Determination of the Effect of Diameter of the Sac on Prognosis in 64 Cases Operated for Meningomyelocele
Article information
Abstract
Objective
To examine the effect of meningomyelocele sac size on prognosis by retrospective review of 64 cases operated for meningomyelocele between January 2009 and December 2012.
Methods
We evaluated newborn babies operated for meningomyelocele by retrospectively reviewing their files for head circumference, location and with of the defect, accompanying anomalies, treatments administered, drugs that mother used during pregnancy. Based on the defect size, 3 patient groups were created as 0–24 cm2 (group I), 25–39 cm2 (group II), and 40 cm2 and above (group III).
Results
Throughout the study, 64 babies were evaluated. Mean head circumference was 37.4 cm (range, 30.7–50 cm). Based on their location, 49 of the defects (76.5%) were lumbar, 7 (10.9%) were thoracolumbar, 4 (6.2%) were thoracic, 3 (3.1%) were sacral, 1 (1.5%) was cervical. Mean size of the meningomyelocele sac was 4.7 cm×5.8 cm (range, 1 cm×1 cm—10 cm×8 cm), 13 of the babies (20.3%) had skin defect requiring flap. According to accompanying anomalies, 47 of the babies (73.4%) had hydrocephalus, 7 (10.9%) had club foot, 1 (1.5%) had diastematomyelia, 1 (1.5%) had tethered cord. Thirty-nine of the babies (60.9%) had paraplegia, 10 (15.6%) had paraparesis, 8 (12.5%) had monoplegia; neurological examination in the remaining 7 babies was normal.
Conclusion
In our study, increased diameter of meningomyelocele sac was associated with greater amount of neural tissue within the sac, which worsens the prognosis. Sac localization was not changing prognosis but infection rates, hospitalization duration were increased in babies with bigger diameter of sacs.
INTRODUCTION
Myelemeningocele is a congenital central nervous system(CNS) anomaly in which a part of the spinal cord, together with the surrounding meningeal structures, protrudes outward through the defected bone and skin as a sac1). The cause is not exactly clear, but genetic and environmental factors are thought to play role together3). While its frequency in European countries is 0.1%, the rate in Turkey, according to various studies, range from 0.3% to 0.58%10). Meningomyelocele can be named according to its location. Studies indicate the most common location as lumbar area with 69% ratio8). Hydrocephalus accompanies meningomyelocele in 80% of all cases8). The diameter of the meningomyelocele sac is among the most important prognostic factors6). The greater the amount of neural tissue inside the sac, the worse is the neuologic deficit and prognosis. Larger sacs require skin flaps more, giving rise to complications such as flap-related infection, reoperation, and wound-site problems6). Treatment in meningomyelocele involves closure of the neural tissue as soon as possible, repair of the skin defect, and placement of ventriculoperitoneal (V/P) shunt in cases with accompanying hydrocephalus.
MATERIALS AND METHODS
In our study, we retrospectively reviewed 64 cases operated for meningomyelocele (Figs. 1, 2) between January 2009 to December 2012, noting data including sex, birthweight, diameter of the sac, and presence of additional anomalies. Patient age varied from neonatal period to 6 months. Regarding distribution of sex, 34 (53.1%) were female, and 30 (46.8%) were male patients. All patients were evaluated with magnetic resonance imaging (MRI) in order to localize the sac, and were examined to determine diameter of the sac accurately (Table 1). Patients were divided into 3 groups based on the size of the defect: group I, 0–24 cm2: group II, 25–39 cm2: group III, 40 cm2 and above (Table 2). As the sacs were circular, we used the following formula for calculating the sac area: ω r2 (ω : pi number, r: radius). Serum urea and creatinine levels were measured during the first 72 hours in order to assess renal functions in all babies. Neurological examination was performed in every patient in order to determine motor deficit. Patients were evaluated for infection, and we performed surgery for those patients without any sign of infection, and the patients were followed up at the postoperative period. We evaluated the effect of sac diameter on hospital stay length, duration of anti-biotherapy, and early morbidity. Babies were followed up throughout the newborn period, and prognosis and complications at the early period were evaluated.
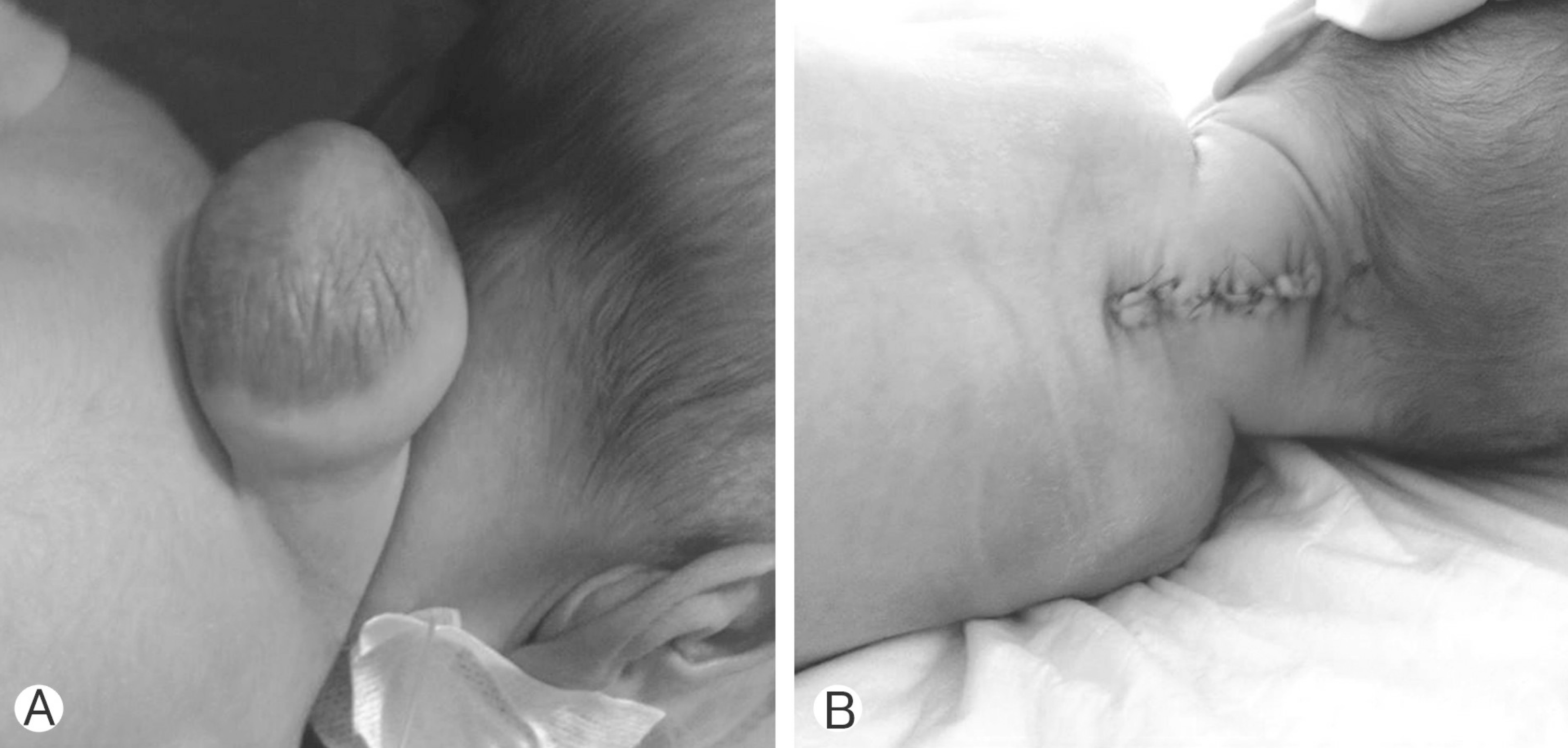
Preoperative (A) and postoperative (B) images of the patient who were operated on for the cervical meningomyelocele.

Preoperative (A) and postoperative (B) images of the patient who were operated on for the thoracolumbar meningomyelocele.
The study data was analyzed with demographic statistics method. Data was expressed as mean±standard deviation. Using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA), nonparametric data were analyzed with chi-square test, Kruskal-Wallis H-test and Mann-Whitney U-Test. Determination of risk factors and relation between variables were analyzed with correlation analysis. A p-value of <0.05 was accepted as statistically significant.
RESULTS
Table 1 shows detailed properties of our cases along with the surgical intervention performed and postoperative conditions. None of the mothers used folic acid supplement, or were exposed to radiation before or during their pregnancy. None of the families receiving antenatal diagnosis accepted medical termination recommendation. In the study group, it was determined that most of the mothers were undereducated, and 89% of the mothers were either primary school graduate or did not go to school at all. A great proportion of meningomyelocele location, in 50 of 64 patients (78.1%), was lumbar region. Sac size varied between 1 cm×1 cm and 10 cm×8 cm. MRI was performed to aid in the diagnosis. Seven cases did not have neural tissue within the sac. None of these patients had neurological deficit or hydrocephalus. Three of these cases were in group 1, and 4 were in group 2 according to the sac diameter.
The defect was covered with flap in 12 cases, while preserving dorsal intercostal artery. All of these cases had neural tissue within the sac. Of these patients, 11 were in group 3, and 1 was in group 2.
The surgery was performed with prone positioning. Neural tissue within the sac was preserved. New dura was formed using fascia, and the skin was closed. For cases with large skin defects, skin was closed with flap. Hydrocephalus was diagnosed in 47 cases (73.4%). Thirty-nine out of 47 patients were treated with V/P shunt. Parents of 8 cases did not approve the recommended shunt surgery. No mortality was observed among our cases at postoperative period, and during the follow-up. Comparison of mean sac diameter with neurological condition, hospital stay length and additional anomalies showed significant difference in Mann-Whitney U-test (p<0.05). As the sac size increases, the length of stay increases (r=0,91, p<0,05). Smaller sac diameter was associated with better neurological condition, and significantly lower hospital stay and infection rates in our study (p<0.05) (Table 2). In our study we observed that localization of sac was not affecting prognosis (p>0.05) (Table 1) and diameter and having neural tissue within the sac were more important prognostic factors than the localization.
DISCUSSION
Meningomyelocele (MM) is one of the severe congenital malformations of CNS; it is a midline line closure defect categorized in spina bifida aperta group. Its frequency varies between 1 and 9 in 1,000 live births10). Spinal cord or nerve roots can protrude through a vertebra defect into a sac containing meninges as well, or they may be exposed without being covered with any meninx or skin. This congenital anomaly can result in severe neurological dysfunction, and it may be accompanied by other anomalies of CNS4). The most frequent accompanying anomaly is hydrocephalus. For live-born cases, an effective reconstruction is essential to close the exposed neural elements and to protect the patient from sepsis5).
Low socioeconomical state is a risk factor for meningomyelocele development. Studies from all around the world and Turkey have reported higher frequency of neural tube defect among families with lower educational states2,7). Van 100. Yıl University is located in eastern part of Turkey and educational level of families in this area is not high. 89% of the mothers had low educational level in our study.
Studies report most frequent location of meningomyelocele as lumbar region (69%)3). This was supported with our observation in our study that 78.1% of cases had MM in lumbar region. The most common additional anomaly in MM is hydrocephalus8). In our study group, the most frequent accompanying anomaly was also hydrocephalus, observed in 73.4% of cases.
During our review of the literature, we did not encounter any study directly investigating the effect of diameter of the meningomyelocele sac on prognosis. In cases with meningomyelocele, increasing sac diameter is associated with greater amount of neural structures within the sac, which worsens the neurological condition9). Diameter of the sac affects prognosis significantly in cases with meningomyelocele6). Neurological condition in group I patients, who have the smallest sac diameter, was better in comparison to group III patients who have larger sac diameter (p<0.05). While all of the group III patients had paraplegia, this ratio was 38% in group I patients, who have sac diameter below 24 cm2. Müslüman et al.6) stated that they closed the defects via primary closure without flap requirement in babies who had sac smaller than 25 cm2, and that these babies had shorter hospital stay length, lower infection risk, and better prognosis. In our study we determined that localization of sac was not affecting prognosis (Table 1), we observed that sac diameter and having neural tissue within the sac were more important prognostic factors than the sac localization.
In our series, infection rate and hospital stay length in group I patients (defect size smaller than 24 cm2) were significantly lower in comparison to groups II and III patients (p<0.05). In our study group, sac diameter at newborn period was a borderline 2×2-cm size. We observed that these babies with a sac size below this borderline had better outcomes than the group with larger defect in terms of hospital stay length, duration of antibiotherapy, as well as neurological conditions. In their series including 35 cases with meningomyelocele, Wilson et al.9) reported that apart from 1 case, all patients had sac size below 24 cm2, and only 8 cases (22.9%) had poor neurological function during follow-up. In our study group, the ratio of paraplegic cases among babies with sac size below 25 cm2 was 38.2%.
CONCLUSION
Babies with smaller meningomyelocele sac have shorter hospital stay length and fewer complications at early period, and more favorable neurological signs. Increasing size of the sac is associated with greater amount of neural tissue within the sac, requiring flap for skin repair and worsening the prognosis.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.

