Perioperative Anesthesia Lean Implementation IS Associated With Increased Operative Efficiency in Posterior Cervical Surgeries at a High-Volume Spine Center
Article information
Abstract
Objective
Lean management strategies aim to increase efficiency by eliminating waste or by improving processes to optimize value. The operating room (OR) is an arena where these strategies can be implemented. We assessed changes in OR efficiency after the application of lean methodology on perioperative anesthesia associated with posterior cervical spine surgeries.
Methods
We utilized pre- and post-lean study design to identify inefficiencies during the perioperative anesthesia process and implemented strategies to improve the process. Patient characteristics were recorded to assess for differences between the 2 groups (group 1, prelean; group 2, post-lean). In the pre-lean period, key steps in the perioperative anesthesia process were identified that were amenable to lean implementation. The time required for each identified key step was recorded by an independent study coordinator. The times for each step were then compared between the groups utilizing univariate analyses.
Results
After lean implementation, there was a significant decrease in overall perioperative anesthesia process time (88.4 ± 4.7 minutes vs. 76.2 ± 3.2 minutes, p = 0.04). This was driven by significant decreases in the steps: transport and setup (10.4 ± 0.8 minutes vs. 8.0 ± 0.7 minutes, p = 0.03) and positioning (20.8 ± 2.1 minutes vs. 15.7 ± 1.3 minutes, p = 0.046). Of note, the total time spent in the OR was lower for group 2 (270.1 ± 14.6 minutes vs. 252.8 ± 14.1 minutes) but the result was not statistically significant, even when adjusting for number of operated levels.
Conclusion
Lean methodology may be successfully applied to posterior cervical spine surgery whereby improvements in the perioperative anesthetic process are associated with significantly increased OR efficiency.
INTRODUCTION
With healthcare costs reaching nearly 20% of the gross domestic product in the United States [1], pressure to control costs and improve efficiency are increasing. Methods of increasing efficiency, most notably Six Sigma and Lean [2], have spread from the manufacturing world to the healthcare sector.
Six Sigma refers to the reduction of production defects to 6 standard deviations from the mean. This was first introduced by the Motorola Corporation in order to keep up with Japanese production and quality standards. In order to compete, Motorola set a goal of producing less than 3.4 defects per 1 million products with Six Sigma. While the end goal may be obtainable in manufacturing, it seems a daunting task in medicine, a field with more variability and less predictability. However, the methodology underlying Six Sigma remains important.
Lean process strategy, first pioneered by Toyota and developed by Taiichi Ohno, seeks to achieve process improvement via the identification of specific types of manufacturing waste. The goals of lean are to (1) identify processes that consume time and effort but do not add to overall value and (2) eliminate those processes. By increasing efficiency via waste elimination and improving processes, lean management strategies aim to optimize value. A key tenet of lean lies in the belief that every step of any one process should meaningfully contribute towards the end goal. Lean was embodied by 5 key foci [3]: value, value stream, flow, pull and perfection [4]. In expanded format, Ohno’s 5 foci are to identify the value of every step, to manage the “value stream,” to make flow more efficient by using “pull” mechanisms, and to aim for perfection to reduce waste; these are the principles that may be applied toward improving efficiency.
The methodologies underlying Six Sigma and Lean are often complementary and are referred to as a single strategy: Lean Six Sigma (LSS) [4]. LSS emphasizes improving efficiency and effectiveness with data and use of the define, measure, analyze, improve, and control approach. This approach provides a useful framework for implementing quality improvement (QI) by defining a problem, measuring important relevant parameters, analyzing data, and determining the root causes of a problem, improving the process with provided solutions, and controlling a situation by assessing the effect of a solution on a focused problem.
This LSS methodology has been applied effectively across multiple medical disciplines, but to the best of our knowledge, has not yet been applied to the neurosurgical operating room (OR) [5-9]. This is an attractive target as strategies that streamline OR turnover time may increase operative productivity and improve profitability.
In the present study, we applied LSS methodology for perioperative anesthesia associated with posterior cervical spine surgeries to assess for associations with OR efficiency.
MATERIALS AND METHODS
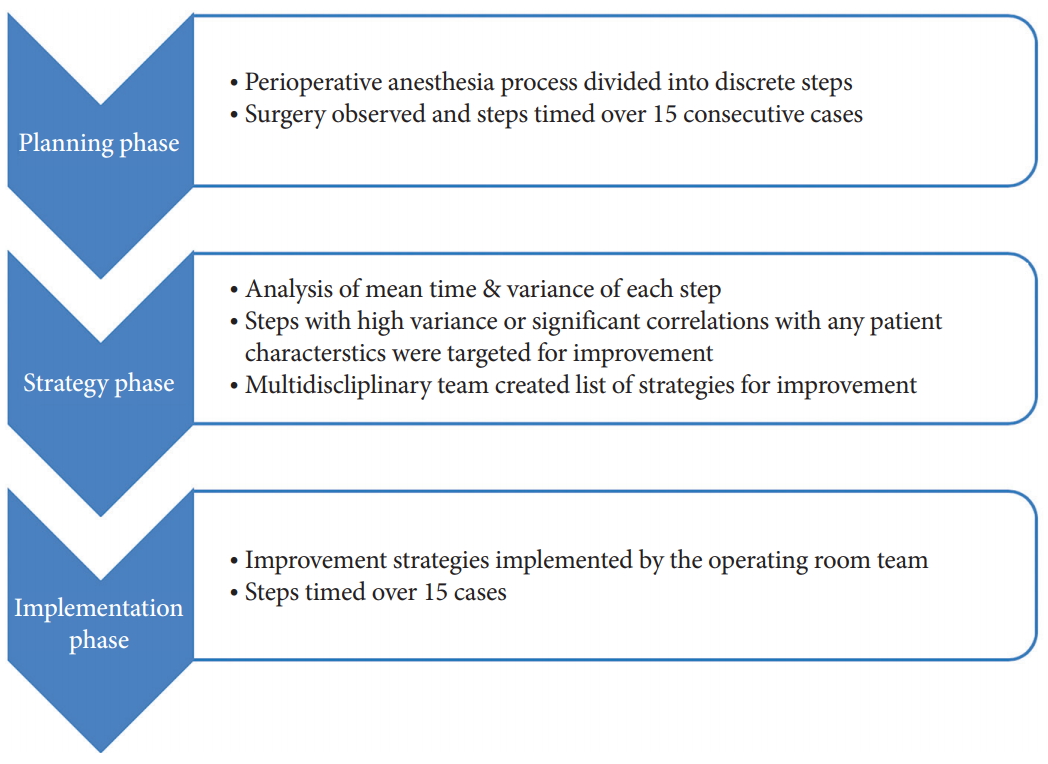
Institutional Review Board approval of University of California San Francisco and Informed Consent were not required as this study was considered a QI initiative. Between April 2017 to April 2018, we identified 30 posterior cervical spine surgeries for the planning, strategy and implementation phases of LSS methodology. Fig. 1 illustrates the overall LSS process including planning, strategy, and implementation phases. Patients were limited to those with degenerative pathology. No trauma, infectious or tumor cases were selected.
During the planning and strategy phases, the authors identified 7 key steps during the perioperative anesthesia process. These key steps were determined by a multidisciplinary team of surgeons, anesthesiologists, nurses, and technicians. Fig. 2 illustrates a detailed process map of the perioperative anesthesia process constructed during the planning and strategy phases. Fifteen consecutive surgeries (group 1 – pre-LSS implementation) were analyzed and the time and notes were collected by an independent study coordinator.
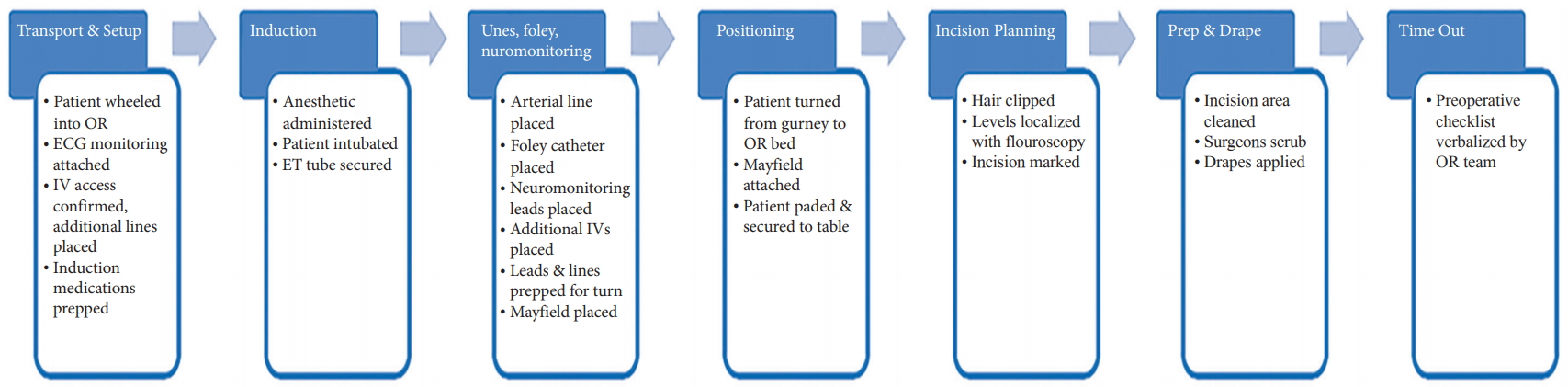
Depiction of the key steps throughout the perioperative anesthesia process. OR, operating room; ECG, electrocardiogram; IV, intravenous; ET, endotracheal.
The multidisciplinary team identified areas of waste within these steps where LSS improvements could be applied. This was determined by identifying the steps which had the largest variation in time. Moreover, root causes of time variation were identified. Thereafter, a list of LSS improvement strategies for increasing the efficiency of the perioperative anesthesia process was formulated. Fig. 3 shows the LSS improvement strategies developed to combat the identified root causes/problems within the perioperative anesthesia process.

Description of the lean improvement strategies implemented. PIV, peripheral intravenous; CRNA, certified registered nurse anesthetist; OR, operating room; A-line, arterial line; mJOA, modified Japanese Orthopaedic Association.
Subsequently, during the implementation phase, 15 surgeries were identified for LSS implementation (group 2 – post-LSS implementation) (Fig. 1), and the LSS strategies were executed. To ensure the perioperative team (e.g., preoperative nurses, anesthesia, scrub techs, patient care assists, etc.) were abreast of the LSS implementation, multiple announcements and meetings were conducted for relevant staff prior to LSS implementation.
Patient characteristics were recorded from both groups including age, sex, body mass index (BMI), modified Japanese Orthopaedic Association (mJOA) score, and American Society of Anesthesiologists (ASA) physical status classification. We also identified whether there was a resident or certified registered nurse anesthetist (CRNA) to assist the anesthesiologist. The time, in minutes, of each key anesthesia step (Fig. 2) was recorded by an independent study coordinator not directly associated with the project during the pre- (group 1, n= 15) and postimplementation periods (group 2, n= 15).
Descriptive statistics were also performed to summarize group characteristics. Univariate comparisons, consisting of Wilcoxon rank-sum tests, assessed for significant differences between groups 1 and 2. Subgroup analyses were conducted for resident and CRNA involvement as well as the type of posterior cervical surgery (laminoplasty versus laminectomy and fusion) that was performed. Two-way analysis of variance was performed on overall duration of procedure with (1) the number of operated levels and (2) whether the procedure occurred pre- or post-LSS implementation. Means and standard errors and percentages are reported as appropriate. The alpha level was set at 0.05. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA).
RESULTS
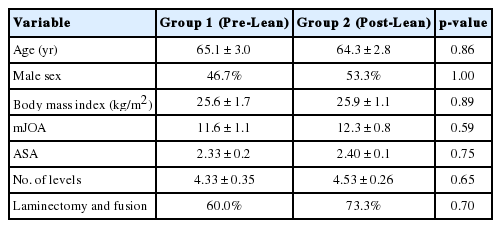
Regarding patient and surgery characteristics in the 2 groups (Table 1), there were no differences between the 2 groups with regards to age (65.1 ± 3.0 years vs. 64.3 ± 2.8 years, p = 0.86), male sex (46.7% vs. 53.3%, p = 1.00), BMI (25.6 ± 1.7 kg/m2 vs. 25.9 ± 1.1 kg/m2, p = 0.89), mJOA score (11.6 ± 1.1 vs. 12.3 ± 0.8, p = 0.59), ASA physical status classification (2.33 ± 0.2 vs. 2.40 ± 0.1, p = 0.75), type of surgery (laminectomy and fusion vs. laminoplasty) (laminectomy and fusion: 60% vs. 73.3%, p = 0.70), and number of levels operated (4.33 ± 0.35 vs. 4.53 ± 0.26, p = 0.65).
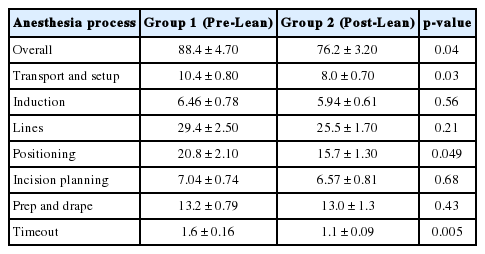
After the implementation of LSS strategies (Table 2), there was a statistically significant decrease in the amount of time taken in the overall perioperative anesthesia process (88.4 ± 4.7 minutes vs. 76.2 ± 3.2 minutes, p = 0.04). This was driven by significant decreases in the following steps: transport and setup (10.4 ± 0.80 minutes vs. 8.0 ± 0.70 minutes, p = 0.03), positioning (20.8 ± 2.10 minutes vs. 15.7 ± 1.30 minutes, p = 0.049), and timeout (1.6 ± 0.16 minutes vs. 1.1 ± 0.09 minutes, p = 0.005).
The remaining steps were not significantly different between groups 1 and 2: induction (6.46 ± 0.78 minutes vs. 5.94 ± 0.61 minutes, p = 0.56), lines (29.4 ± 2.5 minutes vs. 25.5 ± 1.7 minutes, p = 0.21), incision planning (7.04 ± 0.74 minutes vs. 6.57 ± 0.81 minutes, p = 0.68), and prep and drape (13.2 ± 0.79 minutes vs. 13.0 ± 1.3 minutes, p = 0.43). Of note, the total time spent in the OR (i.e., from room entrance to exit) was lower for group 2 (270.1 ± 14.6 minutes vs. 252.8 ± 14.1 minutes) but the result was not statistically significant (p = 0.55). Total time was also adjusted for the number of levels performed on and showed no significant difference between the 2 groups (p = 0.65).
Further subgroup analysis was conducted with procedures stratified by anesthesia team (CRNA versus anesthesia resident involvement). In the CRNA group (group 1, n=4; group 2, n=4), there were no differences in any of the steps: transport and setup (12.0 ± 1.6 minutes vs. 8.5 ± 1.4 minutes, p = 0.20), induction (5.8 ± 0.44 minutes vs. 4.8 ± 1.2 minutes, p = 0.34), lines (25.6 ± 3.7 minutes vs. 25.6 ± 4.0 minutes, p = 0.89), positioning (21.2 ± 4.2 minutes vs. 12.6 ± 0.9 minutes, p = 0.06), incision planning (5.3 ± 0.83 minutes vs. 6.5 ± 2.1 minutes, p = 0.89), prep and drape (10.7 ± 1.9 minutes vs. 15.9 ± 3.5 minutes, p = 0.20), time out (1.3 ± 0.28 minutes vs. 0.98 ± 0.19 minutes, p = 0.49), total perioperative anesthesia time (82.1 ± 9.0 minutes vs. 74.9 ± 4.8 minutes, p = 1.00), and total time spent in the OR (308.3 ± 25.9 minutes vs. 240.3 ± 17.4 minutes, p = 0.11).
In the anesthesia resident group (group 1, n = 11; group 2, n= 6), there was a significant difference in time out (1.67 ± 0.19 minutes vs. 1.21 ± 0.18 minutes, p = 0.02), but not in transport and setup (9.8 ± 0.84 minutes vs. 8.8 ± 1.3 minutes, p = 0.58), induction (6.7 ± 1.1 minutes vs. 7.0 ± 1.2 minutes, p = 0.96), lines (30.7 ± 3.1 minutes vs. 26.3 ± 2.8 minutes, p = 0.29), positioning (20.7 ± 2.5 minutes vs. 17.5 ± 2.5 minutes, p = 0.45), incision planning (7.0 ± 1.1 minutes vs. 6.5 ± 0.68 minutes, p = 0.66), prep and drape (14.2 ± 0.69 minutes vs. 12.5 ± 1.4 minutes, p = 0.12), total perioperative anesthesia time (90.8 ± 5.5 minutes vs. 79.9 ± 6.6 minutes, p = 0.25), nor total time spent in the OR (256.3 ± 16.2 minutes vs. 276.7 ± 21.9 minutes, p = 0.51).
Further subgroup analysis was conducted with patients stratified by type of surgery. In the laminectomy and fusion group (group 1, n= 9; group 2, n= 11), there was a significant decrease in time for transport and setup (11.6 ± 0.93 vs. 8.2 ± 1.1, p = 0.03). positioning (23.3 ± 3.0 minutes vs. 16.7 ± 1.6 minutes, p = 0.051) as well as total perioperative anesthesia time (91.7 ± 5.9 minutes vs. 77.5 ± 4.2 minutes, p = 0.06) phases were also associated with decreased time, though the results did not reach statistical significance. There were no significant differences for induction (5.8 ± 0.77 minutes vs. 6.7 ± 0.81 minutes, p = 0.66), lines (29.8 ± 3.3 minutes vs. 25.4 ± 2.1 minutes, p = 0.26), incision planning (6.3 ± 0.59 minutes vs. 6.6 ± 0.91 minutes, p = 0.82), prep and drape (13.6 ± 1.2 minutes vs. 13.4 ± 1.6 minutes, p = 0.92), time out (1.4 ± 0.14 minutes vs. 1.1 ± 0.11 minutes, p = 0.10), nor total time spent in the OR (289.8 ± 14.7 minutes vs. 274.6 ± 12.9 minutes, p = 0.45). Of note, there was no difference in the number of operated levels between the 2 groups (4.67 ± 0.50 vs. 4.82 ± 0.30, p = 0.79). There remained no significant difference for total time in the OR when adjusting for number of operated levels (p = 0.83).
In the laminoplasty group (group 1, n= 6; group 2, n= 4), there was a trend for decreased time for time out (1.8 ± 0.34 minutes vs. 0.88 ± 0.11 minutes, p = 0.07). There were no significant differences for transport and setup (8.7 ± 0.76 vs. 7.5 ± 1.0, p = 0.40), induction (7.7 ± 1.5 minutes vs. 5.3 ± 0.67 minutes, p = 0.28), lines (28.8 ± 4.1 minutes vs. 25.9 ± 2.9 minutes, p = 0.61), positioning (17.1 ± 2.3 minutes vs. 12.9 ± 1.8 minutes, p = 0.22), incision planning (6.95 ± 1.9 minutes vs. 8.3 ± 1.1 minutes, p = 0.60), prep and drape (12.7 ± 0.88 minutes vs. 11.9 ± 2.3 minutes, p = 0.73), total perioperative anesthesia time (83.6 ± 7.7 minutes vs. 72.6 ± 3.0 minutes, p = 0.31), nor total time spent in the OR (240.7 ± 26.1 minutes vs. 193.0 ± 17.7 minutes, p = 0.22). Of note, there was no difference in the numbers of operated levels between the 2 groups (3.83 ± 0.40 vs. 3.75 ± 0.25, p = 0.88). There remained no difference in total time spent in the OR when adjusting for number of levels (p = 0.41).
DISCUSSION
This study demonstrates the application of LSS methodology to improve OR efficiency as it pertains to perioperative anesthesia for posterior cervical spine cases. We divided the perioperative anesthesia time into discrete steps (Fig. 2). This facilitated our multidisciplinary team in determining areas of high-yield intervention. Following are some examples of notable inefficiencies and strategies for improvement:
In the transport and setup phase, we identified that patients did not have intravenous (IV) access prior to being brought into the OR, which delayed transitions into the induction phase. To address this inefficiency, we required that IV access be obtained in the preoperative area prior to being transported, eliminating a potentially time-consuming step in the OR. This was particularly helpful in patients with higher BMI given the increased difficulty in obtaining IV access for these patients.
Within the lines phase, arterial line placement consumed the most amount of time. We observed that placements were often attempted without the assistance of an ultrasound machine. However, in the event of a difficult placement, an ultrasound machine was often requested—consuming time when the ultrasound would be delivered from a separate equipment room. To mitigate this problem, an ultrasound was placed in each OR prior to patient arrival.
Within the positioning phase, we noted increased difficulty in turning obese patients into the prone position. Because of this, OR staff often called for additional moving assistance to complete this phase—consuming time during the wait for additional persons to arrive. To mitigate this inefficiency, patient care assistants (PCAs) were provided advanced notification of obese patients who would require moving help. PCAs were available in the OR prior to the positioning phase for the procedures. This solution avoided the need to call for additional assistance after the patient was ready for turning to the prone position.
Within the incision planning phase, inefficiencies occurred when inexperienced X-ray technicians were unable to obtain useful fluoroscopic images despite multiple attempts. In response, we scheduled more experienced X-ray technicians to perform the imaging for these posterior cervical surgeries. This reduced time of this phase and limited the use of additional radiation.
After implementation of our LSS improvement strategies, the perioperative anesthesia time was significantly reduced by over 12 minutes, on average (p = 0.04). This represents nearly a 14% reduction in time. There was also a decrease in variation of times between the 2 groups shown with the decrease in standard error in most of the steps (Table 2). While a 12-minute improvement may seem minor, OR time is a valuable resource and major driver of hospital cost with some studies estimating a cost of $70–$80 per minute [9,10]. This improvement scaled across many spinal surgeries at a high-volume center would result in substantial cost savings.
Interestingly, the reduction in overall time and variation was mainly driven by the reductions in the transport and setup phase and the positioning phase. Surprisingly, the lines phase and the incision planning phase were 2 areas where we did not observe significant improvement. While times did decrease, the results were not statistically significant. It is possible that we were underpowered to detect such differences.
This study demonstrates that LSS methodology can (1) successfully be applied to the spinal surgical OR and (2) result in significant improvement in OR efficiency. Similar findings have been reported previously in other surgical disciplines. For example, Cima et al. [11] showed the successful execution of LSS and increased OR efficiency at the Mayo Clinic with gynecologic oncology, general thoracic, and general colorectal surgeries. Their implementation of process maps and execution of the changes in those maps—notably bolstered by leadership support and staff engagement—resulted in a reduction in late case starts and cases lapsing beyond 5 o’clock PM. As is often the case, there were associated improvements in the financial performance of the OR. In other examples, LSS implementation has been associated with improved OR turnover times [7] and ontime first starts [12-14]. LSS implementation can also result in improvements that extend beyond the OR. For example, with the implementation of a specific clinical pathway for hip fractures, Niemeijer et al. [15] revealed the simultaneous reduction of operating time by 57 minutes and—outside of the confines of the OR—a decrease in length of stay by over 4 days. Similarly, after a lumbar fusion specific clinical pathway was implemented, Bradywood et al. [16] demonstrated a decreased length of stay and superior discharge disposition.
Improving the perioperative anesthesia process is only one way to increase OR efficiency. We selected posterior cervical spine cases since they often take over one hour for setup and positioning. Other groups have identified other areas of OR waste and have successfully implemented changes leading to substantial cost savings. For example, studies by Lunardini et al. [17] and Farrokhi et al. [18] demonstrated how LSS methods can increase OR efficiency by reducing unnecessary instrumentation, thereby decreasing setup times and leading to cost savings.
Our study is unique in that it shows a step-by-step methodology for applying LSS to the neurosurgical OR. This provides clinicians with a framework for implementing LSS methods in their own institutions. In an era of increased scrutiny within healthcare spending, methods to increase efficiency and decrease costs are timely. We hope that our study demonstrates how LSS methods can be applied to a commonly performed neurosurgical operation to improve OR efficiency.
The present study is not without several limitations including bias inherent to QI projects. Namely, participants may have been influenced by the Hawthorne Effect. We are also sensitive to the fact that improvements revealed in QI studies may extinguish over time as personnel revert back into old habits and lose the improvement that was created with the initiative. Lastly, as with other QI projects, there is a lack of randomization and thus there is risk of selection bias. Nonetheless, we provide a basic framework for process improvement that can be extrapolated to other surgeries as well. Other surgical teams can apply LSS to their specific surgeries, by identifying potential areas for OR inefficiency outside of the perioperative anesthetic process, such as the intraoperative and postoperative phases of the surgery. Identifying and improving inefficiencies at an incremental level, follows the Plan-Do-Study-Act process of QI [19].
Further studies should involve scaling up our approach as outlined above. As we performed this study in a single, high-volume spinal academic practice and a small sample size, it is unknown if our approach will be generalizable.
CONCLUSION
LSS methodology may be successfully applied to posterior cervical spine surgery whereby improvements in the perioperative anesthetic process is associated with significantly increased OR efficiency. This increased efficiency is particularly evident for patient transportation and positioning steps of posterior cervical surgery. This has important implications for multiple stakeholders, including clinicians, patients, and hospitals.
Notes
Dr. Chan: research support for unrelated study from Orthofix. Dr. Mummaneni: consultant for DePuy Spine, Globus, and Stryker; direct stock ownership in Spinicity/ISD; clinical/research support for unrelated study from NREF; royalties from DePuy Spine, Thieme Publishers, and Springer Publishers; grant from AOSpine; and honoraria from Spineart; research support for non-related study from ISSG. Except for that, the authors have nothing to disclose.
Acknowledgements
This manuscript has been presented at the 46th Annual Meeting of the Cervical Spine Research Society, Scottsdale, Arizona (December 8th, 2018).