Etiopathogenesis of Traumatic Spinal Epidural Hematoma
Article information
Abstract
Spinal epidural hematoma (SEH) is a rare cause of nerve root or cord compression; its pathogenesis is not always clearly recognizable. The aim of this paper was to investigate possible etiopathological factors in a consecutive series of patients affected by traumatic SEH treated at our institution. Seven patients with neurologic impairment due to traumatic SEH were retrospectively analyzed after diagnosis and surgical treatment. Thoracic localization was found in 5 cases, and lumbar and cervical localization were found in 1 patient each. One patient was affected by ankylosing spondylitis and one by diffuse idiopathic skeletal hyperostosis. SEH was associated with spine fractures in 6 cases. Only 2 cases of traumatic SEH resulted from high-energy trauma. All patients underwent surgical decompression within 24 hours after admittance to the hospital. Three patients recovered completely, 3 remained paraplegic, and 1 remained monoplegic. Several concomitant conditions are suggested to be predisposing factors for the development of SEH, although its inherent mechanism is still unknown. Two patients in the present series were affected by rheumatologic disorders, confirming the elevated incidence of hematomas in such patients compared to the normal population. Three very unusual cases of SEH occurred in senile patients affected by osteoporotic fractures. Early diagnosis and urgent decompression of the hematoma remain mandatory.
INTRODUCTION
Spinal epidural hematoma (SEH) is an infrequent but very serious cause of acute neurologic compression that needs early diagnosis and rapid surgical treatment. In the Literature a hematoma of the epidural space is defined as “idiopathic” in case of unknown etiology and “spontaneous” when it occurs as a consequence of minor traumas yet not severe enough to cause vertebral fractures [1]. Spontaneous epidural hematoma represents at least 40% of all epidural bleeding and can occur in patients with artero-venous malformations of the epidural plexus, hemangiomas, spinal tumors or sistemic disease with a high risk of bleeding such as hemophilia in a pediatric population, multiple myeloma, lymphoma, pregnancy, alcoholism and other hepatic diseases, lupus and immunologic diseases or for anticoagulant therapy [2,3]. According to the different series, traumatic SEH occurs between 0.5% to 1.7% of all spinal injuries but the incidence grows up to 9% in cases of patients affected by ankylosing spondylitis or rheumatoid arthritis [4]. A SEH can occur also postoperatively as an unexpected complication of surgical procedures such as mini-invasive spine approach like vertebro- and kyphoplasty, after spine manipulation therapy, or simply for lumbar epidural puncture with an estimated incidence of 0.1%–0.24% [5,6].
In the present study, authors retrospectively analyze a series of 7 consecutive patients affected by SEH who were admitted into our Emergency Department for neurologic deficit of unknown cause. Clinical findings are evaluated and pathogenetic aspects are discussed.
MATERIALS AND METHODS
Seven consecutive patients, 3 females and 4 males, with a mean age of 66 years (range, 32–82 years) affected by SEH and surgically treated in our Division of Spinal Surgery were analyzed between September 2008 and July 2017 (Table 1). Idiopathic and postoperative iatrogenic hematomas were excluded from the present study. All patients were accepted by our Emergency Department for neurologic impairment and all underwent clinical and radiological examinations trough standard X-ray, computed tomography (CT) scan and magnetic resonance imaging (MRI). After the diagnosis of epidural bleeding patients underwent surgical decompression as soon as possible. A spinal stabilization was carried out in the same session in case of associated instability spinal fracture. For each patient clinical aspects, neurologic deficit, medical history, hematologic values, comorbidities and imaging findings were analyzed.
Five patients had a hematoma localized in the back side of the epidural space in the thoracic spine, in 1 case hematoma was localized in the lumbar spine, in another case the hematoma was localized in the cervical spine region. In 6 patients hematoma was associated with spine fractures classified as: 2 type C (1 was C1.1 and 1 C1.3), 1 type B (B1.2), and 3 type A (1 was type A1.1 and 2 were A3.1) according to Magerl’s classification (Table 2) [7]. One type C and 1 type B fractures occurred in patients affected by pre-existing spinal disease. All type C fracturedislocation occurred as a consequence of high-energy trauma. In one case the patient had a long history of ankylosing spondylitis (Fig. 1) while the second one was occurred in a young patient without comorbidities. Type B fracture occurred in a patient affected by diffuse idiopathic skeletal hyperostosis (DISH) as a consequence of a minor trauma (Fig. 2). Three patients had type A fracture from a low-energy trauma that were classified as “fragility fractures” according to the radiographic morphology of the fracture, age of the patients, the insignificance of the trauma and the presence of a clear osteoporosis. Only one patient reported a hematoma as a consequence of head injury with cervical spine hyperextension without any recognizable fractures. At admission to the hospital, all patients of type A group presented clinical comorbidities, the most relevant of which was the high levels of international normalized ratio (INR) due to oral anticoagulant therapy (3 patients had previously undergone cardiac surgery). In 1 case (fragility fracture of T11) hematoma and fracture occurred in a patient treated with chemotherapy (due to an active phase of non-Hodgkin lymphoma) and oral anticoagulants (Fig. 3).
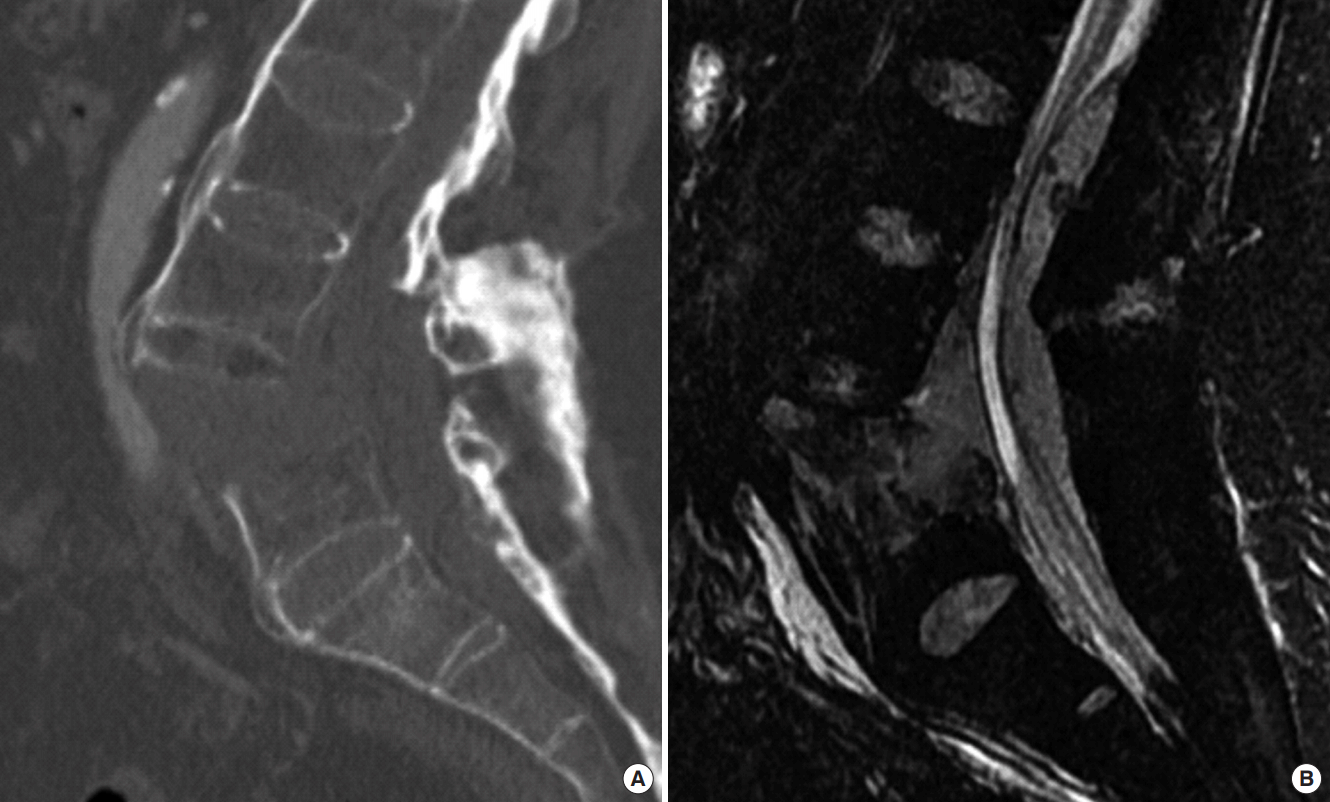
Sagittal computed tomography (A) and T2-weighted magnetic resonance imaging (B) of the lumbar spine in a 59-year-old male patient affected by ankylosing spondylitis, showing the displaced fracture of L5 after a high energy fall, associated to a hematoma in the posterior part of the epidural space.

Fracture of T3 in a 78 years old male patient affected by diffuse idiopathic skeletal hyperostosis and an epidural hematoma extended from the C6–7 intervertebral disc to T9 vertebral body. The hematoma shows its maximum size and the greatest compressive effect on the spinal cord at the level of the fracture of T3 vertebral body.

Fracture of T11 from accidental fall in a 79-year-old woman during chemotherapy for non-Hodgkin lymphoma. Sagittal T1 (A) and T2 (B) weighted and axial magnetic resonance imaging (C) images show an expanding mass in the epidural space with the typical signal intensities due to the catabolism of meta-hemoglobin.
Although CT-scan is the exam of routine in case of spinal trauma in our Emergency Department, MRI is the exam of choice in case of neurologic deficits and it is able to recognize the cause of cord compression also allowing a differential diagnosis with other potential mass occupying space in the vertebral canal. In fact the MRI images of a hematoma typically present a spindle-shaped mass, generally located in the back side of vertebral canal behind the spinal cord, with a mosaic appearance and a different intensity area induced by hemoglobin degradation. Generally, hematoma is wider in the affected site and tends to expand in the adjacent spinal segment. In the present series, hematomas were always extended to more than 1 segment. In one case hematoma extended from L5 as far as the vertebral body of L2 (ankylosing spondylitis) while in another case (patient affected by DISH) it extended from the C6–7 intervertebral disc as far as T9 vertebral body although the maximum compressive effect was at T3, at the level of the fractured vertebral body.
Neurological status at admission was classified according to the Frankel cord impairment grading system (Table 2). Three patients had a complete neurologic deficit, grade A (2 type A, and 1 C type fracture), 2 had grade C (1 type A and 1 type B fracture), and 1 grade D (type C fracture in the lumbar spine of spondylitic patient). In 1 patient with complete paraplegia (type A fracture) the hematoma developed at the level of the fracture and extended proximally for one segment. In this case a severe degree of lumbar stenosis below the fracture was clearly evident. It is highly probable that the devastating effects of bleeding were worsened by pre-existing sufferance of cauda equina nerve roots. In 1 patient there was Brown-Sequard syndrome with emiplegia and motor paralysis on the right side, where the lesion is found, while on the left side there were deficits in pain and temperature sensation.
Four patients underwent cord decompression by simple laminectomy and removal of the hematoma; in 3 patient a laminectomy and spine fixation for potential instability of the spine was performed (Fig. 4, Table 2).
RESULTS
All patients underwent cord decompression within 24 hours of admittance into our Emergency Department although in 4 cases first signs of neurologic impairment appeared more than 24 hours before hospital admittance. Both Frankel C and Frankel D patients showed a complete neurologic recovery after surgical decompression and postoperative rehabilitation. The patients with Frankel A neurological status did not show any recovery and remained completely paraplegic. Instead the patient with Brown-Sequard syndrome caused by cervical spine hematoma remained monoplegic. The patient with lymphoma, belonging to the Frankel A group died several months after decompression for medical causes related to hematologic disease. The patient with cervical spine hematoma died of postoperative complication and comorbilities few days after surgery.
DISCUSSION
SEH is an infrequent disease that generally could be divided into 2 main groups, spontaneous and traumatic, depending on the etiopathogenesis, even though in some cases it is impossible to recognize the true pathogenesis of the epidural bleeding [8]. Spontaneous hematoma is more frequent in male patients over 50 years and the thoracic spine is the most frequent localization, while lumbar localization is more frequent in youth. Cervical spine is involved in 16% of cases with frequent extension of the hematoma to the proximal thoracic tract. The pathogenesis of the hematoma is not completely understood but the vast majority of the authors believe that posterior epidural venous plexus is the most important anatomical structure from which the bleeding takes place [9]. It is well known that pregnancy and all causes of rapid increase of inner abdominal and thoracic pressure such as coughing, sneezing, vomiting and urination, can cause a lesion of venous epidural plexus [2,10]. Traumatic SEHs occur frequently in adult (≥40 years old) mostly in the cervical and proximal thoracic spine and, when associated to vertebral fractures, they generally occur from high energy trauma and typically in patients affected by rheumatologic diseases such as ankylosing spondylitis, rheumatoid arthritis, psoriasic spondylitis [8,11,12].
The reason why a SEH is statistically more frequent in rheumatologic patients rather than normal healthy people is not clear but in our small series 2 patients had ankylosing spondylitis and DISH syndrome while one of them reported a high-energy trauma after high fall. Other three patients belonging to the traumatic group had a minor trauma with associated fragility fractures classified as type A according to Magerl’s classification [7].
Regarding the cause of bleeding in the epidural space some hypothesis have been done. It is reasonable to suppose that when a hematoma is associated with a vertebral fracture the bleeding could originate in fractured spongy bone or from damaged vessels of the epidural space with a secondary collection of blood around the spinal cord [13]. Although this hypothesis seems to be logic, the incidence of hematoma in the epidural space in case of traumatic spinal fractures remains surprising low if compared to the huge number of traumatic injuries of the spine. In our series only 2 cases occurred as a consequence of a real highenergy trauma while the other patients sustained only minor traumatic incidents. Further, in 3 cases, the patients reported vertebral body fractures classified as “fragility fracture” due to osteoporosis. Nowadays osteoporotic fractures are becoming even more common given the increase in mean age, nevertheless, only very few cases of epidural hematoma has been reported in literature associated with fragility fractures in the elderly population [2,14].
A further case of associated fragility fracture and SEH was published but in this case the hematoma was the consequence of the percutaneous approach to the spine for vertebroplasty rather than directly related to the traumatic event and, for this reason, it has to be classified as iatrogenic [5]. Based on the above mentioned considerations, it could be reasonable to hypothesize that other factors than traumatic events alone can concur to facilitate bleeding in a closed space such as the epidural space. In our patients with minor trauma we were able to find adjunctive medical factors able to induce a high risk of bleeding such as oral or parenteral anticoagulant drugs and comorbidities (2 patients had non-Hodgkin lymphoma, 1 of them was treated with chemotherapy; and 3 patients underwent cardiac surgery some years before). Although a close relationship between blood coagulation properties and hematoma seems easily recognizable, only very few papers in the literature, with a large series of patients, focused their attention on the pathogenetic mechanisms of bleeding, rather than on the challenging diagnosis, timing of treatment and evaluation of the prognostic factors [15,16].
On the other hand, there are a lot of case reports that speculate, case by case, on the patho-genetic aspects suggesting various hypotheses. From literature review, underlining the uncertainty of SHE pathogenesis, the most reasonable explanation seems to be that hematoma develops as a consequence of a combination of more than one risk factor among which the most important is certainly the alterations of the normal blood coagulative properties caused by iatrogenic and paraneoplastic factors. Six patients from our series had high levels of INR upon admission to hospital from badly conducted anticoagulation therapy.
Finally, it is very difficult to find any relationship between cancer and hematoma although few reports in the literature describe the association of SEH and tumors especially hematologic cancer. Pathogenetic factors which are suggested to be responsible for the hematoma are epidural inflammation, hematic stasis, venous epidural vessels fragility due the presence of neoplastic tissue, microfractures, chemotherapy or hematoma for pathologic fractures. We do not know exactly the pathogenesis of the hematoma in neoplastic cases of this series (non-Hodgkin lymphoma) but we are prone to think that modifications of coagulative properties for anticoagulant therapy was the most relevant factor in development of hematoma fragile patients.
Concerning diagnosis, MRI is the gold standard in recognizing the real cause of cord compression. The mosaic appearance of the fusiform mass in the epidural space due to the irregular alternation of different intensity areas caused by the methaemoglobin degradation is a pathognomonic sign of disease [10]. SEH may have a wide clinical presentation from radiculopathy to more severe quadriplegia depending on the rapidity of the onset [17]. Sometimes it is possible to find rare clinical conditions such us Brown-Sequard syndrome with motor paralysis on the same side as lesion and deficits in pain and temperature sensation on the opposite side.
The etiology of neurological deficits is not only caused by compression but also by secondary damage due to local flogosis [18,19]. Surgical decompression carried out as soon as possible is widely accepted as the reference treatment of the disease although it depends on the severity of neurologic impairment at hospital admission [16]. Adhering to a general concept, valid in every case of cord compression, an early surgical decompression after the achievement of correct diagnosis is the best way to ensure a chance of neurologic recovery [20]. Three patients from our series regained complete neurologic recovery. Another three patients, all affected to high-severity neurologic impairment (Frankel grade A), did not improve at all; 1 patient with Brown-Sequard syndrome showed a partial recovery of the upper right arm but not in the lower. A conservative treatment could be take into consideration only in case of very mild and nonprogressive neurologic impairment or in hemophilic patients because of high risk of surgical bleeding [9].
CONCLUSION
A SEH, spontaneous or traumatic, is a very infrequent event that, if not rapidly recognized and rapidly treated, can cause high-grade neurologic disability. A really surprising aspect is the disproportion which exists between the huge number of traumatic spine injuries and the low incidence of associated SHE. What is difficult to understand is the true mechanism of blood collection inside a confined space such as the spinal canal. It is highly probable that more than one factor can converge in causing epidural space bleeding. In our series we had 2 patients affected by rheumatologic diseases (ankylosing spondylitis and DISH) that confirmed the reported high incidence in Literature of hematomas in rheumatologic patients and two patients who had high energy trauma. Because all patients had oral anticoagulant therapy with very high values of INR at the time of hospital admittance, we are forced to suggest that the decreased coagulation properties induced by medical treatment could play the major role in causing the epidural bleeding. Although this hypothesis seems to be the most suitable, it is a fact that every day in the world, millions of elderly people suffer fragility fractures and, because of the age and concomitant diseases, the vast majority of them take drugs that act on blood coagulation, the incidence of epidural hematomas remains however very low. Therefore the occurrence of SEH in three of seven patients of our series, affected by osteoporotic fractures, has to be regarded definitely as relatively uncommon.
Notes
The authors have nothing to disclose.