INTRODUCTION
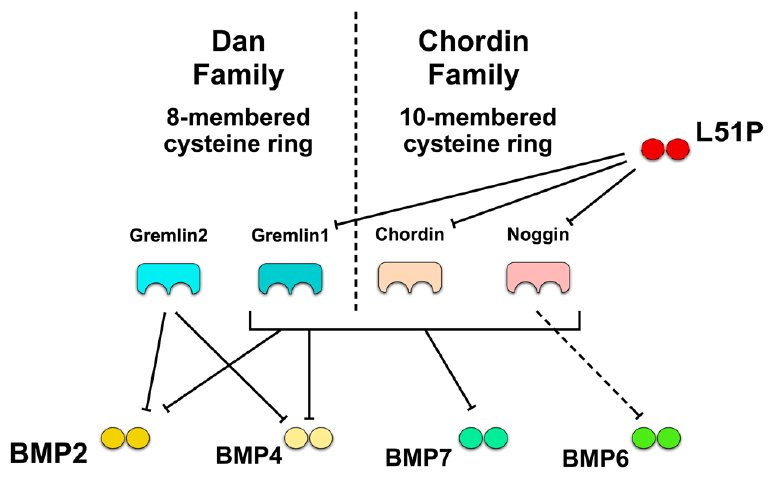
Spinal fusion surgery is a common treatment modality for various pathologic conditions of the spine, including degenerative disorders, trauma, tumours and deformities. Anterior, posterior or combined anteroposterior fusion are technically distinguished. Anterior fusion includes removal of the intervertebral disc (IVD), followed by insertion of a structural graft or spacer/cage [1]. Additional fixation with pedicle screw/rod constructs or plating are frequently used to maintain mechanical stability until osseous fusion is reached. Although lumbar fusion is an effective clinical treatment, the prevalence of nonunion and/or pseudoarthrosis ranges between 5% and 35% [2]. Nonunion is defined as lack of solid osseous fusion 1 year after surgery [3]. Predictors of failure of a union are excessive motion, metabolic abnormalities, smoking, trauma and infection. Lately, clinical observations indicate that partial removal of the disc could lead to nonunion [4,5]. Recent studies have shown that mesenchymal stromal cells (MSCs) are prevented from undergoing osteogenesis when cocultured with IVD cells [6,7]. Several bone morphogenetic proteins (BMPs) are effective stimulators of osteogenesis. BMP2 and 7, which are U.S. Food and Drug Administration approved, are used in clinics as osteoinductive factors during spinal fusion [8]. However, the applied supraphysiological doses of BMPs raise safety concerns [9]. One essential pathway to induce osteogenic differentiation is the BMP signalling pathway [10]. After the discovery of BMPs in 1965 by the surgeon Marshall R. Urist, many studies aimed to prove the beneficial effects of these proteins in the skeleton [11]. BMPs bind to serotonin/threonine kinase receptors; the activated BMP receptors phosphorylate carboxyterminally the Smad 1/5/8 complex, which is then forming heteromers with Smad 4. The complex then translocates to the nucleus, where activation or inhibition activity is initiated [12]. BMP antagonists like GREM1 (gremlin1), GREM2 (gremlin2), NOG (noggin), CHRD (hordin), FLST (follistatin), and TWSG (twisted gastrulation) bind with different affinities to BMPs, thus preventing the interaction of BMPs with their specific receptors (Fig. 1). One possible reason for the inhibition of bone formation during spinal fusion could be the presence of different BMP antagonists, which might be secreted from remaining IVD tissue. We and others have recently shown that BMP antagonists are expressed in IVD cells [4,7,13]. It has been debated whether supraphysiological doses of BMP2 should be applied in clinics. Furthermore, these high doses of BMPs often lead to adverse effects [14]. Additionally, BMPs are known for their pleiotropic character, which means that they might induce inadvertent effects in other tissues [15]. The BMP2 variant L51P could be a possible solution for the reduction of BMP dosis and efficacy in clinics. This analogue has one amino acid substituted with leucine to proline at position 51. L51P has a lower affinity to BMPR type I (BMPRI) but binds to BMPR type II (BMPRII) with similar affinity as does wild-type BMP2 [16,17]. The affinity for binding to the BMP antagonists, however, remains the same as for the wild-type [16]. Several studies have demonstrated the increased bioefficacy of exogenous and endogenous BMP2 if L51P was applied in combination in primary murine osteoblasts, MC3T3-E1, ATCDC5 and pro-myoblasts [18-20]. Hauser et al. [21] have even demonstrated improved bone formation by L51P and BMP2 in an in vivo mouse model. So far, most studies investigated the effect of BMP2 on IVD cells for regenerative purposes. It has recently been speculated that IVDs could probably demonstrate plasticity and undergo osteogenic differentiation under certain conditions or might resist to osteogenic stimuli by an unknown mechanism. Recent studies have shown chondrogenic, others even osteogenic, differentiation of IVD cells if stimulated with BMP2 [22]. However, our group demonstrated an apparent deblocking effect of L51P in the case of MSCs in coculture with IVD cells [7]. Thus, this study aimed to investigate whether exogenous stimulation of L51P and BMP2 has a stimulating effect on metabolism, gene expression and extracellular matrix (ECM) production on 3 different IVD cells, that is, nucleus pulposus cells (NPCs), annulus fibrosus cells (AFCs), and cartilaginous endplate cells (CEPCs).
MATERIALS AND METHODS
1. IVD Donor Material and Cell Isolation
IVDs were obtained from patients undergoing spinal surgery. All procedures were approved by the ethics committee of the Canton of Bern and were performed with the patients’ written consent. All tissue was processed within 24 hours after surgery. Human IVD tissue was separated into nucleus pulposus, annulus fibrosus and cartilaginous endplate tissue by an experienced surgeon. IVD cells were obtained from 5 donors from 22 to 32 years in age (31.8±3.4 years). One patient was the donor of 2 IVDs from different levels (Table 1).
Subsequently, IVD cells were isolated from their native ECM as described previously [13,23]. Briefly, cells were isolated by pronase (Roche, Basel, Switzerland) followed by collagenase type 2 (Worthington, London, UK) enzymatic digestion as previously reported. NPCs, AFCs and CEPCs were expanded in proliferation medium (low-glucose [1g/L] Dulbecco’s Modified Eagle Medium supplemented with 10% foetal bovine serum [FBS] and penicillin/streptomycin [P/S]).
2. Cell Encapsulation and Stimulation
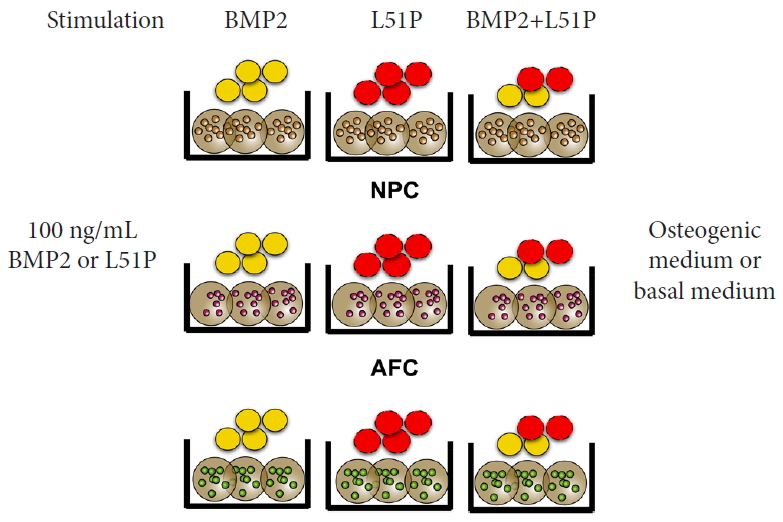
NPCs, AFCs, and CEPCs were encapsulated at a density of 4 Mio/mL in 1.2% alginate (Fluka, Sigma-Aldrich, Buchs, Switzerland). The cell-alginate suspension was dropped into 102 mM CaCl2 using a syringe (22-G needle) [24] and a syringe pump to set the flow rate (1.8 mL/min). NPC, AFC, and CEPC beads were then stimulated with 100 ng/mL (33 nM) of either BMP2, L51P, and BMP2+L51P. The cytokines were supplied in osteogenic medium (OM, α-minimal essential medium (α-MEM) supplemented with 10% FBS, P/S, 50 µg/mL Vit C, 10 nM dexamethasone and 5 mM β-glycerophosphate [all purchased from Sigma-Aldrich]) or basal medium (BM, α-MEM, supplemented with 10% FBS and P/S). The 3 control groups included BM and OM only stimulation and β-glycerophosphate control (αMEM supplemented with 10% FBS, P/S, and 5 mM β-glycerophosphate [β-GP control]). The experimental groups are depicted in Fig. 2.
3. Histological Staining of Proteoglycans
The beads were embedded on day 21 in Tissue-TEK O.C.T. compound (Sysmex, Horgen, Switzerland), frozen in liquid nitrogen, stored at -80°C and then cut into 12-µm sections using a cryostat (microm HM560, Thermo Fisher Scientific, Basel, Switzerland). Sections were then stained with Alcian Blue (Sigma-Aldrich) (pH, 1) solution containing 0.1 M CaCl2 for 30 minutes at 37°C to visualize sulfated GAGs. Generally, all solutions for the staining contained CaCl2, to avoid dissolution of the beads. After mounting in Aquatex (cat# 108562, Sigma-Aldrich) sections were examined by light microscopy.
4. Analysis of Specific Gene Expression in IVD 3D Culture With Quantitative Polymerase Chain Reaction
Snap-frozen alginate beads were pulverized using liquid nitrogen and a precooled mortar and pestle. Pulverized beads were then suspended in TRI reagent (Molecular Research Center, Cincinnati, OH, USA). The total RNA extraction method was performed as described before using a combined phase separation and silicon column method [13]. After RNA isolation, possible remaining DNA was digested using DNase I (AMPD1, Sigma-Aldrich) for 15 minutes. Reverse transcription (RT) was performed using all-in-one cDNA Synthesis SuperMix (Bimake, Houston, TX, US; distributed by LuBioScience GmbH, Lucerne, Switzerland). Real-time quantitative polymerase chain reaction (qPCR) was performed in duplicate using SYBR Green PCR master mix on a CFX96 touch qPCR system (all from BioRad, Reinach, Switzerland). Genomic (no RT) and negative controls were included to ensure the quality of RNA extractions and the PCR. The IVD cells were investigated for IVD marker genes, that is, aggrecan (ACAN), collagen type 1 (COL1) and collagen type 2 (COL2; Table 2). Microsynth Inc. (Balgach, Switzerland) synthesized all primers, all of which were tested for efficiency. qPCR was conducted using a 2-step protocol with an annealing temperature of 61°C for 40 seconds and 95°C for 15 seconds for 45 cycles. Amplicon specificity was ensured by conducting melting curve analysis after cycling. CFX-96 cycler software v 3.1 (Bio-Rad) was used for the quantification analysis of relative gene expression. The number of PCR cycles needed for each sample to reach the threshold level was recorded as the Cq value [25]. ∆∆Cq values were normalized relative to day 0 or negative control and transformed into relative mRNA values using the formula 2−∆∆Cq and 2 reference genes (18S and GAPDH, respectively) [26].
5. Mitochondrial Activity
On days 7, 14, and 21, 3 beads were collected and immersed in 50 µM resazurin sodium salt solution (Sigma-Aldrich) and incubated for 2 hours at 37°C. Mitochondrial activity of osteoblasts and MSC monolayers was measured after 21 days by incubating the cells for 30 minutes at 37°C. Relative fluorescence units were read on a plate reader at an excitation wavelength of 547 nm and an emission wavelength of 582 nm using an M5 Spectramax plate reader (Molecular Devices, LLC., distributed by Bucher Biotec, Switzerland).
6. DNA, GAG, and Hydroxyproline Content
Beads were digested overnight at 60°C in a 4.9-IE/mL papain solution containing papain from Papaya latex (Sigma-Aldrich). Three beads were digested in 450-µL papain solution. Subsequently, DNA was determined using a bisbenzimide fluorescent dye (Hoechst 3258, Sigma-Aldrich) and a standard curve from calf thymus DNA (Sigma-Aldrich). The fluorescence was measured at a wavelength of 350 nm (excitation) and 450 nm (emission) using an M5 Spectramax plate reader.
GAG content of the papain-digested samples was quantified using 1,9-dimethylmethylene blue (Sigma-Aldrich) binding assay [27]. Absorbance was measured at 600 nm using an M5 Spectramax plate reader. A standard curve obtained using chondroitin sulfate (Sigma-Aldrich) allowed calculation of the GAG concentrations.
Hydroxyproline (OHP) content was measured to determine the total amount of collagen within the papain-digested samples. To release OHP from the digested sample, hydrolysation was induced with 12M HCl at 95°C for 20 hours followed by neutralisation by adding equimolar NaOH to the sample. The solution was pretreated with a 1:1 mix of charcoal (Fluka, Sigma-Aldrich) and resin (Bio-Rad) to eliminate interfering substances or colour inhibition. The colour reaction was induced by adding chloramine T and para-dimethylaminebenzoaldehyde to the sample. A standard curve was obtained with L-4-hydroxy-proline (Sigma-Aldrich). The absorbance was measured at 560 nm using an M5 Spectramax plate reader.
7. Statistics
All assays were run in technical duplicates. Statistical analysis was performed on biological replicates, that is, donor cell repetitions. The statistical analysis was performed using PRISM 7.0 software for Mac OS X (GraphPad, La Jolla, CA, USA). Values are given as means±standard error of the mean. Shapiro-Wilk normality test for parametric distribution was performed. As some data were not-normally distributed and the sample sizes (n = 4) were nonparametric, Kruskal-Wallis test was performed to test statistical significance followed by Dunn multiple comparison test.
8. IVD Cells in 3D Culture
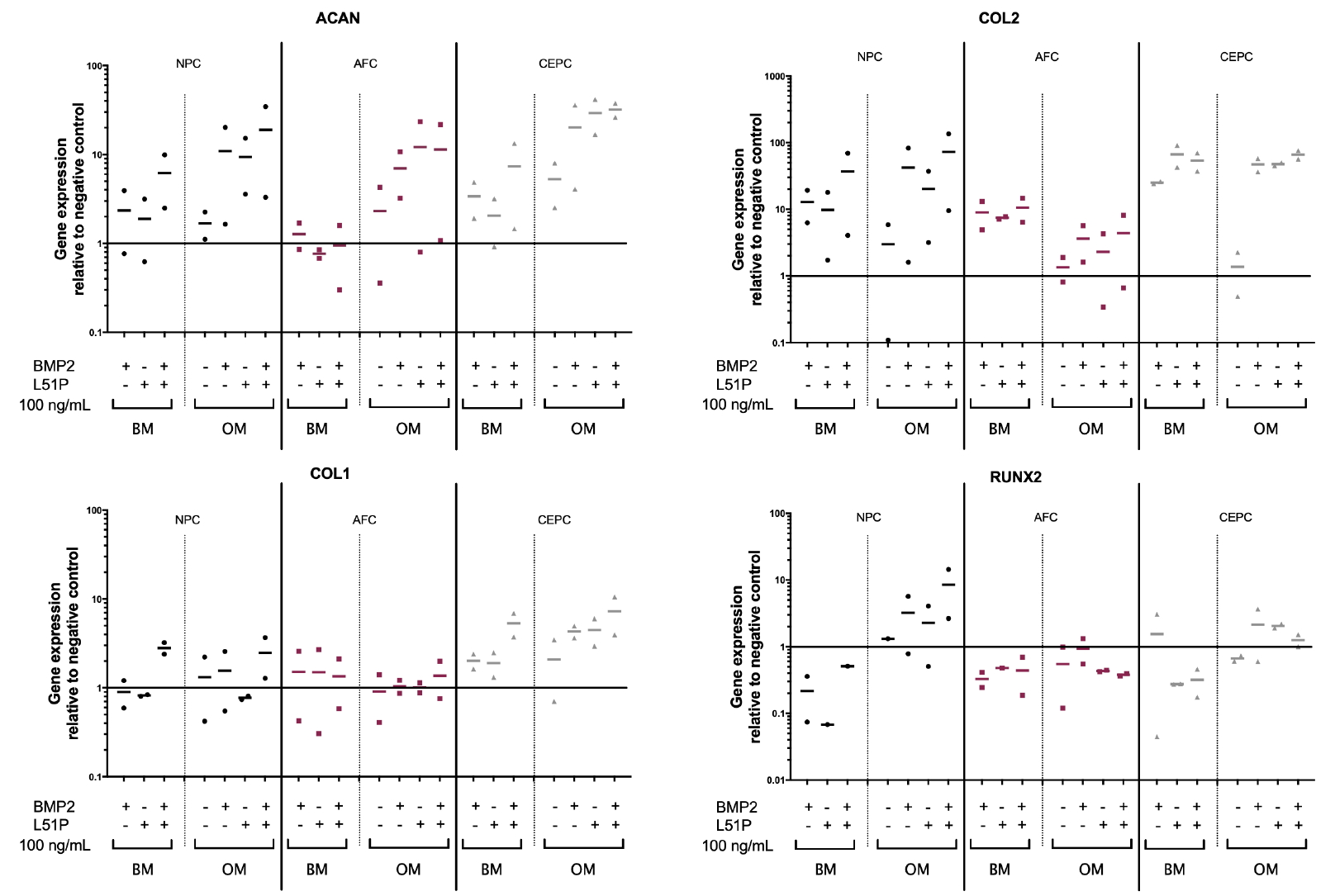
Gene expression measured from 2 donors after 7 days in culture showed a trend for upregulation of ACAN in groups with BMP2 or L51P stimulation. In all 3 cell types (NPCs, AFCs, and CEPCs) ACAN expression was the most upregulated if cells were stimulated with OM and 100-ng/mL BMP2 and L51P compared with negative control (BM only: mean NPCs, 19.0; AFCs, 11.4; and CEPCs, 31.9-fold increase). The same could be observed for COL2 expression (NPCs, 72.5; AFCs, 4.4; CEPCs, 66.1). However, COL1 changed only slightly, again with the highest COL1 expression in groups stimulated with OM supplemented with BMP2 and L51P (NPCs, 2.5; AFCs, 1.4; CEPCs, 7.2). RUNX2 also showed upregulation in NPCs stimulated with OM and L51P or BMP2 and costimulation (Fig. 3).
9. GAG, DNA, and OHP Content of IVD Cells in 3D Culture
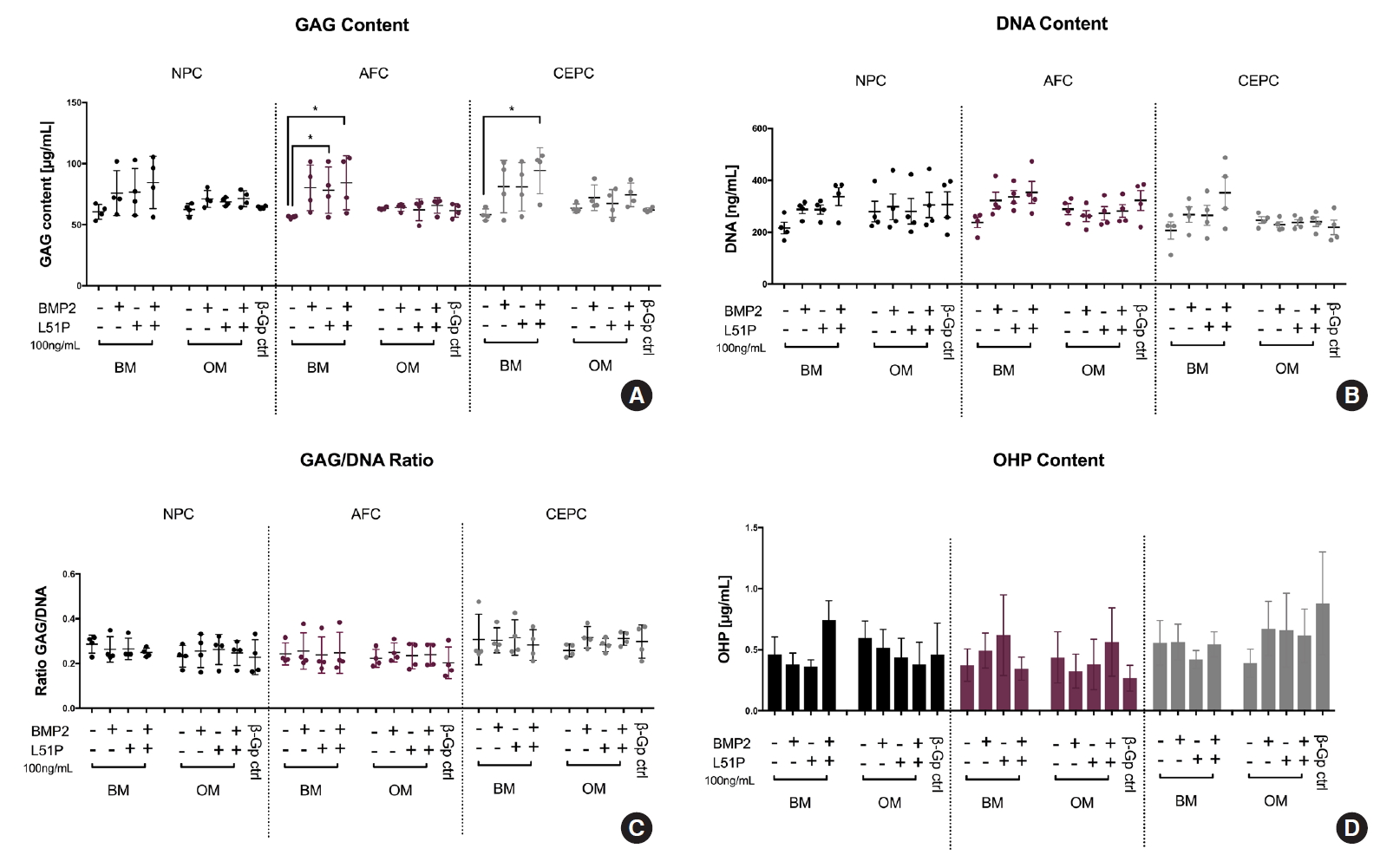
BM supplemented with BMP2 and L51P showed a significantly higher GAG content in AFC (p = 0.00347) and CEPC (p = 0.0115) compared with respective control groups in BM (Fig. 4A). Also, the DNA content of CEPCs and AFCs stimulated with BM and BMP2 + L51P showed the trend to be higher than in the respective group stimulated with BM only (Fig. 4B). The DNA/GAG ratio was relatively stable in all groups (Fig. 4C). The measured OHP content was rather low in all groups in NPCs; however, a similar trend like that in DNA and GAG content was detected. When costimulated with BMP2 and L51P, an increase was observed (Fig. 4D).
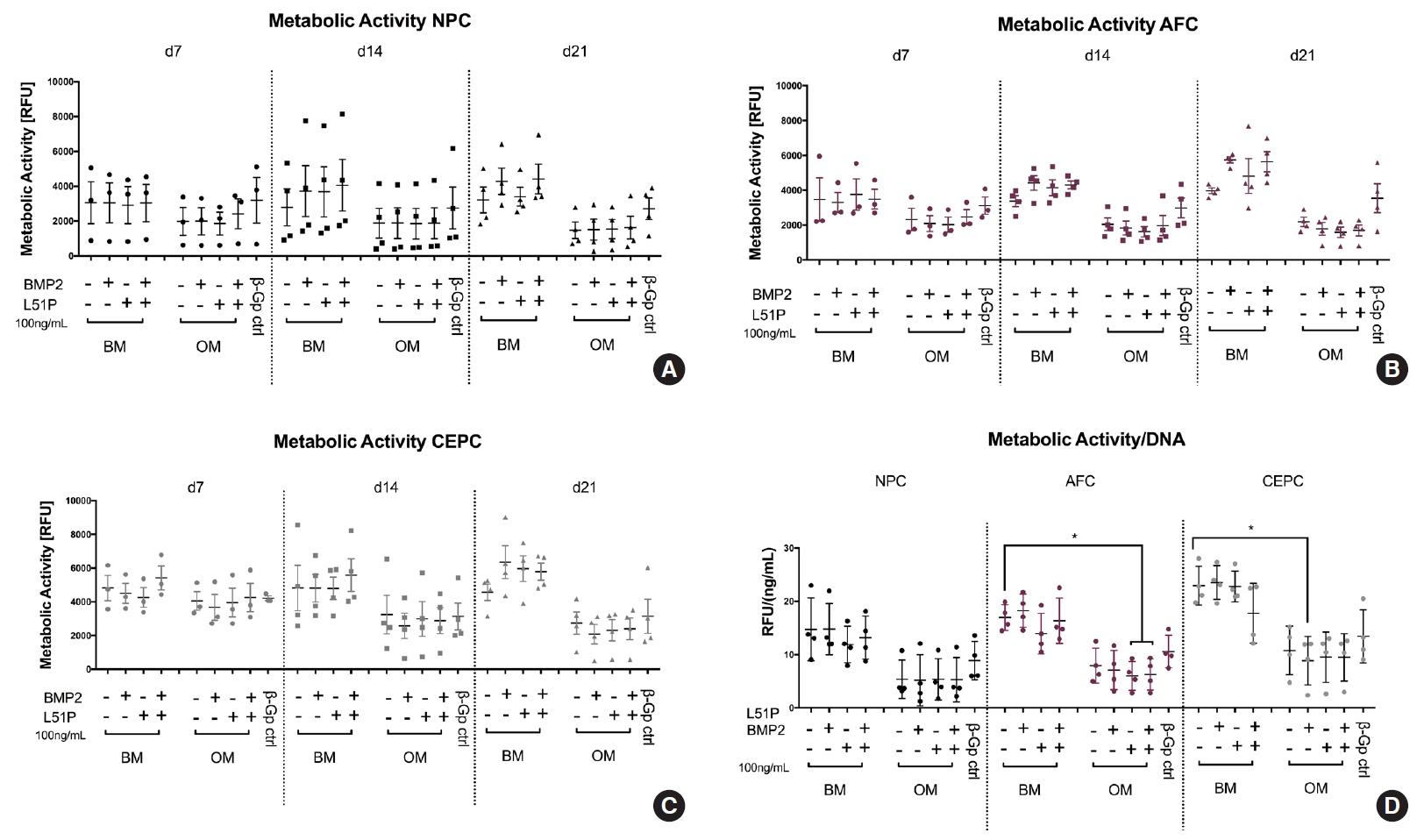
10. Metabolic Activity of IVD Cells in 3D Culture
Cell viability was measured every week (d7, d14, and d21) and normalized to the DNA content. The data demonstrated that the groups stimulated with OM possessed lower cell viability at all 3-time points. When normalized to DNA content at day 21, groups stimulated with BM showed the trend of higher cell viability than OM groups (AFCs stimulated with CM: 17.0±1.2 and AFCs stimulated with OM: 7.9±1.6. CEPCs showed overall the highest cell viability in the 3D culture (NPCs stimulated with CM: 14.7±2.9, AFCs stimulated with CM: 17.0±1.2, AFC stimulated with CM: 23.0±1.8; Fig. 5D).
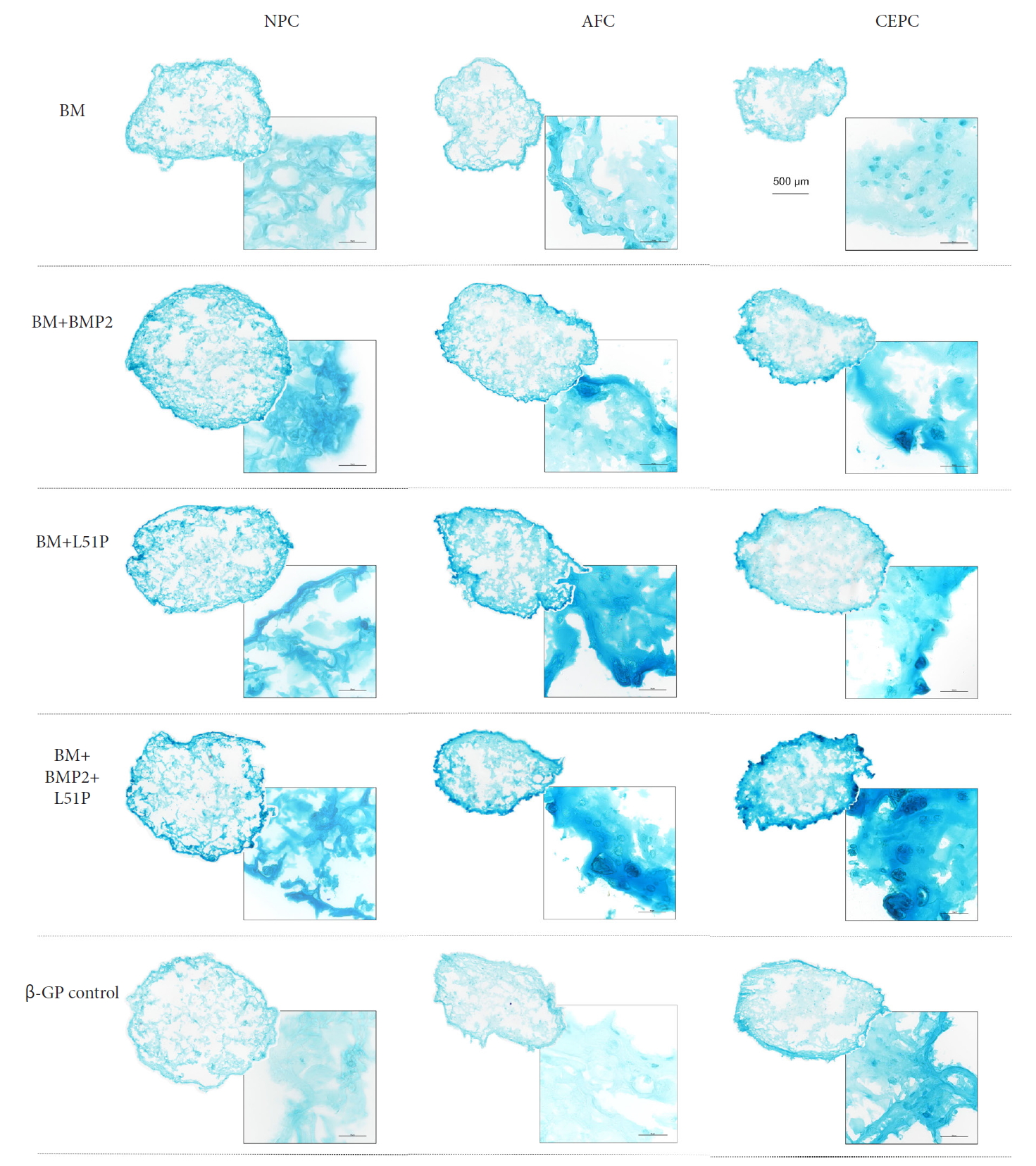
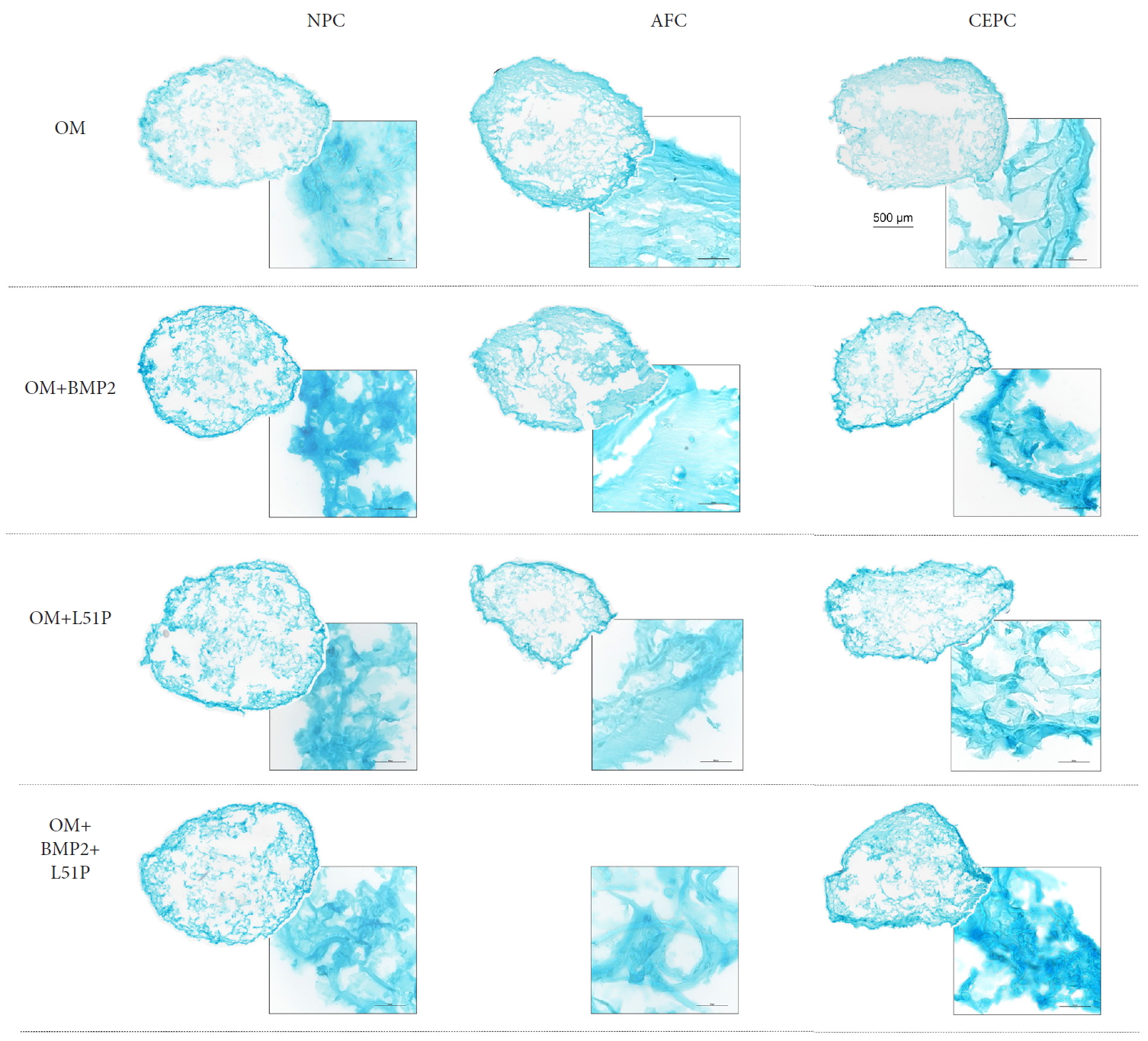
11. Visualization of Proteoglycans of IVD Cells in 3D Culture
Beads stained with Alcian blue for proteoglycan visualization showed the most robust proteoglycan staining in beads stimulated with BM and supplemented with BMP2 and L51P (Fig. 6). This was especially the case for the CEPCs group. Also, beads stimulated with BM and supplemented with BMP2 or L51P showed more proteoglycan aggregation than those in other groups (Fig. 7). Generally, groups stimulated with OM and L51P and/or BMP2 showed weaker Alcian blue staining than groups stimulated with BM and L51P and/or BMP2.
DISCUSSION
In this study, we investigated the effect of BMP2 and L51P on primary human IVD cells, that is, NPCs, AFCs, and CEPCs. So far, some studies have been conducted on the effect of L51P in combination with BMP2 on primary murine osteoblasts, MC3-T3-E1, ATCDC5 and pro-myoblasts [18-20]. However, studies on primary human cells are very rare. Tekari et al. [7] have shown that osteogenesis of human mesenchymal stem cells cocultured with human IVD cells can be rescued with the addition of L51P. Generally, all stimulations—BMP2 and L51P in CM and OM but also OM only—showed a tendency for ACAN and COL2 upregulation. Interestingly, ACAN and COL2 showed the highest expression in NPCs in both groups stimulated with L51P and BMP2 (in BM and OM) compared with the corresponding media with single treatment. COL1 expression showed the same trend in NPCs stimulated with L51P and BMP2 in OM and accordingly to this RUNX2 expression was upregulated. This finding for RUNX2 discriminated NPCs from AFCs and CEPCs under OM and could be related to their different origin during the development of NPCs. It was repeatedly demonstrated that NPCs are notochord-derived cells, which here were more responsive to OM than AFC and CEPC.
One of the main findings was also the generally higher upregulation of ACAN in all groups stimulated with OM compared with CM. ACAN and COL2 are among the most important products expressed by NPCs and were thus monitored in these cultures. Anabolic effects of ACAN and COL2 because of BMP2 or BMP7 administration have been confirmed in various studies on different species (dogs, rabbits, rats, cows, and humans) [22,28-30]. For COL1 expression, however, inconsistency prevails. Multiple groups like Kim et al. [30], Haschtmann et al. [22], and Lee et al. [31] have shown an upregulation in COL1 in human IVD 3D culture, rabbit IVD explants and rabbit NPCs, all stimulated with exogenous BMP2 (rabbit IVD explants additionally with TGF-β3). However, others like Li et al. [28] observed no changes in COL1 gene expression after stimulation of rat AFC in vitro or after BMP2/BMP7 stimulation of bovine NPCs in a 3D fibrin-hyaluronan culture [29]. Our findings showed that COL1 expression in NPCs stimulated with BMP2 and L51P was slightly upregulated. The COL1 expression level in CEPC generally showed the tendency for upregulation. Again, groups treated with BMP2 and L51P tended to show the strongest effects.
Generally, beads cultured in OM lost transparency; they start to have a cloudy appearance and become brittle. Pravdyuk et al. [32] also observed this with the cultivation of MSCs in alginate microspheres and stimulation with OM. Westhrin et al. [33] also showed that mineralization of MSCs encapsulated beads because of OM stimulation.
GAG quantification of different groups matches the observation made in gene expression analysis only partially. GAG content was the highest in groups stimulated with BMP2 and L51P. However, in contrast to gene expression at day 7, GAG content measurement after 21 days showed higher concentrations in BM groups than in OM groups. The reduction of this chondrogenic marker might be the effect of extensive β-glycerophosphate stimulation, which is contained in the OM. Continuing stimulation with the OM might decrease chondrogenic differentiation and instead induce matrix mineralization. Wu et al. [34], also observed this when stimulating ATDC5 cells with β-glycerophosphate; they confirmed early chondrogenesis with an upregulation of COL2 and ACAN and the production of a GAG-rich matrix within the first 7 days. Beta-glycerophosphate delivery after early chondrogenesis further upregulated hypertrophic markers [34]. GAG content was significantly higher in AFCs and CEPCs stimulated with BM + BMP2 and L51P than in BM control treatment. The same was observed for DNA content. Other studies have shown the beneficial effect of BMPs on cell proliferation [35]. Interestingly, IVD cells cultured in OM generally presented the lower metabolic activity to DNA ratio than cells in BM.
Previous studies using L51P in combination with BMP2 have shown improved bone formation in a rat animal large bone defect model and rat calvaria cells [16,19-21]. In these studies, no osteoinductive effect could be obtained with L51P application alone. Surprisingly, in our experiment, we observed a comparable effect of BMP2 and L51P single application in gene expression and GAG content. While the ligand-binding affinity of BMPRI to L51P is lower compared with BMP2 [16,17], the binding affinity of L51P to BMPRII is comparable to wild-type binding of BMP2. The binding affinity of L51P to BMP antagonists, such as NOG and GREM, was retained [17]. The comparable results are explained by natural BMP antagonist expression, which has recently been shown [4,13]. BMP antagonist expressed by IVD cells, which block regular BMPs, are inhibited by the presence of L51P and hence remain endogenous. BMP2 induces anabolic effects.
Newer studies have highlighted a new perspective that parts of the IVD tissue undergo osteogenic differentiation [4,22]. This ignited a debate among researchers about the possible use of BMP stimulation for IVD regeneration. The fact that IVD can ossify opens up a new direction for a possible treatment strategy in spinal fusion surgery. Many studies show the anabolic effect of BMPs on IVD, without consensus if BMPs facilitate chondrogenic or an osteogenic phenotype of the cells. We studied BMP2 and the BMP analogue L51P, which is a BMP antagonist inhibitor but not an inducer of BMP signalling, and found that NPCs, AFCs, and CEPCs tended towards a chondrogenic rather than an osteogenic phenotype expression. However, few studies have been conducted to date with the BMP2 variant L51P in human cells, and further in vitro studies are in demand. Additionally, open questions remain, such as understanding the underlying reason for L51P behaving in a similar manner to BMP2 in our experiments, or which L51P can inhibit BMP antagonists.
CONCLUSION
• Exposure of BMP2 and L51P did not show any inhibiting effects on cyto-compatibility for any of the primary IVD cells, that is, NPCs, AFCs, and CEPCs.
• Conversely, BMP2 in the combination of L51P enhanced mainly cell proliferation as revealed by an increase of DNA content, which resulted in an increased GAG production after 21 days.
• However, the GAG/DNA ratio remained unchanged over time.
• In OM, the IVD cells produced less GAG and were less metabolically active, possibly because of the onset of differentiation or inhibiting factors of chondrogenesis.
• For clinical relevance, these data confirm that application of the BMP2 analogue L51P does not negatively interfere with IVD cells but has a synergistic effect if applied in combination of BMP2 onto IVD cells.
• L51P remains an interesting therapeutic molecule for the clinical application of improved spinal fusion.