in spina bifida: developing a research agenda, May 9-10, 2003. Washington, DC: Spina Bifida Association of America; 2003.
10. Fletcher JM. Spina bifida: a multidisciplinary perspective. Hoboken (NJ): Wiley-Blackwell; 2010.
11. Blencowe H, Cousens S, Modell B, et al. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol 2010 39 Suppl 1:i110-21.



12. De-Regil LM, Fernandez-Gaxiola AC, Dowswell T, et al. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2010 (10)::CD007950.



13. Castillo-Lancellotti C, Tur JA, Uauy R. Impact of folic acid fortification of flour on neural tube defects: a systematic review. Public Health Nutr 2013 16:901-11.


14. Bruner JP, Tulipan NE, Richards WO. Endoscopic coverage of fetal open myelomeningocele in utero. Am J Obstet Gynecol 1997 176(1 Pt 1):256-7.

15. Verbeek RJ, Heep A, Maurits NM, et al. Fetal endoscopic myelomeningocele closure preserves segmental neurological function. Dev Med Child Neurol 2012 54:15-22.


16. Blumenfeld YJ, Belfort MA. Updates in fetal spina bifida repair. Curr Opin Obstet Gynecol 2018 30:123-9.


17. Wataganara T, Seshadri S, Leung TY, et al. Establishing prenatal surgery for myelomeningocele in Asia: The Singapore Consensus. Fetal Diagn Ther 2017 41:161-78.


18. Begeer JH, Meihuizen de Regt MJ, HogenEsch I, et al. Progressive neurological deficit in children with spina bifida aperta. Z Kinderchir 1986 41 Suppl 1:13-5.



19. Tamaki N, Shirataki K, Kojima N, et al. Tethered cord syndrome of delayed onset following repair of myelomeningocele. J Neurosurg 1988 69:393-8.


20. Oi S, Yamada H, Matsumoto S. Tethered cord syndrome versus low-placed conus medullaris in an over-distended spinal cord following initial repair for myelodysplasia. Childs Nerv Syst 1990 6:264-9.



21. Bowman RM, McLone DG, Grant JA, et al. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg 2001 34:114-20.


22. Linder M, Rosenstein J, Sklar FH. Functional improvement after spinal surgery for the dysraphic malformations. Neurosurgery 1982 11:622-4.



23. Ohe N, Futamura A, Kawada R, et al. Secondary tethered cord syndrome in spinal dysraphism. Childs Nerv Syst 2000 16:457-61.



24. Hertzler DA 2nd, DePowell JJ, Stevenson CB, et al. Tethered cord syndrome: a review of the literature from embryology to adult presentation. Neurosurg Focus 2010 29:E1.

25. Søreide K, Winter DC. Global surgery in an ecosystem for worldwide health. Br J Surg 2019 106:e12-e13.


26. Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr Neurosurg 2007 43:236-48.


27. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009 6:e1000097.



28. McKibbon A, Eady A, Marks S. PDQ evidence-based principles and practices. Hamilton (ON): BC Decker; 1999.
29. The United Nations Statistics Division. Methodology. Geographic Regions [Internet]. New York, United Nations. 2018 [2018 Jun 11]. Available from: https://unstats.un.org/unsd/methodology/m49/.
30. Khoshhal KI, Murshid WR, Elgamal EA, et al. Tethered cord syndrome: a study of 35 patients. J Taibah Univ Med Sci 2012 7:23-8.

31. Balkan E, Kilic N, Avsar I, et al. Urodynamic findings in the tethered spinal cord: the effect of tethered cord division on lower urinary tract functions. Eur J Pediatr Surg 2001 11:116-9.



32. Geyik M, Alptekin M, Erkutlu I, et al. Tethered cord syndrome in children: a single-center experience with 162 patients. Childs Nerv Syst 2015 31:1559-63.



33. Kumar R, Garg P, Kalra SK, et al. Management of multiple tethering in spinal dysraphism. Childs Nerv Syst 2010 26:1743-7.



34. Yun-Hai S, Nan B, Ping-Ping G, et al. Is repair of the protruded meninges sufficient for treatment of meningocele? Childs Nerv Syst 2015 31:2135-40.




35. Cha S, Wang KC, Park K, et al. Predictive value of intraoperative bulbocavernosus reflex during untethering surgery for post-operative voiding function. Clin Neurophysiol 2018 129:2594-601.


36. Shang AJ, Yang CH, Cheng C, et al. Microsurgical efficacy in 326 children with tethered cord syndrome: a retrospective analysis. Neural Regen Res 2019 14:149-55.



37. Shahjouei S, Hanaei S, Habibi Z, et al. Randomized clinical trial of acetazolamide administration and/or prone positioning in mitigating wound complications following untethering surgeries. J Neurosurg Pediatr 2016 17:659-66.


38. Sasani M, Asghari B, Asghari Y, et al. Correlation of cutaneous lesions with clinical radiological and urodynamic findings in the prognosis of underlying spinal dysraphism disorders. Pediatr Neurosurg 2008 44:360-70.


39. Bademci G, Saygun M, Batay F, et al. Prevalence of primary tethered cord syndrome associated with occult spinal dysraphism in primary school children in Turkey. Pediatr Neurosurg 2006 42:4-13.


40. Nejat F, Radmanesh F, Ansari S, et al. Spina bifida occulta: is it a predictor of underlying spinal cord abnormality in patients with lower urinary tract dysfunction? J Neurosurg Pediatr 2008 1:114-7.


41. Alamdaran SA, Mohammadpanah N, Zabihian S, et al. Diagnostic value of ultrasonography in spinal abnormalities among children with neurogenic bladder. Electron Physician 2017 9:4571-6.




42. Seki T, Hida K, Yano S, et al. Surgical outcome of children and adolescents with tethered cord syndrome. Asian Spine J 2016 10:940-4.



43. Iqbal N, Qadeer M, Sharif SY. Variation in outcome in tethered cord syndrome. Asian Spine J 2016 10:711-8.



44. Kural C, Solmaz I, Tehli O, et al. Evaluation and management of lumbosacral myelomeningoceles in children. Eurasian J Med 2015 47:174-8.



46. Dias L. Myelomeningocele and intraspinal lipoma. In: Richards S, Sponseller PD editors. American Academy of Orthopaedic Surgeons, Pediatric Orthopaedic Society of North America. Orthopaedic knowledge update: pediatrics. 2nd ed. Rosemont (IL): American Academy of Orthopaedic Surgeons; 2002. p.249-59.
47. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011 364:993-1004.



48. Worley G, Greenberg R, Dicianno B, et al. Cohort comparison study of neurosurgical procedures after closure of myelomeningocele by fetal surgery versus by neonatal surgery in patients in the national spina bifida patient registry. Dev Med Child Neurol 2018 60:1-87.

49. Danzer E, Thomas NH, Thomas A, et al. Long-term neurofunctional outcome, executive functioning, and behavioral adaptive skills following fetal myelomeningocele surgery. Am J Obstet Gynecol 2016 214:269. e1-269.

50. Nelson MD Jr. Ultrasonic evaluation of the tethered cord syndrome. In: Yamada S editors. Tethered cord syndrome. Park Ridge (IL): AANS Publ Committee; 1996. p.71-8.
51. Sahin F, Selcuki M, Ecin N, et al. Level of conus medullaris in term and preterm neonates. Arch Dis Child Fetal Neonatal Ed 1997 77:F67-F69.



52. George TM, Fagan LH. Adult tethered cord syndrome in patients with postrepair myelomeningocele: an evidencebased outcome study. J Neurosurg 2005 102(2 Suppl):150-6.


53. Herman JM, McLone DG, Storrs BB, et al. Analysis of 153 patients with myelomeningocele or spinal lipoma reoperated upon for a tethered cord. Presentation, management and outcome. Pediatr Neurosurg 1993 19:243-9.


54. Klekamp J, Raimondi AJ, Samii M. Occult dysraphism in adulthood: clinical course and management. Childs Nerv Syst 1994 10:312-20.



55. Filler AG, Britton JA, Uttley D, et al. Adult postrepair myelomeningocoele and tethered cord syndrome: good surgical outcome after abrupt neurological decline. Br J Neurosurg 1995 9:659-66.


56. Albright AL, Pollack IF, Adelson PD, et al. Outcome data and analysis in pediatric neurosurgery. Neurosurgery 1999 45:101-6.


57. van Leeuwen R, Notermans NC, Vandertop WP. Surgery in adults with tethered cord syndrome: outcome study with independent clinical review. J Neurosurg 2001 94(2 Suppl):205-9.


58. McLone DG, Bowman RM. Overview of the management of myelomeningocele (spina bifida). Waltham (MA): UpToDate; 2018.
59. Caldarelli M, Boscarelli A, Massimi L. Recurrent tethered cord: radiological investigation and management. Childs Nerv Syst 2013 29:1601-9.



62. Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet 2017 390:415-23.


63. Lohr KN. Comparative effectiveness research methods: symposium overview and summary. Med Care 2010 48(6 Suppl):S3-S6.


64. Moynihan R, Henry D, Moons KG. Using evidence to combat overdiagnosis and overtreatment: evaluating treatments, tests, and disease definitions in the time of too much. PLoS Med 2014 11:e1001655.



65. Berenson RA, Pronovost PJ, Krumholz HM. Achieving the potential of health care performance measures. Timely Anal Immed Heal Pol 2013 1-30.

- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























