Intradural Extramedullary Capillary Hemangioma In the Upper Thoracic Spine with Simultaneous Extensive Arachnoiditis
Article information
Abstract
Capillary hemangiomas are common benign vascular tumors on skin and soft tissues, but developing as an intradural and extramedullary (IDEM) tumor in spine is extremely rare. In this report, we present IDEM tumor compressing thoracic cord in T2–3 level with extensive arachnoiditis below the tumor level in a 60-year-old man. The lesion was removed and histological diagnosis was capillary hemangioma. Prompt diagnosis and resection are important to avoid neurological deterioration from acute hemorrhagic condition. Simultaneous arachnoiditis may be originated from old subarachnoid hemorrhage associated tumor before diagnosis, and we suggest it as a helpful diagnostic feature to suspect vascular tumors such as capillary hemangioma.
INTRODUCTION
Vascular lesions make up 2%–7% of all spinal intradural tumors. These include cavernous/capillary hemangioma (CH), arteriovenous malformations, capillary telangiectasias, and venous malformation. Hemangioma can be subdivided into capillary, cavernous, mixed, and cellular, depending on capillary and cavernous predominating15,17,23).
Customarily CH is benign vascular neoplasms, most of them develop in the skin or soft tissue but on rare occasions occurring in the intradural space of the spine1,17,21). CH as intradural extramedullary (IDEM) tumor in spine is extremely rare and difficult to differentiate with other common pathologies10,21). Despite of the low incidence, prompt diagnosis and complete resection area important, because acute hemorrhage leading to sudden neurologic deterioration can be occurred17).
It is known to be commonly located near conus medullaris or attached to nerve roots of cauda equina. We review a rare case of IDEM CH in the upper thoracic spine accompanying extensive arachnoiditis below the tumor in this report.
CASE REPORT
A 60-year-old man presented with 1-month history of hypoesthesia in the trunk below T4 sensory dermatome, progressive gait disturbance and thoracic girdle pain. On the neurological examination, he demonstrated sensory impairment below T5 dermatome and increased deep tendon reflex bilaterally. The patient did not have any previous history of trauma or spine surgery before.
Magnetic resonance (MR) imaging demonstrated an oval shaped and well defined IDEM mass lesion centered with compressing thoracic cord in T2–3 level. The lesion was iso-intensity on T1-weighted images (T1WI), relatively hyper-intensity on T2-weighted images (T2WI), and homogenous well enhancement of entire mass with considering dural tail sign on contrast-enhanced T1WI. There were signal voids, suspicious vascular structures around the tumor on T2WI. Additionally, extensive arachnoiditis on thoracolumbar region below the tumor level and small sized and round shaped syrinx on the conus medullaris were noted (Fig. 1).
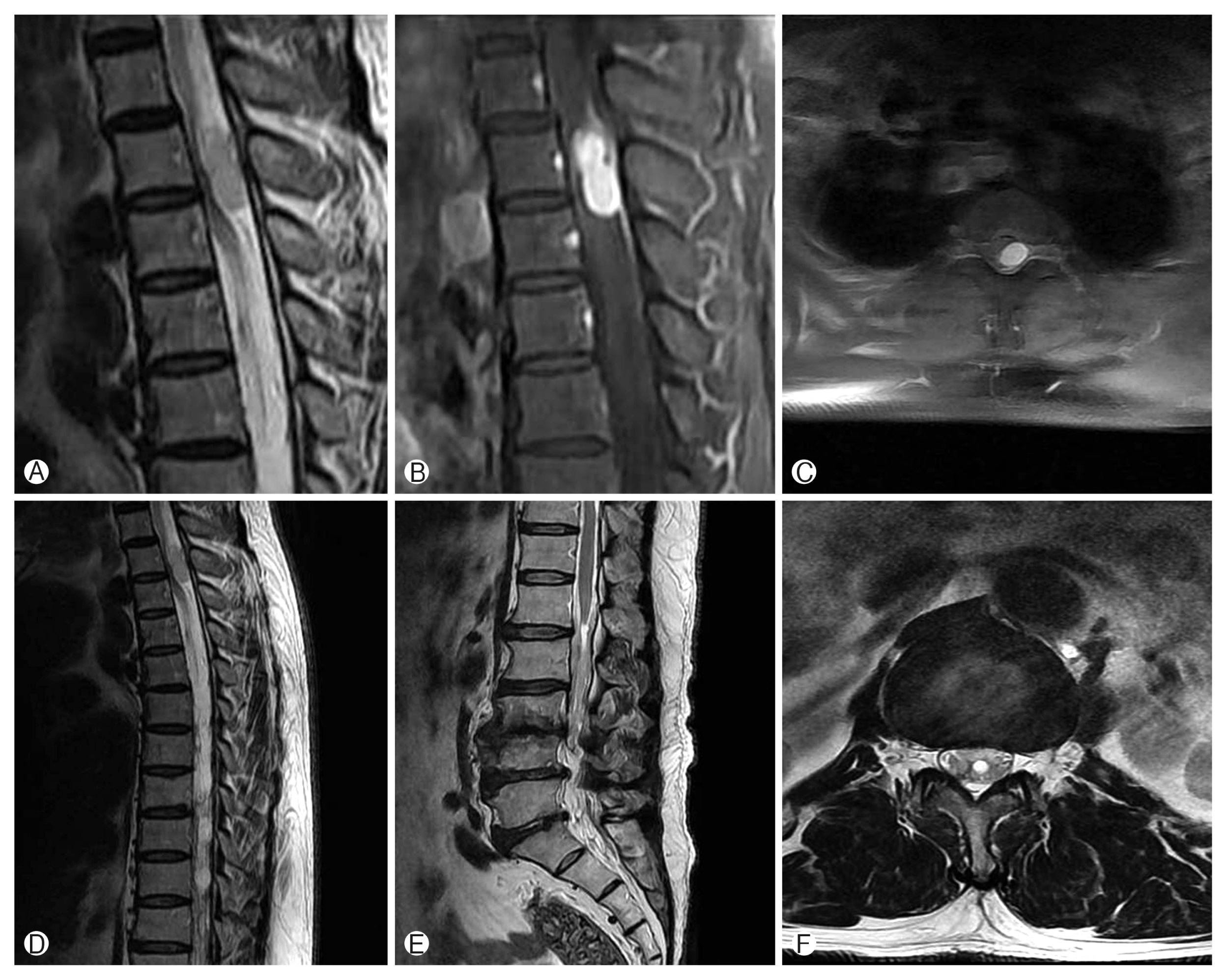
Preoperative magnetic resonance images. (A) Sagittal T2-weighted image shows a round well-circumscribed lesion with relatively hyperintensity with compressing spinal cord at the T3 level. Contrast-enhanced T1-weighted image shows enhancement of the whole part of the mass with the enhancement lesion such as dural tail sign (B) and tumor occupying the most of the spinal canal with replacing the thoracic cord (C). (D–F) There are also extensive arachnoiditis below T3 and small sized and round shaped syrinx of conus medullaris.
The preoperative diagnosis was a meningioma due to homogeneous enhancement across the whole mass lesion and attached dura considered as dural tail sign. The patient underwent T2 total laminectomy and T3 subtotal laminectomy. On opening the dura, friable oval shaped, dark reddish colored mass compressing spinal cord was noted. The mass was covered by the thickened arachnoid membrane, and strongly matted and adherent to pia mater of thoracic cord and involving left T2 nerve root. There was an ill-defined boundary between the tumor and the thoracic cord without attaching to the dura. Careful dissections and coagulating the surrounding tissues were performed to divide and dissect the spinal cord and nerve root from the tumor under the microscope. There was some amount of bleeding by the violation of tumor capsule during resection. Cranial and caudal poles of the mass contained dilated vessels traveling along the direction of left T2 nerve roots. Complete resection was achieved with detachment of around neural components and coagulation of dilated vessels.
Histological examination revealed hemangioma consists of a myriad of tightly packed well-formed capillaries lined by a single layer of endothelial cells. There are also variably dilated cavernous formations within the clustered capillaries. Immuno-histochemical staining showed positive response on CD31 (Fig. 2).

(A) Complete resection was achieved. There was some amount of bleeding by the violation of tumor capsule during dissection. (B) Histological examination (H&E, ×100) revealed hemangioma consists of a myriad of tightly packed well-formed capillaries lined by a single layer of endothelial cells. There are also variably dilated cavernous formations within the clustered capillaries. Immuno-histochemical staining (×200) showed positive response on CD31.
The patient’s symptoms including gait disturbance and sensory change improved noticeably 2 weeks after the surgery.
DISCUSSION
The incidence of CH on the upper thoracic cord is extremely rare, and there were only 9 cases of CH in the literature review even though including middle thoracic cord (Table 1). CH may arise from the blood vessels of nerve roots, the inner surface of the dura, or the pial surface of the spinal cord7,18,19,21). CH usually behaves as space occupying mass lesion, and its most common clinical course does not differ from that of other spinal lesions with slowly and progressive spinal cord compression producing myelopathy and/or radiculopathy. Acute symptoms are probably caused by newly developed hemorrhages within or around the mass. However, the incidence of bleeding is relatively lower than that of typical cavernous hemangioma14,17).

Nine cases of intradural extramedullary capillary hemangioma on upper and middle thoracic spine previously reported
On MR images, the differential diagnosis for IDEM tumors includes meningioma, schwannoma, paraganglioma, hemangioblastoma, cavernous angioma, and metastasis. Among these tumors, schwannoma and meningioma are the most common pathologies developing at thoracic spine, and show similar MR imaging features with CH21). Especially, CH arises from the inner surface of the dura mater, and it reveal similar feature such as a dural tail sign4,21). There are difficulties to differentiate CH from the other IDEM tumors, and still are risks of misdiagnosis1,9). Tekin et al.22) suggested the importance of identifying draining vein detecting as signal voids on T2WI might be a clue to suspect a vascular IDEM preoperatively. In these respect, a retrospective review of preoperative MR imaging of our patient shows round shaped mass lesion accompanying no cystic lesion and relatively iso-intensity on T1WI and hyper-intensity on T2WI, which is differ from typical features of meningioma and schwannoma. There were signal voids on the cranial and caudal poles of the mass. This feature could be a clue to deserve further attention to consider vascular lesions.
Additionally, the patient showed extensive arachnoiditis and syrinx on the conus medullaris below the tumor level. These conditions mean the disturbance of the movement of cerebrospinal fluid (CSF) in the spinal cord just distal to the tumor. The pressure of CSF transmits inside the spinal cord through the central canal causing interstitial edema, it becomes relatively larger than that of outside. Repetitive formation of this pressure gradient leads to development of the syrinx3). Swelling of conus medullaris in this patient may be a progress of developing syrinx with the aggravation of extensive thoracolumbar arachnoiditis. Spinal arachnoiditis is a chronic inflammation of the pia-arachnoid that occurs after meningitis, spinal trauma, subarachnoid hemorrhage, and intrathecal injection12,20). Holtzman et al.7) reported that the intense arachnoiditis that was noted to surround the tumor may have resulted from minute bleeding or the diapedesis of erythrocytes.
Although the incidence of hemorrhage in CH relatively rare among the vascular tumors, there was a cerebral CH presenting acute massive hemorrhage was reported as a case report13). We guess the accompanied spinal arachnoiditis may have resulted from the thickened arachnoid membrane and strong adhesion of CH into pia mater of thoracic cord, which might be developed by old subarachnoid hemorrhage from CH. From these backgrounds, we think the accompanying arachnoiditis can be an additional diagnostic clue to suspect vascular tumor.
Piecemeal resection should be avoided during the operation, because excessive intraoperative bleeding can result in incomplete resection. Subtotal resection may lead to recurrence of neurological symptoms related with regrowth of mass or bleeding from residual lesion8,14,17). Untethering the spinal cord with removing CH and restoring CSF flow disturbance can improve arachnoiditis. However, intraoperative bleeding during the surgery aggravated arachnoiditis below the tumor level and enlarged syrinx on conus medullaris 1 year postoperatively (Fig. 3).
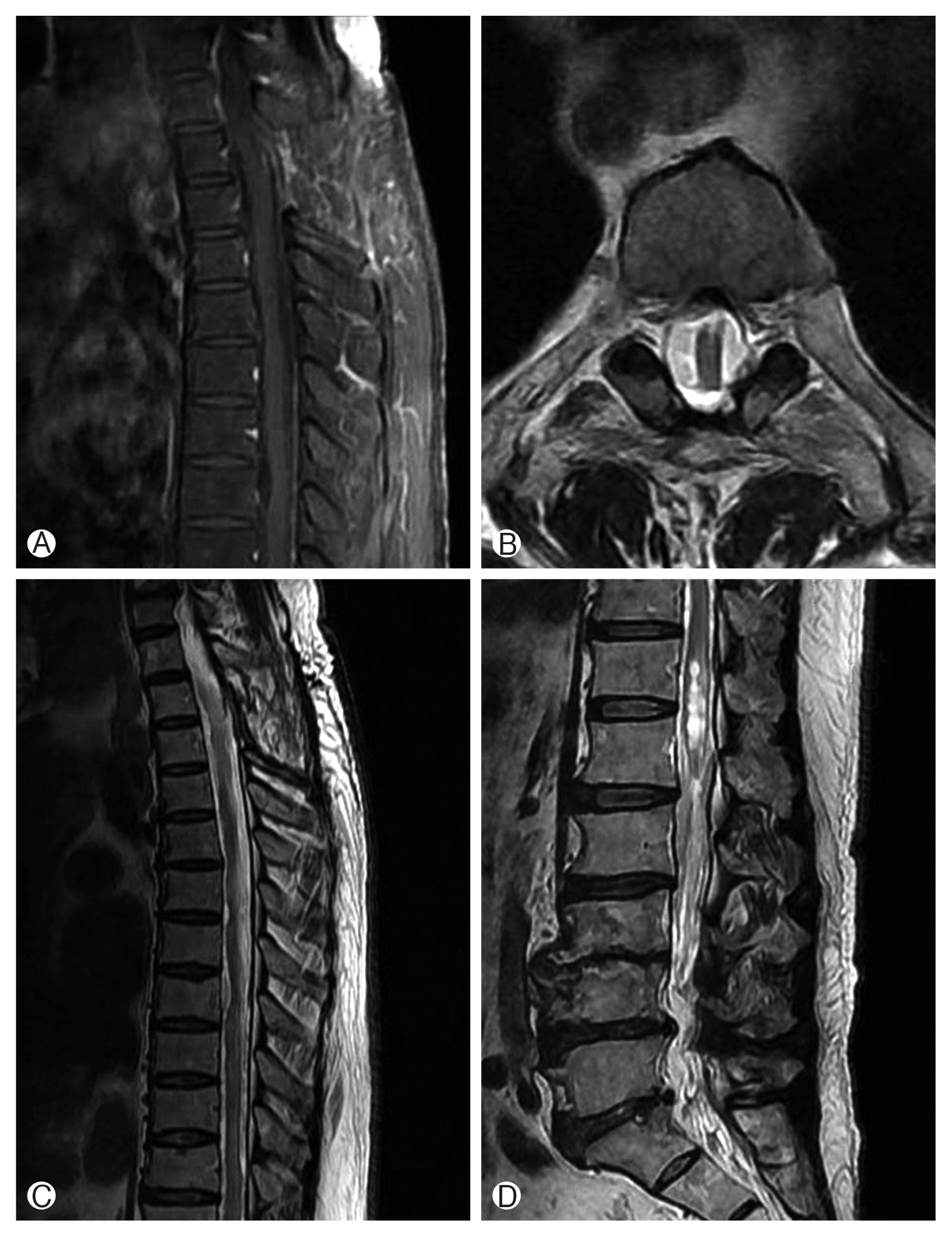
Postoperative magnetic resonance images. (A, B) Complete resection of the tumor was achieved under total laminectomy. (C, D) There are sustained arachnoiditis and progression of syrinx on the conus medullaris.
The conclusive treatment modality for spinal arachnoiditis has not been established. Resolution of spinal cord tethering and CSF flow disturbance via surgery are regarded as effective treatment method. Arachnoid dissection and subarachnoid space decompression with duroplasy for a focal arachnoiditis was suggested, but less satisfactory results were obtained in the patients with extensive arachnoiditis involving greater than two vertebral levels11). Mitsuyama et al.16) introduced arachnoid microdissection and ventriculo-subarachnoid shunting for longitudinally extensive spinal adhesive arachnoiditis.
CONCLUSION
IDEM CH on upper thoracic spine is extremely rare, and it has similar clinical and radiological features compared with other usual IDEM tumors. CH should be included as a differential diagnosis for IDEM tumors for prompt diagnosis and surgical resection. We suggest simultaneous arachnoiditis as a helpful diagnostic feature to suspect vascular tumors such as CH.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.