Comparison of the Clinical Efficacy of Transforaminal Endoscopy and Microtubular Technology for the Treatment of Lumbar Dumbbell-Shaped Tumors
Article information
Abstract
Objective
To analyze differences in feasibility and efficacy between the paravertebral approach and microtubular tumorectomy (PAMT) or percutaneous transforaminal endoscopic tumorectomy (PTET) for the treatment of lumbar dumbbell-shaped tumors.
Methods
Clinical data of dumbbell-shaped lumbar tumors in patients treated with PAMT or PTET in our hospital between June 2015 and November 2020 were retrospectively analyzed. The gross total resection (GTR) rate, operation time, estimated blood loss, postoperative hospital stay (PHS), postoperative neurological function, and spinal stability were compared between the 2 surgical methods. Neurological improvement was assessed using the pain visual analogue scale (VAS) and the Japanese Orthopaedic Association (JOA) score.
Results
Fifteen cases of GTR (93.8%) and 1 case of subtotal resection were included in the PTET group, whilst all 18 patients in the PAMT group achieved GTR. There was no significant difference in the GTR rate, operation time, and PHS between the PAMT and PTET groups. The estimated blood loss was significantly lower in the PTET group than in the PAMT group. At the last follow-up, there was no significant difference in the VAS or JOA scores between PTET and PAMT. No tumor recurrence or spinal instability was observed in either group during the follow-up period.
Conclusion
Both PAMT and PTET can achieve Eden type III-IV lumbar 1-stage tumor resection without additional spinal internal fixation due to reduced muscle, ligament, and facet joint damage. No lumbar instability and tumor recurrence occurred, and neurological function was improved.
INTRODUCTION
Dumbbell-shaped tumors of the lumbar spine are rare spinal canal tumors that often compress the peripheral nerve tissue, of which surgical resection is the preferred treatment method. Eden’s classification and pathological types of dumbbell-shaped lumbar tumors aid surgeons in their decision regarding the appropriate surgical strategy [1,2]. The traditional treatment of lumbar spine dumbbell-shaped tumors achieves adequate exposure and resection of the tumor by excising the facet joint, opening the intervertebral foramen, and performing fusion and internal fixation to maintain spinal stability [3]. With the development of minimally invasive techniques and the concept of minimally invasive spine surgery, many surgeons elect to use the anatomical features of the spine to remove tumors while reducing the impact of surgical operations on the stability of the spine and the impact of internal fixation on the functional activities of the patient’s spine [4-6].
In 2015, Chunmei et al. [7] detailed the paravertebral approach and microtubular tumorectomy (PAMT) for the treatment of lumbar spinal tumors and reported that lumbar spinal tumors could be completely removed, and damage to the paravertebral muscles, articular processes, and spines could be avoided. They highlighted that PAMT for the treatment of lumbar spinal tumors resulted in less trauma, faster recovery, less complications, and good lumbar spine stability. In recent years, the percutaneous transforaminal endoscopic technique has been widely used in the treatment of lumbar disc herniation [8]. In addition, we have reported on full-endoscopic surgery to remove lumbar dumbbell tumors [9,10]. Alternatively, percutaneous transforaminal endoscopic tumorectomy (PTET) is a novel, safe, and effective surgical approach for lumbar spine dumbbell-shaped tumors, which can help avoid the spine and minimize the damage to the normal structure of the spine while completely removing the tumor inside and outside the lumbar intervertebral foramen. Spinal stability is conducive to the early recovery of patients [9]. At present, with regards to the minimally invasive surgery of intraspinal tumors, there is no published work detailing the difference between endoscopic spinal surgery and tubular spinal surgery. This study assuming that PTET is not less efficacious compared with PAMT in patients with lumbar dumbbell-shaped tumors, and aimed to compare the gross total resection (GTR) rate, perioperative period data, postoperative neurological function, and complications to evaluate their safety and efficacy.
MATERIALS AND METHODS
1. Patients
Patients with lumbar dumbbell-shaped tumors treated with PAMT or PTET (according to their decision) in our hospital between June 2015 and November 2020 were retrospectively studied.
The inclusion criteria for this study were as follows: (1) patients with tumor classified as Eden type III or IV and a tumor located in the epidural layer; (2) the diameter of the intraspinal canal tumor was ≤ 2 cm; (3) the diameter of the paravertebral tumor was ≤ 5 cm; and (4) the patient has signed an informed consent form. The exclusion criteria were as follows: (1) infectious lesions in the surgical path, (2) tumor invading ≥ 2 intervertebral foramina, (3) the segment where the tumor was located with a history of surgery, (4) vascular tumor or tumor with rich blood supply, (5) lumbar instability, and (6) severe lumbar scoliosis.
2. Ethics Approval
This study has been granted approval by the Ethics Committee of Fujian Medical University Union Hospital, Fuzhou, China (approval number: 2021YF022-01).
3. Clinical Data and Imaging Examination
The clinical manifestation and presentation of each patient were also assessed. Radiographic examination, 3-dimensional computed tomography (CT) reconstruction, and magnetic resonance imaging (MRI) with enhanced scanning were performed on the lumbar vertebrae before surgery to obtain a definitive diagnosis.
4. Intervention: PTET
The puncture point and path of the intervertebral foramen were designed based on MRI and 3-dimensional reconstruction CT images taken prior to surgery, including the distance from the skin to the tumor surface and the distance from the skin to the intervertebral foramen. After the induction of general anesthesia, the patient was placed in the prone position and x-ray fluoroscopy was used to locate the surgical segment and puncture point. The puncture point was located 15 cm lateral to the posterior midline (adjusted according to the patient’s body shape), the intervertebral foraminal puncture path pointed to the paravertebral tumor, and intervertebral foramen in the extending direction. Under x-ray fluoroscopy guidance, when the puncture needle reached the paravertebral tumor tissue, a guidewire was inserted. A skin incision of approximately 7 mm was made, and a soft tissue dilator and a tubular working sheath were sequentially placed along the guidewire. Tumor resection (from outside to the inside of the spinal canal) under endoscopy (Spinendos, Germany) was performed as follows: (1) First, the paravertebral tumor was excised after the nerve roots, tumor capsule, and normal muscle tissues were identified in the tubular working sheath. Electrocoagulation was used to cut off the blood vessels supplying the tumor, and the paravertebral tumor tissue and the tumor-bearing nerve root were excised to protect the normal nerve root. (2) If the intervertebral foramen was small, foraminoplasty was performed using an endoscopic burr or trephine. In most cases, tumor growth led to the expansion of the intervertebral foramen, and the tubular working sheath can easily enter the spinal canal. (3) Resection of tumors in the intervertebral foramen and spinal canal to avoid a dural tear. The direction of the tubular working sheath was adjusted such that the endoscope could gradually penetrate the intervertebral foramen and enter the spinal canal in the tumor capsule, and the tumor tissue could be removed in pieces. A drainage tube was placed outside the intervertebral foramen, the working sheath was removed, and the wound was sutured (Figs. 1-3). A typical case was presented (see Supplementary materials).
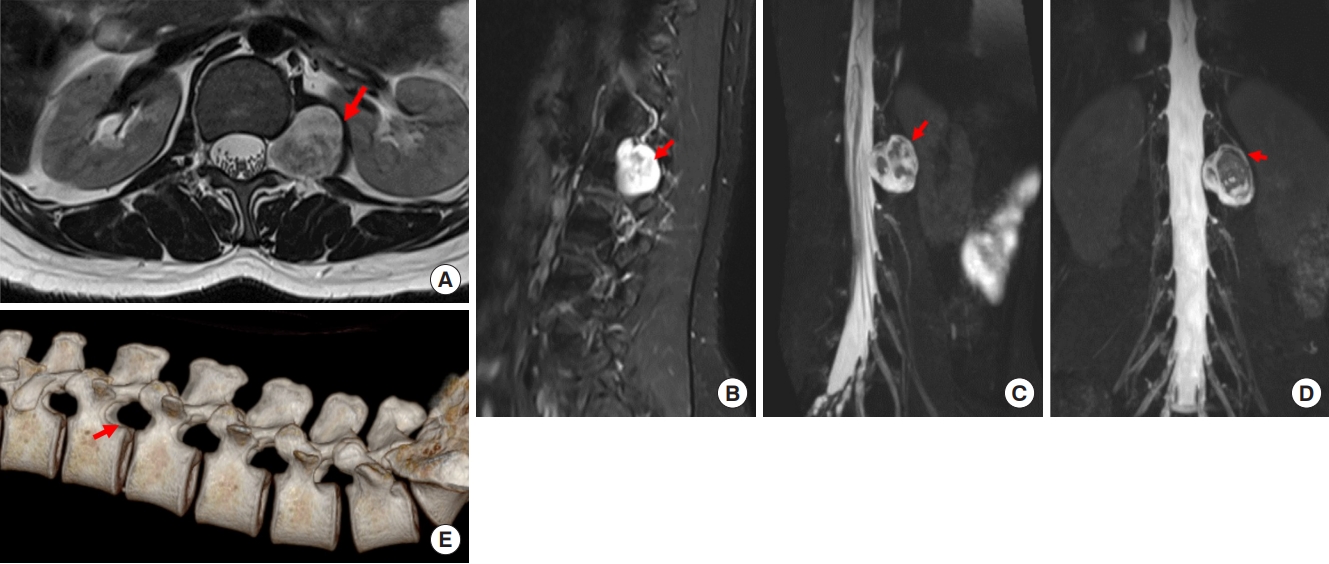
Preoperative T2-weighted magnetic resonance imaging on axial (A) plane, T1-weighted enhanced sagittal (B) plane, 3-dimentional (3D) sagittal (C), and 3D coronal (D) planes, revealing a dumbbell tumor in the left L1–2 foramen (arrows). (E) 3D computed tomography scan shows the enlarged foramen (arrow).

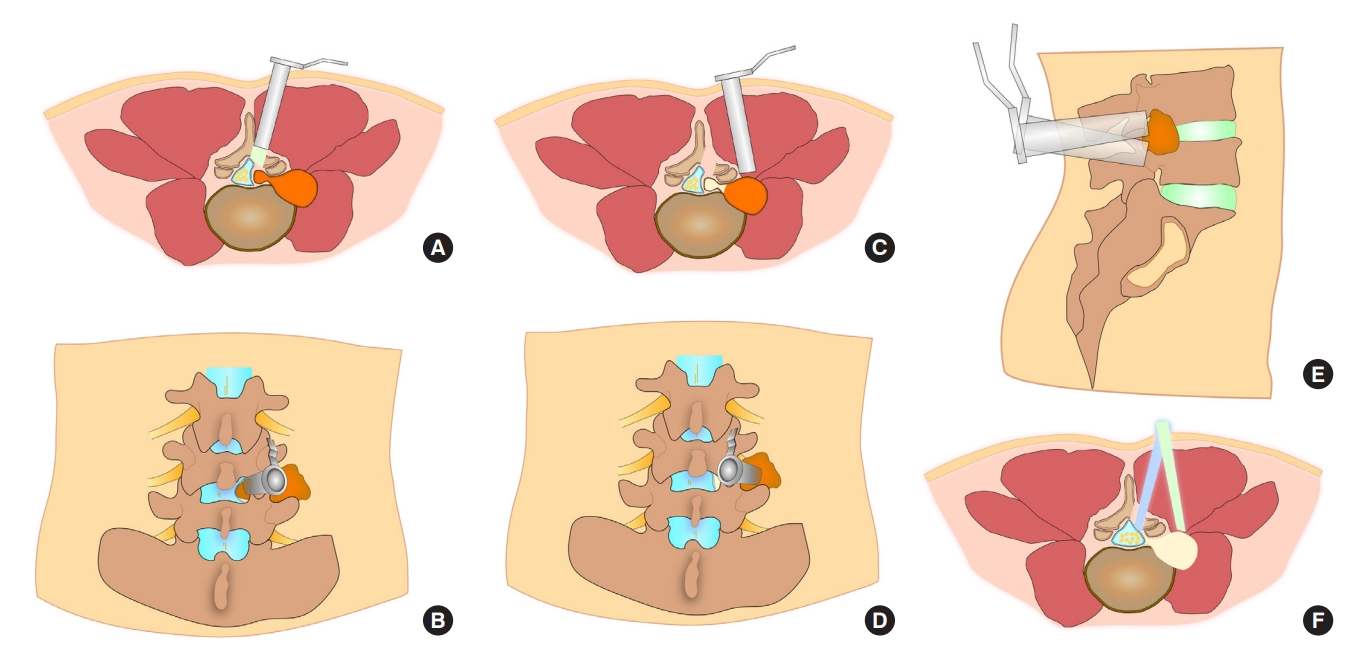
Surgical diagrams illustrating the percutaneous transforaminal endoscopic tumorectomy operation process. (A) A tubular working sheath was placed along the guidewire above the surface of the paravertebral part of the dumbbell tumor in lumbar vertebrae on the axial plane. (B) The paravertebral tumor and the tumor-bearing nerve root were excised after the nerve roots, tumor capsule, and normal muscle tissues were identified in the tubular working sheath on the sagittal plane. (C) The tubular working sheath reached the internal space of the foramen after foraminoplasty on the axial plane. (D) The tubular working sheath’s direction was adjusted so that the endoscope could gradually penetrate the intervertebral foramen and enter the spinal canal in the tumor capsule. The tumor tissue could be removed in pieces on the coronal plane. (E) After confirming that the tumor was removed entirely, the working sheath was withdrawn.
5. Intervention: PAMT
1) Anesthesia and position
After the induction of general anesthesia, patients were placed in the prone position to minimize lumbar lordosis/thoracic kyphosis and avoid compressing the abdomen.
2) Approach and exposure
A 20- to 25-mm skin incision was made approximately 20–35 mm lateral to the midline (adjusted according to the patient’s body habitus), and the accurate target level was confirmed by x-ray fluoroscopy.
The paravertebral approach involved the paravertebral muscles, which were bluntly dissected using the muscle splitting technique. After the smallest dilator was inserted to reach the lamina or peripheral bone structure, the dilators were sequentially placed on top of each other, and a working tubule (diameter, 14 mm or 16 mm) was inserted over the dilators. The dilators were removed, and a tubular surgical path was established. The tubule was fixed using a flexible arm mounted on the operating table. Due to the flexible fixed arm, the tubule was able to be angulated to expand the operating field. The tumor resection was performed under a microscope (OPMI Pentero, Carl Zeiss AG, Oberkochen, Germany).
In Eden type III tumors, tumor resection was first performed in the intraspinal tumor, followed by the paraspinal tumor. The microtubules first reached the lamina to facilitate intraspinal tumor removal. A high-speed drill combined with Kerrison punches was used to remove part of the lamina, and the ligamentum flavum was excised to expose the dura and the spinal nerves. The intraspinal canal and intervertebral foramen tumors were excised which was located in the epidural area. Any obvious tumor-bearing nerves were removed. The bone window of the spinal canal was temporarily sealed using gelatin sponge. If the diameter of the paravertebral tumor is ≤ 2 cm, the direction of the tubule can be directly adjusted to expose and remove the paravertebral tumor. If the diameter of the paravertebral tumor (Figs. 4-6). was ≥ 2 cm, the microtubule was required to be reinserted to reach the region between transverse processes and establish a second paravertebral muscle tubular path (dual-tubule path); if necessary, remove the part of the transverse process and expose and remove the extraforaminal tumor and paravertebral tumor. For Eden IV tumors, the microtubule directly reached the paravertebral transverse process, exposing and resecting the extraforaminal tumor and paravertebral tumor. The paravertebral muscles were repositioned, and the muscle fascia, subcutaneous tissue, and skin were sutured layer-by-layer.

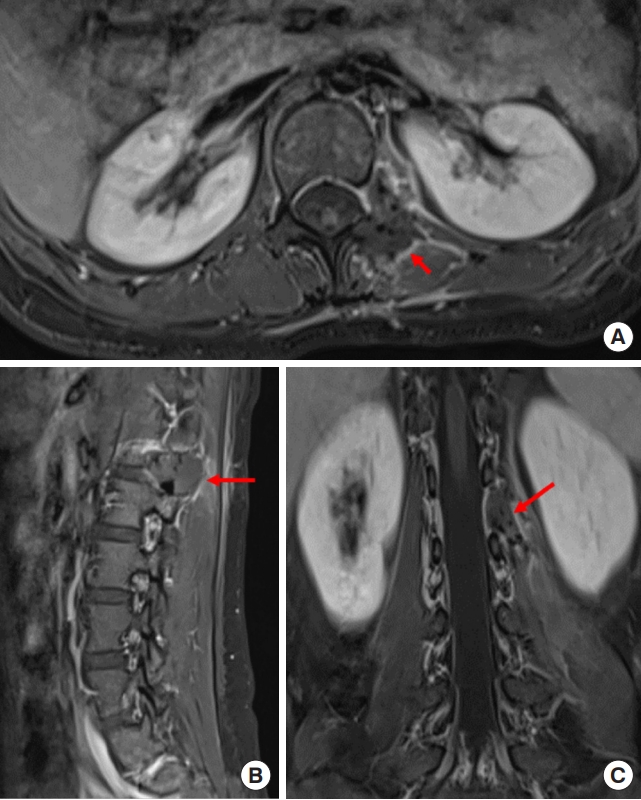
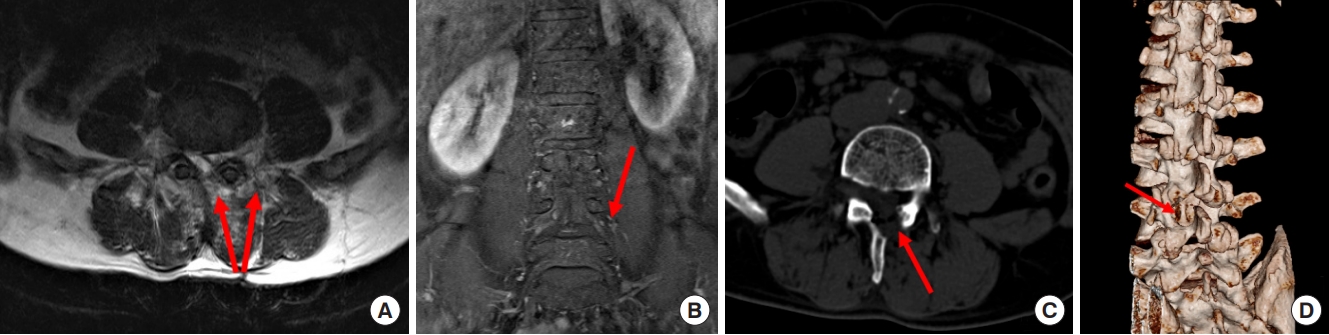
Preoperative T1-weighted enhanced magnetic resonance imaging on sagittal (A) plane, coronal (B) plane, and axial (C) plane, revealing a dumbbell tumor (Eden II) in the left L4–5 foramen (red arrows).
Surgical diagrams illustrating the paravertebral approach and microtubular tumorectomy operation process. (A, B) A working tubule (diameter, 14 mm or 16 mm) reached the lamina by dissecting the paravertebral muscle bluntly. The intraspinal canal and intervertebral foramen tumors were excised, which were located in the epidural area after removing part of the lamina and the ligament flavum. (C, D) The microtubule was reinserted to reach the region between transverse processes and establish a second paravertebral muscle tubular path (dual-tubule path); if necessary, remove the part of the transverse process and expose and remove the extraforaminal tumor and paravertebral tumor. (E) The microtubule can be angulated to expand the operating field. (F) After confirming that the tumor was completely removed, the microtubule was withdrawn.
Postoperative T2-weighted magnetic resonance imaging on axial (A), and T1-weighted enhanced on coronal (B) planes. Any remnant of the dumbell tumor could not be identified after 6 months (red arrows). (C, D) The axial plane and 3-dimensional computed tomography scan showed the bone window of L4 and L5 lamina (red arrows).
3) Postoperative measurements
The drainage tube was removed within 24 hours after surgery, and patients underwent passive lower extremity and walking training protected by a belt brace as soon as possible. A belt brace was used for 1–2 weeks after surgery.
6. Outcome Measurements and Data Collection
Neurological improvements were assessed using the pain visual analogue scale (VAS) and Japanese Orthopaedic Association (JOA) scores. The baseline data included sex, age, body mass index, comorbidities, target segment, clinical performance, and preoperative VAS and JOA scores. The primary outcome measure was the GTR rate. The extent of resection was defined as GTR if there was no residual tumor on postoperative MR images and subtotal resection (STR) if the residual tumor was present [11]. Secondary outcome indicators included operation time, estimated blood loss (EBL), postoperative hospital stay, postoperative VAS score, postoperative JOA score, tumor recurrence rate, and spinal stability during the follow-up period.
7. Statistical Analysis
Data were analyzed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). For continuous variables, Student ttest was used for independent samples. Contingency tables will be constructed for the categorical variables, assessing the independence between variables using Fisher exact test. Statistical significance was set at p<0.05. An exploratory subgroup analysis was performed to investigate whether the treatment effect varied across subgroups of patients.
RESULTS
1. Participants’ characteristics
Thirty-four patients were included in this study in accordance with the inclusion and exclusion criteria. The PTET group included 16 cases, 8 of which were Eden type III and 8 were Eden type IV, with an average age of 42.81±16.86 years. The PAMT group included 18 cases, 12 of which were Eden type III and 6 were Eden type IV, with an average age of 48.17±17.31 years. The pathological report showed 24 cases of schwannoma, 3 cases of inflammatory granuloma, 1 case of a cyst, 1 case of cavernous hemangioma, 1 case of ganglionoma, 1 case of neurofibroma, and 3 cases of metastases. The 3 metastatic cases included 1 small-cell lung tumor, 1 acute lymphocytic leukoma (B cell), and 1 acute leukemia metastasis (myeloid) (Table 1).
2. Primary and Secondary Outcomes
1) GTR rate
There were 15 cases of GTR (93.8%) and 1 case of STR in the PTET group whilst all 18 patients in the PAMT group achieved GTR. Due to an insufficient sample size, the Fisher exact test was used and determined no statistically significant difference in the GTR rate between the 2 groups (p=0.47) (Table 2).
2) Surgical data
The mean operative time in the PTET group was 101.3±25 minutes, and the mean operative time in the PAMT group was 112.2±18.96 minutes (-10.97 minutes; 95% CI, -26.37 to 4.42; p=0.16). There was no significant difference in postoperative hospital stay between the PAMT and PTET groups (-0.59 days; 95% CI, -1.33 to 0.15; p=0.11). However, the EBL in the PTET group was significantly lower than that in the PAMT group (-32.71 mL; 95% CI, -63.53 to -1.89; p=0.04) (Table 2).
3) Neurological function improvement
Lumbar or leg pain, lower-limb weakness, and other symptoms were significantly relieved in both the PTET and PAMT groups, and no new neurological dysfunction was observed. There was no significant difference in preoperative VAS (0.88; 95% CI, -0.40 to 2.15; p=0.17) and JOA (-1.92; 95% CI, -4.75 to 0.90; p=0.16) scores between PTET and PAMT, indicating that data were comparable between groups. At the last follow-up, there was no significant difference in the VAS (-0.19; 95% CI, -0.58 to 0.20; p=0.34) and JOA (0.47; 95% CI -0.24, 1.18; p=0.19) scores between the PTET and PAMT groups (Table 2).
4) Adverse events
Postoperative complications included cerebrospinal fluid leakage in 1 case (PAMT group), cavity effusion in 4 cases (2 cases in the PTET group and 2 cases in the PAMT group), wound infection in 3 cases (2 cases in the PAMT group and 1 case in the PTET group), and relief after management and medication. No tumor recurrence or spinal instability was observed in both groups (Table 2).
5) Subgroup analysis
Subgroup analysis was performed according to the different Eden types. For Eden type III lumbar tumors, the JOA score at 6 months after surgery was significantly lower in the PTET group than in the PAMT group (-2.29; 95% CI, -4.48 to -0.1; p=0.04), while the EBL in the PTET group was significantly less than that in the PAMT group (47.08 mL; 95% CI, -85.45 to -8.72; p=0.02). For Eden type IV lumbar tumors, there was no significant difference in operation time, EBL, PHS, VAS score or JOA score between the PTET and PAMT groups (p>0.05) (Table 3).
DISCUSSION
Based on preliminary reports on the feasibility and safety of PAMT and PTET, this study aimed to compare the efficacy of the 2 approaches and summarize the advantages and disadvantages of each technique in dumbbell-shaped lumbar tumors. Our findings indicated that both the microtubular and full-endoscopy approach can achieve reliable efficacy of conventional surgery and also fully utilize the features of minimally invasive surgery with less injury, less bleeding, fewer complications, and faster recovery. Therefore, we compared the safety and efficacy characteristics of the microtubular technique and full-endoscopic technique in lumbar dumbbell tumor surgery and found that both tubular spine surgeries can achieve satisfactory outcomes, which is a novel addition to current literature.
Most dumbbell-shaped lumbar tumors are benign. The tumors were located below the facet joints and reached the spinal and extravertebral canals through the intervertebral foramen. They can stimulate or damage the spinal nerves and cause clinical symptoms, including pain. Total tumor resection is often accomplished by open surgery for dumbbell-shaped lumbar tumors. Open surgeries result in sufficient exposure and reduced tumor recurrence, but they can result in damage to the paravertebral muscles and ligaments and removal the facet joints and lamina, which may cause spinal instability and require additional fusion and internal fixation [12-14]. Additional fixation surgeries significantly aggravate the extent of damage to the paravertebral muscles and ligaments, which is not necessary for tumor resection and fixation instruments, greatly increasing medical costs. In addition, patients have been reported to recover slowly, suffer from chronic low back pain, and have limited lumbar movement after open surgery. We have previously performed intraspinal subdural schwannoma and thoracic dumbbell tumor resections using the microtubular technique, and we have also attempted lumbar dumbbell tumor resection under spinal endoscopy [9,15,16].
For Eden type III-IV lumbar tumors with a single segment and a maximum diameter of < 5 cm, both PTET and PAMT achieved 1-stage total resection. In PTET approach, the tumor resection procedure extends from the paravertebral tumor to the intraspinal canal tumor. Therefore, we chose to reach the paravertebral tumor directly after intracapsular resection of the tumor, using the intervertebral foramen bony channel to enter the spinal canal and remove the tumor in the spinal canal, which can reduce the damage to the bony structure and paravertebral muscles of the spine. Puncture site, puncture path, and modified foraminoplasty are key to the success of PTET [11,17-19]. PTET differs from the lumbar disc herniated endoscopic transforaminal approach (PTED) in terms of the puncture technique and foraminoplasty; the design of the puncture path made the working sheath easier to pass the intervertebral foramen and remove the tumor tissue in the spinal canal after paravertebral tumor resection [4,20]. Since the dorsal root ganglion was mostly located in the intervertebral foramen, lumbar dumbbell-shaped tumors compressed the exiting nerve roots or dorsal root ganglion, causing low back and leg pain and movement disorders. Another effect of modified foraminoplasty was that the exiting nerve and dorsal root ganglion were adequately decompressed to relieve the symptoms of low back and leg pain.
During PTET surgical tumor resection, when the paravertebral tumor diameter was > 3 cm, endoscopic tumor resection spent more time; for schwannomas, the bearing-tumor nerve often accompanied the tumor supply blood vessels, which helped to find blood vessels, and under the “navigation” of the tumorbearing nerve (exiting nerve), the endoscope can more easily enter the intervertebral foramen and remove tumor tissue in the spinal canal. Furthermore, under the influence of the lavage water pressure of the endoscope, it is often difficult to distinguish between the blood vessels supplying the tumor and the internal foraminal venous plexus. Alternately closing and opening saline can compare the morphological characteristics of blood vessels and help distinguish different blood vessels and nerve root branches. Tumors with rich blood supplies were prone to bleeding, which made it difficult to identify the tissue under the endoscope and increased the risk of surgery. Therefore, endoscopic tumor resection still has a relatively long learning curve, even for experienced spinal surgeons [5,6,21].
Tubular spine surgery involves the application of expandable tubules or nonexpandable tubule using tubular retractors of different diameters (14–28 mm) to treat various spinal diseases. This technique is safe and effective and does not increase the risk of nerve damage after resolving spinal diseases. In the PAMT approach, we achieved resection of intraspinal, foraminal, and paravertebral tumors by adjusting the position and angle of the paravertebral muscle path under the nonexpandable tubule (diameter, 14/16 mm). For larger Eden type III tumors, it is necessary to establish 2 paravertebral muscle tubular paths (dual-tubular paths) to remove intraspinal and paravertebral tumors, which can protect the facet joints and isthmus, avoid excessive traction and separation of muscles, and reduce the risk factors for spinal instability [22–24]. However, for tumors with diameters > 5 cm or vascularized tumors, we still recommend open surgery or mini-open surgery [13].
For Eden III-IV lumbar dumbbell-shaped tumors, the EBL in the PAMT group was higher than that in the PTET group, but there was no significant difference in the operation time, JOA or VAS scores, or PHS. When subgroup analysis was performed according to different Eden types, it was found that for Eden type III tumors, the PTET group was significantly lower than the PAMT group’s JOA score at 6 months after surgery, but the EBL was lower than that of the PAMT group. The reason for the difference in EBL between the 2 groups may be that PTET was performed under saline perfusion pressure, which helped to reduce the bleeding of the venous plexus in the intervertebral foramina, but the number of cases in the 2 groups was small. Furthermore, the PAMT group included patients with spinal canal metastases and other pathological types of tumors. Therefore, it is difficult to ascertain whether there was a difference in EBL between the 2 procedures. The JOA score of the PAMT group was higher than that of the PTET group at 6 months after the operation, indicating that the early postoperative efficacy of the PAMT group may be better than that of the PTET group. However, there was no difference in the JOA scores at other follow-up periods and the last follow-up, indicating that the efficacy of the 2 groups was not significantly different. For Eden type IV, there were no significant differences in the operative time, EBL, PHS, VAS, and JOA scores between the PAMT and PTET groups. Therefore, for single-segment lumbar dumbbell-shaped tumors (Eden types III and IV) with a maximum diameter of < 5 cm, we believe that both PAMT and PTET are alternative, effective, and minimally invasive treatment options.
This study analyzed the clinical results of PAMT and PTET in the treatment of single-segment lumbar dumbbell-shaped tumors (Eden types III and IV), but there are still several limitations. This research was a retrospective study with a low level of evidence. The number of cases collected in this study was small, and the follow-up time in some cases was < 2 years. Both PTET and PAMT are not suitable for all lumbar spine dumbbell tumor surgeries. For example, in cases of large paravertebral tumors (diameter, > 5 cm), such as tumors invading multiple intervertebral foramina, tumors with abundant blood supply, and scoliosis deformity, combined surgery or mini-open surgery is recommended for tumor removal. Although no tumor recurrence occurred during the follow-up period, it is undeniable that there is the possibility of tumor recurrence for PTET because the tumor is resected in pieces. PTET may result in residual tumor tissue for tumors that are too large, irregularly shaped, or incompletely enveloped. Finally, physicians cannot determine the pathological type of the tumor before surgery. If the pathological type of the tumor is a non-neurogenic tumor, physicians may be difficult to distinguish the tumor tissue from the normal tissue under spinal endoscopy, and intraoperative pathological analysis is often used to identify the tumor tissue. In the future, more cases and longer follow-up times are needed to verify the advantages and disadvantages of the 2 tubular spinal surgical methods.
CONCLUSION
PTET and PAMT are safe and effective surgical methods for Eden type III and IV lumbar dumbbell-shaped tumors, that can achieve 1-stage total tumor resection, reduce facet joint damage, and do not require fusion and internal fixation surgery. Furthermore, PAMT and PTET are alternative, effective, and minimally invasive treatments for dumbbell-shaped lumbar tumors.
SUPPLEMENTARY MATERIALS
Supplementary Material: Supplementary material can be found via https://doi.org/10.14245/ns.2244152.076.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was funded by Technology and Innovation Foundation of Fujian, China (grant no. 2021Y0021 to RW).
Author Contribution
Conceptualization: RW, CC; Data curation: RW, ZL; Formal analysis: ZL, YC; Funding acquisition: RW; Methodology: RW; Project administration: CC; Writing - original draft: RW, ZL; Writing - review & editing: RW, ZL, YC, CC.