|
 |
- Search
| Neurospine > Volume 17(1); 2020 > Article |
|
|
Abstract
Objective
To determine the effect of anterior plating on postoperative dysphagia (POD) among adult patients undergoing elective anterior cervical discectomy and fusion (ACDF) for cervical spondylosis and determine the potential role of demographic and clinical characteristics in the development of POD.
Methods
Consecutive adults undergoing an elective, single-level, ACDF were randomly assigned to receive a stand-alone CoRoent Cage or a CoRoent Cage with a Helix, or Helix-Mini plate. Patients with a history of cervical spine surgery were excluded. M. D. Anderson Dysphagia Inventory and Bazaz questionnaires were completed at regular intervals for 12 months postoperatively.
Results
Twenty-five patients were recruited over a 2-year period, with 8 allocated to receive a stand-alone cage, 5 to receive a cage and Helix Mini plate, and 12 to receive a cage and Helix plate. The POD rate was 68% at 48 hours, before falling to 16% at 6 and 12 months. A longer retraction time was observed in the Helix plate group compared to the stand-alone cage group (7.88; 95% confidence interval, 0.12–15.63; p = 0.046), although there was no difference in the incidence or severity of dysphagia between cohorts at any timepoint. With the exception of body mass index, there was no difference in patients with and without dysphagia, and each of the interventions was equally efficacious with respect to clinical and radiological endpoints.
Anterior cervical discectomy and fusion (ACDF) is a standard surgical treatment for patients with symptomatic degenerative radiculopathy or myelopathy following failure of conservative management. Although the procedure is generally associated with low rates of perioperative morbidity or mortality [1], the requisite mobilization of oesophageal and paraesophageal structures has been associated with dysphagia [2].
Despite being a well-recognized complication of an anterior cervical approach [3], there is no universally accepted definition for postoperative dysphagia and, in consequence, the incidence is variably reported [3-5]. Similarly, the pathophysiological basis of this process remains incompletely understood and previous investigation of potential risk factors has revealed conflicting results. Indeed, while some have associated female sex [4,6,7], older age [5,8,9], higher body mass index (BMI) [5,6,10], smoking [11,12] and greater preoperative pain [13] with postoperative dysphagia, others have not found any relationship between these variables [3,8,9,11-15]. There is comparable variability with respect to the role of blood loss [8,9], operative time [3-6,8,10,13], and surgical level [3-6,10,12,13,16] in the pathogenesis of this complication.
Given the disagreement regarding risk factors for postoperative dysphagia following ACDF surgery, particularly with respect to the role of anterior plate augmentation, we have designed a prospective, randomized trial that sought to; (1) determine the effect of the presence and type of anterior plate augmentation on postoperative dysphagia among adult patients undergoing elective ACDF for cervical spondylosis, (2) determine the potential role of other demographic and clinical characteristics in the development of postoperative dysphagia, and (3) to determine the influence of the presence and type of anterior plate augmentation on clinical and radiological outcomes following ACDF surgery.
This study had a prospective, controlled, double-blind design with balanced (1:1:1) randomization. Consecutive adult (age ≥ 18 years) patients undergoing an elective, single-level, ACDF for cervical spondylosis were randomly assigned to receive either; a stand-alone CoRoent Interbody Cage, a CoRoent Interbody Cage with a 12-× 2.4-mm Helix Mini plate and 2 countersunk screws, or a CoRoent Small Interbody Cage with the 16-× 2.7-mm Helix plate (NuVasive, San Diego, CA, USA) secured with 4 counter-sunk screws. Eligibility was restricted to adult patients undergoing single-level surgery for degenerative cervical myelopathy or radiculopathy. Patients with impaired ability to consent, any condition that precluded them from completing symptom-based outcome questionnaires, or that may independently lead to dysphagia, and those who have undergone previous ventral cervical spine surgery were excluded (Fig. 1).
Standard block randomization was conducted with the assistance of a computer algorithm. One investigator (LM) was aware of treatment allocation prior to surgery, which was revealed to the surgeon once the surgical approach, decompression, and end-plate preparation had been completed. Surgeons were deliberately blinded to treatment allocation prior to implantation in order to avoid bias during the patient positioning, preparation, and surgical procedure.
All patients were given verbal and written information about the project before providing informed consent to be included. The study was approved by the Human Research Ethics Committee for the participating hospital (HREC-D 115/14).
All operations were performed by one of 2 experienced spinal surgeons (YYW or CT). A standard ventral cervical approach and discectomy was performed through a right hemicollar incision with the assistance of a Shadow-Line (Becton. Dickinson and Company, Franklin Lakes, NJ, USA) fixed retractor system. Stand-alone cages with or without anterior plate augmentation were then positioned according to treatment allocation.
Patient’s allocated to the cage-only cohort received a standalone cage, which is composed of Polyetheretherketone, and were sized on a patient-specific basis. This was then supplemented with either; a Helix Mini plate and 2 counter-sunk screws, or a Helix plate secured with 4 counter-sunk screws, among those randomized to receive anterior plate supplementation. There are 2 primary differences between the plates. Firstly, the Helix Mini plate is narrower and shallower (12 mm× 2.4 mm) than the larger (16 mm× 2.7 mm) Helix plate.
Demographic data were prospectively collected, as were relevant patient comorbidities and operative details, including; duration of surgery, retractor time and estimated blood loss. Data from routine preoperative imaging with magnetic resonance imaging and computed tomography were also recorded.
All patients completed the M. D. Anderson Dysphagia Inventory (MDADI) questionnaire at baseline. This tool comprises of 20 items in 4 domains (global, emotional, functional, and physical). The global assessment is a stand-alone measure, which provides a general insight into the presence of dysphagia and its effect on an individual’s daily routine. The functional, emotional and physical subscales each contain questions that are scored on a scale of 1 to 5 (strongly agree, agree, no opinion, disagree, and strongly disagree). These answers are summed and a mean score is calculated, which is then multiplied by 20 to provide a composite score that ranges from 0 (extremely low functioning) to 100 (high functioning).
A Bazaz score was also determined for all participants at baseline. This tool was developed from a cohort of patients undergoing ACDF surgery [4] and consists of 2 items, each scored on a four-point scale. It has been utilized in a large number of previous studies with similar scope and intention [2,3,15,17,18]. Consistent with previous work [18], the Bazaz score was dichotomized into patients without dysphagia (Bazaz score 1) and with dysphagia (Bazaz scores 2, 3, and 4). The visual analogue scale (VAS), Neck Disability Index (NDI), and the 12-item Short Form Survey (SF-12) were also completed at baseline. The Physical (PCS) and Mental Health Composite Score (MCS) components of the SF-12 are reported independently.
MDADI and Bazaz questionnaires were subsequently completed at 2 days, 2 weeks, 3 months, 6 months, and 12 months. Postoperative outcome was also assessed using the VAS, NDI, and SF-12, which were completed and 3, 6, and 12 months after surgery. Consistent with previous studies [19], fusion was defined by the presence of bony trabeculation across the level of surgery, and an absence of dynamic instability or bony lucency at the graft/vertebral junction. This assessment was performed by an independent consultant radiologist, who interpreted static and dynamic sagittal X-rays at 6 and 12 months after surgery. Continuous data were recorded as exact values, while dichotomous outcomes were coded after extraction.
Descriptive categorical data were summarized using absolute values and proportions, while nonparametric and parametric data were expressed using the median (range) and mean ±standard deviation, respectively.
Dichotomous or multinomial variables were assessed for independence using either a Pearson chi-square test or Fisher exact test depending on the expected cell frequencies. Adjusted standardized residuals were used to further investigate significant results and, as described by Agresti [20], standardized residuals greater than 2 were taken to be significant.
Comparisons between continuous data in dichotomous groups were carried out according to distribution. Normally distributed data, without significant outliers, were compared using an independent 2-sample t-test, whereas a Mann-Whitney test was used to compare data with a nonnormal distribution.
Comparisons of central tendency between normally distributed data with 3 or more groups in the independent variable, and no outlying values, was achieved using a 1-way analysis of variance. Post hoc analysis was conducted using the Tukey-Kramer or Games-Howell tests, according to the presence of homogeneity, or otherwise, of variances. A Kruskal-Wallis test was used to assess for differences among 3 or more groups with respect to ordinal variables or continuous data with a nonparametric distribution. Significant results were further interrogated using pairwise comparisons according to Dunn procedure. A Bonferroni correction for multiple comparisons was made and the adjusted p-values are presented.
Statistical analyses were conducted with IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). Graphs were produced using GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA) and Microsoft Excel 2016 (Redmond, WA, USA). A p-value of < 0.05 was considered to be statistically significant.
A total of 25 consecutively presenting patients were recruited into this study over a period of 2 years, with 8 patients (32.00%) allocated to receive a stand-alone cage, 5 (20.00%) to receive a cage and Helix Mini plate, and 12 (48.0%) to receive a cage and Helix plate. All patient’s tolerated surgery well, and there were no significant operative or perioperative complications. After 2 years, the trial as halted due to slow recruitment as a consequence of the increasing acceptance of disc arthroplasty in this cohort of patients.
The mean age and BMI of included patients were 55.79±12.66 years and 28.24±5.48 kg/m2, respectively. A majority (68%, n = 17) of participants were female and 7 patients (28.00%) had a history of smoking. Nearly one-quarter (24.00%) of patients had a history of hypertension, 3 patients (12.0%) were diabetic and the median American Society of Anesthesiologists (ASA) physical status classification grade was 2.0 (1.0–3.0).
The C5/6 (48.0%, n = 12) and C6/7 (44.0%, n = 11) levels were the most frequently operated upon, and the mean surgical time was 54.04±10.49 minutes. The fixed retraction system was in situ for a mean of 28.71±7.50 minutes, and the mean total anesthetic time was 74.16±12.26 minutes. The mean estimated blood loss was 80.42±95.85 mL.
Rates of preoperative dysphagia were very low, with a median score of 1 (1–3) on both the Bazaz liquids and solids scales. Similarly, the median global MDADI score was 5 (3–5) and the mean composite MDADI score was 90.36±9.11 at baseline. Mean NDI, SF physical component score, and SF mental component scores were 18±9.09, 38.54±8.68, and 43.39±10.55, respectively. The baseline characteristics of included patients are detailed in Table 1.
There was no significant difference in age, sex, BMI, smoking status, degree of preoperative pain, surgical time, total time under intubation, or operative level among the 3 groups (Table 2). However, a significant difference was observed in mean retraction time (p = 0.03), with a Tukey-Kramer post hoc analysis revealing that retraction time was greater in the Helix plate group compared to the stand-alone plate group (7.88; 95% confidence interval [CI], 0.12–15.63; p = 0.046). A difference in baseline Bazaz solids score was also detected. Pairwise comparisons with Bonferroni correction for multiple comparisons revealed a significant difference in this variable between patients in the Helix plate and cage-only groups (p = 0.04). This was reflected by a similar difference in the overall proportion of patients complaining of dysphagia of any severity between groups, with investigation of adjusted standardized residuals indicating that the increased proportion of patients with dysphagia at baseline in the stand-alone cage cohort was significant and accounted for the observed disparity.
A sensitivity analysis was performed with exclusion of the 3 patients in the stand-alone cage group with preoperative dysphagia to determine whether this baseline disparity in swallowing function may have concealed a significant reduction in dysphagia in this group at follow-up. However, exclusion of these patients did not result in a difference in dysphagia rates between groups at 48 hours (p = 0.07), 14 days (p = 0.15), 3 months (p = 0.32), or 6 months (p = 0.58).
The overall rate of postoperative dysphagia was 68% (n = 17) on postoperative day 2, before falling to 60% (n = 15) at 2 weeks, 24% (n = 6) at 3 months, and 16% (n = 4) at 6 and 12 months. There were no significant differences in the rate of postoperative dysphagia, Bazaz solids score, Bazaz liquids score, MDADI Global Assessment or MDADI Composite Assessment between cohorts at any timepoints (Fig. 2, Supplementary Table 1). However, the nonsignificant increase in surgical time (63.75±8.62 minutes) among those with, compared to those without (53.17±10.19), dysphagia at 12 months (p = 0.07) requires further investigation in future research.
With the exception of BMI, for which a significant difference was observed between the mean value among patients with (23.72±2.84) compared to those without (29.74±5.35) (p = 0.02) dysphagia at 3 months, there was no difference between age, sex distribution, smoking history, comorbidities, preoperative dysphagia, preoperative pain score, ASA physical status classification, surgical time, intubation time, retraction time, operative level, estimated blood loss, or the presence of anterior plate augmentation among patients with and without dysphagia at any timepoint (Supplementary Table 2A-E).
Each of the interventions was equally efficacious in alleviating symptoms of neck and arm pain, with no significant differences observed in VAS-Arm, VAS-Neck, NDI, SF PCS or SF MCS at any of the follow-up timepoints. All patients achieved bony fusion at 12 months.
Estimates of the incidence of dysphagia following ACDF are variable [5,7,12,14,16], which is multifactorial in etiology. Firstly, there is no universally accepted method for detecting postoperative dysphagia, nor any consensus definition for this complication. Similarly, estimates of dysphagia incidence are highly sensitive to changes in the duration of time that has elapsed since surgery, with most investigators observing a reduction in dysphagia over time [4,6]. Furthermore, it is clear that there is a poor correlation between patient-reported dysphagia surveys and medical records [21], which suggests that the rate of dysphagia may be considerably underreported among retrospective studies that derive incidence estimates from hospital records. Finally, although patient- and surgery-related risk factors for postoperative dysphagia are a source of contention, it seems likely that some variation in the incidence of dysphagia relates to heterogeneity in sample populations and surgical techniques between studies.
In this prospectively conducted, double-blind randomized study, which employed a number of patient-reported outcome measures, the overall rate of dysphagia was 68% (n = 17) on postoperative day 2, before falling to 60% (n = 15) at 2 weeks, 24% (n = 6) at 3 months, and 16% (n = 4) at 6 and 12 months. These findings are consistent with those of previous studies that utilized similar outcome measures and follow-up regimens [4,6].
Although postoperative dysphagia is a well-recognized complication of ventral cervical spine surgery, the pathophysiological and anatomical basis of this condition, as well as the role of various risk factors and preventative strategies, remains incompletely understood.
Some authors have proposed that relatively smaller neck anatomy and differences in swallowing physiology, particularly with respect to swallowing velocity [22], may result in an increased risk of dysphagia among women undergoing ACDF. This is supported by the results of at least 6 studies [4,6,7,11,12], which have all recorded increased rates of dysphagia among female patients. We did not observe any difference in dysphagia rates between patients of different gender at any timepoint, which is concordant with a large number of previous reports and [9,13,14,18], therefore, the role of gender in the generation of postoperative dysphagia after ACDF remains unclear.
There is similar debate surrounding the relationship between age and postoperative dysphagia following ACDF. Consistent with a number of previous studies [3,4,6,7,11,13,15,16], we did not observe a correlation between age and the rate or severity of dysphagia at any timepoint. These results are contradicted by the findings of 2 prospective studies [5,23] and 3 retrospective studies [8,9,24] which have identified older age as a risk factor for postoperative dysphagia.
A large number of studies have reported higher rates of postoperative dysphagia among patients receiving anterior plate augmentation following single- and multilevel ACDF when compared to those receiving a ‘stand-alone’ cage [25], or a ‘zeroprofile’ cage-plate device [17,26]. This has been attributed to the plate’s physical profile leading to mass effect on the adjacent esophagus, increased dissection and oesophageal retraction associated with plate positioning and screw fixation and reduced postoperative cervical motion [27]. Additionally, it has been suggested that the formation of scar tissue around ventral plate systems may lead to delayed dysphagia [18], which is supported by the finding of extensive adhesions between the esophagus and anterior plate in a series of 31 patients who underwent elective plate removal due to persistent dysphagia [28]. Importantly, the vast majority (87.1%) of these patients experienced a significant improvement in symptoms after surgery [28]. Finally, some have proposed that anterior plating is associated with higher rates of adjacent segment ossification, which may result in chronic dysphagia [17], although this remains conjectural. Despite this, many other studies [4-6,13,29], including our own, did not observe any association between anterior plate augmentation and postoperative dysphagia, with others [30] suggesting that any potential difference is likely to dissipate within the first few months after surgery.
The varied results of previous investigations into the relationship between anterior plate augmentation and postoperative dysphagia have provoked interest in the potential role of plate profile and composition as an explanation for the observed heterogeneity. Theoretically, the advantages of this lower plate profile include reduced mass effect on the esophagus and less intraoperative retraction required to position the implant. Secondly, the larger number of screws required to secure the Helix plate is associated with more interrupted surface contour. It has been hypothesised that this may be associated with a larger amount of postoperative scarring and, in this way, could contribute to increased rates of dysphagia [18]. Three prospective [17,18,31] and 3 retrospective cohort studies [8,26,32] have suggested that lower-profile plates or ‘zero-profile’ cage-plate systems are superior to standard anterior plate devices with respect to postoperative dysphagia rates. Among 156 consecutively enrolled patients undergoing single-level ACDF, Lee et al. [18] observed that those receiving anterior augmentation with the thicker and wider Atlantis plate (Medtronic, Minneapolis, MN, USA) had higher rates of postoperative dysphagia than those receiving the smaller Zephir plate (Medtronic) at 2 years after surgery (14.26% vs. 0%, p< 0.04). In a similar study of 89 patients, Miao et al. [31] reported a lower rate of postoperative dysphagia among patients treated with a ‘zero-profile’ plate-cage construct compared with a cage and anterior titanium plate, although the statistical significance, or otherwise, of this observed difference was not reported. Hofstetter et al. [26] reported similar results, with 7 patients (20.0%) treated with an anterior plate experiencing dysphagia, compared to 1 patient (2.86%) treated with a ‘zero-profile’ plate-cage construct (p = 0.027). These results are consistent with those obtained by Qi et al. [32], Yang et al. [17], and Zeng et al. [8], who all reported higher rates of dysphagia in patients treated with anterior plating compared with zero-profile plate-cage constructs or stand-alone anchored spacers. In contrast, neither Chin et al. [12], nor Vanek et al. [33], found any association between plate prominence or the use of anterior plating compared with a ‘zero-profile’ plate-cage construct, and postoperative dysphagia, respectively. These findings are consistent with the results of the presently reported study and, therefore, the role of ventral plate augmentation in the pathogenesis of postoperative dysphagia requires further clarification in larger prospective studies.
The majority of previous studies have not observed any association between operative time [4-8,12-14,16], or retraction time [7], and rates of postoperative dysphagia. Our findings were consistent with these results, although the nonsignificant increase in surgical time among those with, compared to those without, dysphagia at 12 months requires further investigation, particularly given contrary findings among 5 previous studies [3,10,13,29,34]. These discrepant results may relate to a substantially lower mean retraction time in our cohort (54.04 minutes) compared with the aforementioned studies (121.53 minutes), which may have concealed a clinically and statistically significant difference.
Differences in regional anatomy in the cervical spine, and its relevance to swallowing physiology, has led to the hypothesis that each surgical level may be associated with different risks of postoperative dysphagia. The location of the internal branch of the superior laryngeal nerve, and its innervation of the pharyngeal mucosa in the region of C3 or C4 [35], has been used to explain the observation that ACDF surgery involving the upper cervical levels is often associated with higher rates of postoperative dysphagia [3,4,10]. Nevertheless, a number of other studies [4-6,12,13,16], including this one, have not found any relationship between surgical level and dysphagia and, as such, this also requires further elucidation in future research.
Although each of the interventions was equally efficacious in alleviating symptoms of arm and neck pain, and in achieving bony fusion, a recent systematic review and meta-analysis [36] has demonstrated that anterior plate augmentation is associated with significantly higher fusion rates (odds ratio [OR], 1.98; 95% CI, 1.16–3.37, I2= 0%) and lower odds of cage subsidence (OR, 0.31; 95% CI, 0.18–0.52; I2= 28%). As was the case in our study, both plated and nonplated constructs were associated with an improvement in functional outcomes, without any clinically significant difference in postoperative arm- or neckpain between groups.
The presently described study has a number of strengths. It had a prospective, randomized design, with surgeons blinded to allocation prior to implantation in order to avoid bias during the patient positioning, preparation and surgical procedure, and patients remained blinded for the entire study duration. Groups were well matched, inclusion and exclusion criteria were not overly restrictive and standard surgical techniques were utilized, which provides the study with external validity. There were no losses to follow-up, and the follow-up was relatively prolonged, with data collected at multiple timepoints, thereby permitting investigation of temporal changes in postoperative dysphagia. Multiple, patient-reported and validated outcome measures were utilised, which captured information pertaining to dysphagia, pain, functional and emotional status. Some may protest the absence of objective outcome assessment tools, such videofluoroscopic swallow evaluations, which provide information regarding the location and extent of mechanical obstruction. However, Frempong-Boadu et al. [16] have demonstrated a poor correlation between radiographic evidence of swallowing dysfunction and patient symptoms, and, therefore, patient-reported outcome measures have been favored in previous studies of similar scope and intention.
The limitations of this study should also be acknowledged. First and foremost, the increasing acceptance of cervical disc arthroplasty for patients requiring surgery for single-level spondylotic disease resulted in slow recruitment and premature termination of this study. In consequence, our sample size may have been insufficient to detect a difference in dysphagia between groups and limited our capacity to investigate risk factors for postoperative dysphagia. It was also difficult to examine other clinical and radiological endpoints between groups. Nevertheless, we did demonstrate the safety and effectiveness of each of the 3 presented surgical strategies, and provided estimates of dysphagia incidence and natural history that are consistent with those produced by previous similar studies [4,6]. It is also important to note that the limitation of our study to single-level ACDF surgery for degenerative pathology may have masked potential benefits of each of the investigated strategies among patients requiring multilevel surgery or treatment for nondegenerative pathology.
This study demonstrates that dysphagia is a common consequence of ACDF surgery. We observed that the placement of a Helix plate was associated with significantly longer retraction time, but that this does not result in higher rates of dysphagia, nor were any differences observed in other clinical or radiological outcome measures between cohorts. When taken with the results of previous research, it is clear that the pathophysiology of, and risk factors for, postoperative dysphagia following ACDF remain poorly understood and larger prospective studies are required to elucidate the role of both patient-specific and surgical factors in the etiology of this complication. This may result in the emergence of perioperative and operative strategies to reduce swallowing dysfunction and improve outcomes for patients requiring ventral cervical surgery.
CONFLICT OF INTEREST
LM was an employee of NuVasive and YYW was an education consultant for NuVasive and Depuy Synthes at the time that this research was undertaken. The remaining authors have no financial or other interests related to this paper to disclose.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1 and 2A-E can be found via https://doi.org/10.14245/ns.1938446.223.
Supplementary Table 2A.
Differences in patients with and without dysphagia at 48 hours postoperatively
Supplementary Table 2B.
Differences in patients with and without dysphagia at 2 weeks postoperatively
Supplementary Table 2C.
Differences in patients with and without dysphagia at 3 months postoperatively
Supplementary Table 2D.
Differences in patients with and without dysphagia at 6 months postoperatively
Supplementary Table 2E.
Differences in patients with and without dysphagia at 12 months postoperatively
Fig. 2.
(A) Frequency of postoperative (preop) ysphagia between cohorts at each follow-up timepoint. (B) Comparison of median (range) Bazaz solids score between cohorts at each follow-up timepoint. (C) Comparison of median (range) MDADI global score between cohorts at each follow-up timepoint. (D) Comparison of median (range) MDADI composite score between cohorts at each follow-up timepoint. MDADI, M. D. Anderson Dysphagia Inventory.
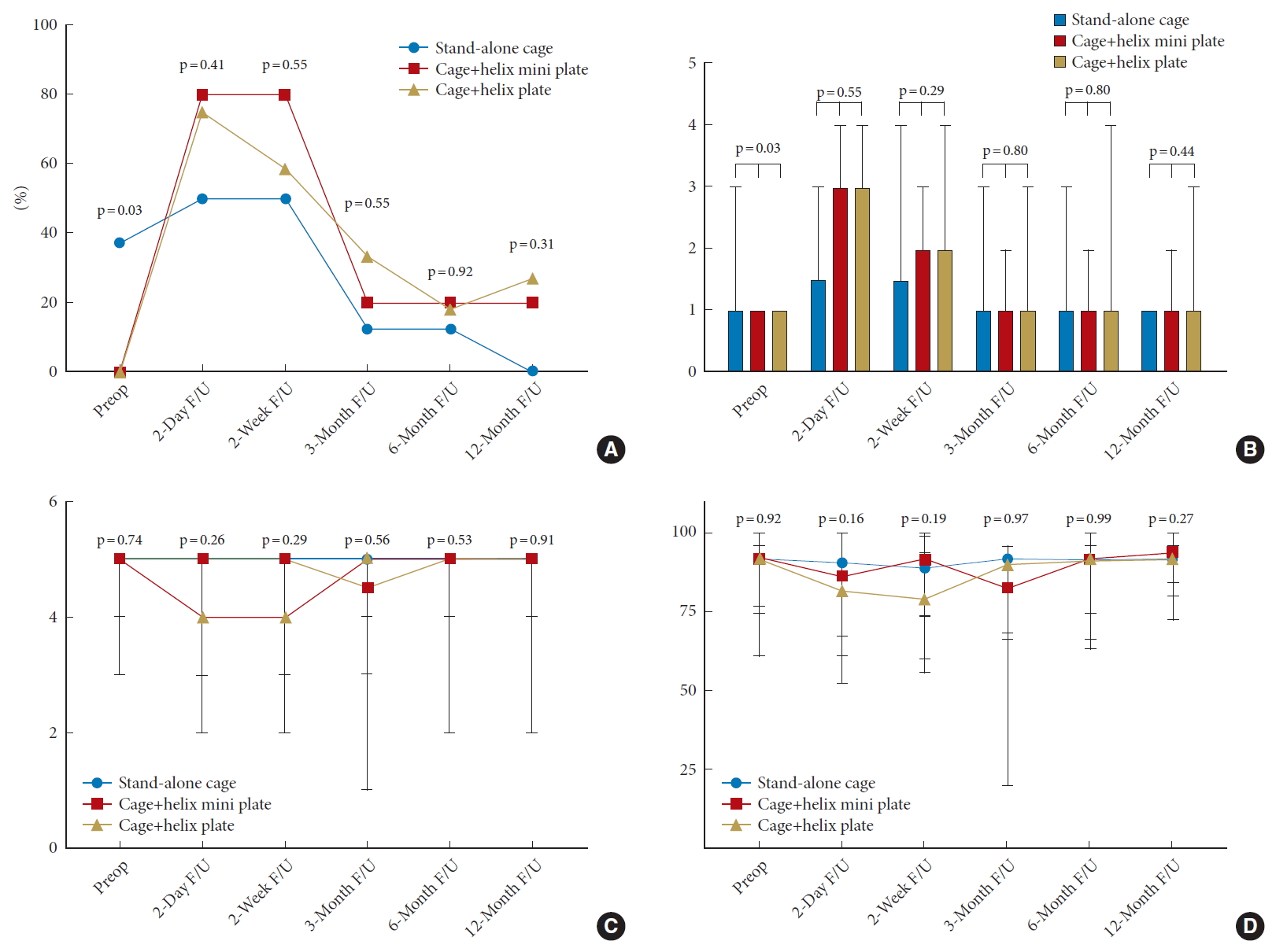
Table 1.
Baseline characteristics of entire cohort
Table 2.
Comparison of baseline characteristics between cohorts
| Variable | Stand-alone Cage (n=8) | Narrow Plate (n=5) | Wide Plate (n=12) | p-value |
|---|---|---|---|---|
| Age (yr) | 55.60 ± 12.79 | 50.31 ± 16.44 | 58.20 ± 11.29 | 0.52 |
| Sex, male:female | 0:8 | 2:3 | 3:9 | 0.77 |
| Body mass index (kg/m2) | 30.58 ± 4.02 | 24.43 ± 3.49 | 28.46 ± 6.27 | 0.16 |
| Smoking | 0.90 | |||
| Current | 6 (75.0) | 4 (80.0) | 8 (66.7) | |
| Previous | 1 (12.0) | 1 (20.0) | 3 (25.0) | |
| Life-time nonsmoker | 1 (12.0) | 0 (0) | 1 (8.3) | |
| Comorbidities | ||||
| Diabetes | 0 (0) | 1 (20.0) | 2 (16.7) | 0.47 |
| Hypertension | 2 (25.0) | 1 (20.0) | 3 (25.0) | 0.94 |
| Preoperative dysphagia | ||||
| Patients with dysphagia | 3 (0.4) | 0 (0) | 0 (0) | 0.03* |
| Bazaz liquids score | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 1.0 (1.0–2.0) | 0.36 |
| Bazaz solids score | 1.0 (1.0–3.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.03* |
| Global MDADI | 5.0 (4.0–5.0) | 5.0 (3.0–5.0) | 5.0 (3.0–5.0) | 0.74 |
| Composite MDADI | 91.45 ± 7.60 | 89.47 ± 7.29 | 90.00 ± 3.21 | 0.92 |
| Preoperative pain | ||||
| VAS score: arm pain | 5.5 (0–10.0) | 5.0 (4.0–8.0) | 5.0 (0–10.0) | 0.98 |
| VAS score: neck pain | 6.0 (0–7.0) | 3.0 (3.0–8.0) | 6.5 (0–9.0) | 0.58 |
| Neck Disability Index | 17.63 ± 9.74 | 21.20 ± 8.87 | 16.92 ± 9.24 | 0.69 |
| SF PCS | 39.48 ± 6.54 | 37.73 ± 14.19 | 38.25 ± 7.93 | 0.93 |
| SF MCS | 44.62 ± 9.99 | 41.95 ± 10.73 | 43.17 ± 11.64 | 0.91 |
| ASA PS classification grade | 2.0 (2.0–3.0) | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 0.80 |
| Surgical time (min) | 49.25 ± 10.79 | 52.40 ± 11.76 | 57.92 ± 9.03 | 0.18 |
| Intubation time (min) | 70.88 ± 14.07 | 66.00 ± 12.75 | 79.75 ± 8.30 | 0.06 |
| Retraction time (min) | 25.13 ± 5.28 | 25.00 ± 3.81 | 33.00 ± 8.17 | 0.03* |
| Operative level | 0.75 | |||
| C4/5 | 1 (12.5) | 0 (0) | 0 (0) | |
| C5/6 | 4 (50.0) | 3 (60.0) | 5 (41.7) | |
| C6/7 | 3 (37.5) | 2 (40.0) | 6 (50.0) | |
| C7/T1 | 0 (0) | 0 (0) | 1 (8.3) | |
| Estimated blood loss (mL) | 55.0 (3.0-100) | 100 (40.0-500.0) | 60.0 (0-500.0) | 0.38 |
REFERENCES
1. Gutman G, Rosenzweig DH, Golan JD. Surgical treatment of cervical radiculopathy: meta-analysis of randomized controlled trials. Spine (Phila Pa 1976) 2018 43:E365-72.


2. Riley LH 3rd, Vaccaro AR, Dettori JR, et al. Postoperative dysphagia in anterior cervical spine surgery. Spine (Phila Pa 1976) 2010 35(9 Suppl):S76-85.


3. Rihn JA, Kane J, Albert TJ, et al. What is the incidence and severity of dysphagia after anterior cervical surgery? Clin Orthop Relat Res 2011 469:658-65.


4. Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976) 2002 27:2453-8.


5. Smith-Hammond CA, New KC, Pietrobon R, et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine (Phila Pa 1976) 2004 29:1441-6.


6. Lee MJ, Bazaz R, Furey CG, et al. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J 2007 7:141-7.


7. Papavero L, Heese O, Klotz-Regener V, et al. The impact of esophagus retraction on early dysphagia after anterior cervical surgery: does a correlation exist? Spine (Phila Pa 1976) 2007 32:1089-93.


8. Zeng JH, Zhong ZM, Chen JT. Early dysphagia complicating anterior cervical spine surgery: incidence and risk factors. Arch Orthop Trauma Surg 2013 133:1067-71.



9. Fehlings MG, Smith JS, Kopjar B, et al. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study. J Neurosurg Spine 2012 16:425-32.


10. Kalb S, Reis MT, Cowperthwaite MC, et al. Dysphagia after anterior cervical spine surgery: incidence and risk factors. World Neurosurg 2012 77:183-7.


11. Siska PA, Ponnappan RK, Hohl JB, et al. Dysphagia after anterior cervical spine surgery: a prospective study using the swallowing-quality of life questionnaire and analysis of patient comorbidities. Spine (Phila Pa 1976) 2011 36:1387-91.


12. Chin KR, Eiszner JR, Adams SB Jr. Role of plate thickness as a cause of dysphagia after anterior cervical fusion. Spine (Phila Pa 1976) 2007 32:2585-90.


13. Riley LH 3rd, Skolasky RL, Albert TJ, et al. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976) 2005 30:2564-9.


14. Mendoza-Lattes S, Clifford K, Bartelt R, et al. Dysphagia following anterior cervical arthrodesis is associated with continuous, strong retraction of the esophagus. J Bone Joint Surg Am 2008 90:256-63.


15. Olsson EC, Jobson M, Lim MR. Risk factors for persistent dysphagia after anterior cervical spine surgery. Orthopedics 2015 38:e319-23.


16. Frempong-Boadu A, Houten JK, Osborn B, et al. Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech 2002 15:362-8.


17. Yang H, Chen D, Wang X, et al. Zero-profile integrated plate and spacer device reduces rate of adjacent-level ossification development and dysphagia compared to ACDF with plating and cage system. Arch Orthop Trauma Surg 2015 135:781-7.



18. Lee MJ, Bazaz R, Furey CG, et al. Influence of anterior cervical plate design on Dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech 2005 18:406-9.


19. Buchowski JM, Liu G, Bunmaprasert T, et al. Anterior cervical fusion assessment: surgical exploration versus radiographic evaluation. Spine (Phila Pa 1976) 2008 33:1185-91.


20. Agresti A. An introduction to categorical data analysis. 2nd ed. Hoboken (NJ): John Wiley & Sons; 2007.
21. Edwards CC 2nd, Karpitskaya Y, Cha C, et al. Accurate identification of adverse outcomes after cervical spine surgery. J Bone Joint Surg Am 2004 86:251-6.


22. Yew AY, Nguyen MT, Hsu WK, et al. Quantitative risk factor analysis of postoperative dysphagia after anterior cervical discectomy and fusion (ACDF) using the Eating Assessment Tool-10 (EAT-10). Spine (Phila Pa 1976) 2019 44:E82-E88.


23. Lovasik BP, Holland CM, Howard BM, et al. Anterior cervical discectomy and fusion: comparison of fusion, dysphagia, and complication rates between recombinant human bone morphogenetic protein-2 and beta-tricalcium phosphate. World Neurosurg 2017 97:674. -83. e1.


24. Singh K, Marquez-Lara A, Nandyala SV, et al. Incidence and risk factors for dysphagia after anterior cervical fusion. Spine (Phila Pa 1976) 2013 38:1820-5.


25. Li Z, Zhao Y, Tang J, et al. A comparison of a new zero-profile, stand-alone Fidji cervical cage and anterior cervical plate for single and multilevel ACDF: a minimum 2-year follow-up study. Eur Spine J 2017 26:1129-39.



26. Hofstetter CP, Kesavabhotla K, Boockvar JA. Zero-profile anchored spacer reduces rate of dysphagia compared with ACDF with anterior plating. J Spinal Disord Tech 2015 28:E284-90.


27. Yang Y, Ma L, Liu H, et al. Comparison of the incidence of patient-reported post-operative dysphagia between ACDF with a traditional anterior plate and artificial cervical disc replacement. Clin Neurol Neurosurg 2016 148:72-8.


28. Fogel GR, McDonnell MF. Surgical treatment of dysphagia after anterior cervical interbody fusion. Spine J 2005 5:140-4.


29. Saville P, Vaishnav AS, McAnany S, et al. Predictive factors of post-operative dysphagia in single-level anterior cervical discectomy and fusion (ACDF). Spine (Phila Pa 1976) 2018 https://doi.org/10.1097/BRS.0000000000002865. [Epub].

30. Fisahn C, Schmidt C, Rustagi T, et al. Comparison of chronic dysphagia in standalone versus conventional plate and cage fusion. World Neurosurg 2018 109:e382-8.


31. Miao J, Shen Y, Kuang Y, et al. Early follow-up outcomes of a new zero-profile implant used in anterior cervical discectomy and fusion. J Spinal Disord Tech 2013 26:E193-7.


32. Qi M, Chen H, Liu Y, et al. The use of a zero-profile device compared with an anterior plate and cage in the treatment of patients with symptomatic cervical spondylosis: a preliminary clinical investigation. Bone Joint J 2013 95-B:543-7.


33. Vanek P, Bradac O, Delacy P, et al. Anterior interbody fusion of the cervical spine with Zero-P spacer: prospective comparative study-clinical and radiological results at a minimum 2 years after surgery. Spine (Phila Pa 1976) 2013 38:E792-E797.


34. Liu JM, Tong WL, Chen XY, et al. The incidences and risk factors related to early dysphagia after anterior cervical spine surgery: a prospective study. PloS One 2017 12:e0173364.