Comparison of Axial Symptoms and Limitations of Activities of Daily Living Accompanying Reduced Neck Mobility After Cervical Laminoplasty Preserving C2 Muscle Attachments With and Without C2 to T1 Instrumented Fusion
Article information
Abstract
Objective
Muscles are usually detached from C2 to facilitate C2 pedicle screw insertion. The aim of this study was to compare 1-year postoperative axial symptoms and limitations in activities of daily livings (ADLs) accompanying reduced neck mobility between 2 procedures in which all C2 muscle attachments are preserved: laminoplasty and C2 to T1 fusion (LPF group: n=15) and laminoplasty alone (LP group: n=26).
Methods
We examined axial symptoms and limitations in ADLs using the Japanese Orthopedic Association Cervical Myelopathy Evaluation Questionnaire. We also examined related factors, including the occiput (O)–C7 angle in extension and flexion, and the rotational and O–C2 ranges of motion (ROM).
Results
The postoperative decreases in the O–C7 angle in flexion (27.8° vs. 9.4°) and rotational ROM (40° vs. 15°), as well as the compensating postoperative increase in the O–C2 ROM (11.7° vs. 2.3°), were significantly greater in the LPF group. Most of the axial symptoms were similar between groups. The ability to perform ADLs tended to worsen more frequently in the LPF group, but the difference did not achieve significance.
Conclusion
Postoperative changes in axial symptoms and loss of ROM were not obstacles affecting patients’ ability to perform ADLs after laminoplasty with muscle-sparing C2 to T1 fusion.
INTRODUCTION
The severity of ossification of the posterior longitudinal ligament (OPLL) can be characterized by its assessment as K-line (+) or (-) [1]. The K-line is a straight line joining the midpoints of the spinal canal at C2 and C7 on a lateral radiograph. In K-line (+) OPLL, the ossified mass does not cross the K-line, whereas in K-line (-) OPLL, some portion of the OPLL mass crosses the K-line posteriorly. In large OPLL, the mass occupies >60% of the area anterior to the K-line. Cervical laminoplasty (LP) has been reported to result in poor outcomes in patients with K-line (-) or large OPLL [2]. Therefore, posterior decompression with instrumented fusion has been used to treat cervical myelopathy with K-line (-) OPLL and has resulted in good neurologic improvement [3]. However, postoperative worsening of axial symptoms [4-8] or limitation of activities of daily livings (ADLs) accompanying reduced neck mobility [9,10] are potential problems after posterior fusion; the added instrumentation in posterior fusion is thought to worsen these problems compared with LP alone.
Problems associated with LP are reduced by preserving the posterior muscles, especially the semispinalis cervicis (SSC) insertion onto the spinous process of C2 [7,10]. At our institution, instead of performing a conventional LP procedure—C3 to C7 LP, with reattachment of the SSC insertion onto C2—we perform C4 to C7 LP with C3 laminectomy, preserving the SSC insertion onto C2 (Fig. 1). Furthermore, in 2011, we changed the surgical procedure for cervical myelopathy with K-line (-) OPLL in neutral or flexed position [11] from a modified LP, preserving the SSC insertion onto C2, to LP with C2 to T1 instrumented fusion (LPF) (Fig. 2), completely preserving all muscle attachments to C2, including the rectus capitis posterior major (RCPM) and obliquus capitis inferior (OCI) origins and the SSC insertion (Fig. 3) [12].

Cervical laminoplasty of C4 to C7 with C3 laminectomy with preserved semispinalis cervicis into C2 (white arrows) seen on 3-dimensional computed tomography (A) and intraoperative photograph (B).

Cervical laminoplasty and C2 to T1 instrumented fusion seen on front radiograph (A) and lateral radiograph (B).

Preservation of all muscle attachments (rectus capitis posterior major [black arrow], obliquus capitis inferior [white arrow], and semispinalis cervicis [black dotted arrow]) to C2 during cervical laminoplasty with C2 to T1 fusion.
We hypothesized that postoperative axial symptoms and limitations in ADLs accompanying reduced neck mobility would not increase after LPF compared with after LP alone despite the added instrumentation in posterior fusion. This retrospective study compared postoperative axial symptoms and limitations in ADLs accompanying postoperative reduced neck mobility after LPF with preservation of all muscle attachments to C2, with those of modified LP with preservation of the SSC insertion onto C2. Furthermore, the pre- and postoperative O–C7 angle, O–C2 ROM, and rotational ROM were measured after LP and LPF.
MATERIALS AND METHODS
1. Ethics Approval
This study was approved by the institutional ethics committee of Odate Municipal General Hospital according to the 1964 Helsinki declaration (approval number: 30-09), and informed consent was obtained from all participants.
2. Subjects
Fifteen consecutive patients treated for K-line (-) OPLL in neutral or flexed position beginning in 2011 were enrolled in the study. All 15 patients (9 men, 6 women) were treated with LPF (Fig. 2) with the preservation of all muscle attachments into the C2 spinous process (LPF group), including the RCPM, OCI, and SSC (Fig. 3). All patients were examined 1 year after surgery. The mean age at the time of surgery was 61 years (range, 41–81 years).
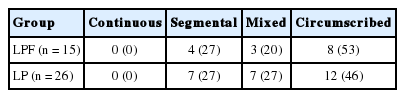
Thirty-one consecutive patients with K-line (+) OPLL in neutral or flexed position were treated at the same hospital with C4 to C7 LP with C3 laminectomy, with preservation of the SSC insertion onto C2 (Fig. 1). Five patients were excluded because of neuromuscular diseases, such as Parkinson disease; fracture of a lifted lamina, and postoperative fracture of the left humerus. The remaining 26 patients (10 men, 16 women) comprised the control group (LP group). The mean age at the time of surgery was 69 years (range, 48–83 years). The LP group was also investigated 1 year postoperatively.
All patients in both groups were evaluated radiographically, by computed tomography, and by magnetic resonance imaging. No patient in either group had any additional cervical spondylotic changes, including osteophyte formation or disc herniation.
All patients in both groups underwent evaluation of preoperative spinal cord evoked potential (SCEP) to determine the anatomic level at which neurologic symptom due to OPLL were evident. The SCEP was measured after the electrical stimulation of the spinal cord, as described by Tani et al. [13] In brief, local anesthesia was applied and platinum-tipped stimulating and recording electrodes were introduced into the dorsal epidural space at the level of the lower thorax and C3, respectively. Recordings were obtained simultaneously at the level of C3, C3/4, C4, C4/5, C5, C5/6, C6, C6/7, C7, and C7/T1. Each test set comprised 50 times of summated potentials, with a stimulation frequency of 30 Hz and a stimulus intensity of 7–10 mA. An evoked potential was defined as neurologically symptomatic at a specific vertebral level when the negative peak increased at the immediately caudal level to and decreased immediately cranial to the vertebra. The anatomic distributions of neurologically symptomatic responses are shown in Table 1. In addition, the type of OPLL present in each patient was evaluated according to the classification system established by the Investigative Committee on the Ossification of the Spinal Ligaments, of the Japanese Ministry of Public Health and Welfare (now the Japanese Ministry of Health, Labour, and Welfare). Lateral plain radiographs were used to classify OPLL of the cervical spine as either continuous, segmental, mixed, or circumscribed [14,15], as shown in Table 2.
3. Operative Technique and Postoperative Management
Both groups underwent laminectomy at C3 with complete preservation of the SSC insertion at C2 [8]. The LP procedure used in both groups was a spinous process-splitting LP (double-door type) performed using a thread-wire saw [16], with the placement of hydroxyapatite spinous process spacers [17] at C4 to C7. In the LPF group, pedicle screws were inserted bilaterally in the C2, C7, and T1 pedicles. Using retractors, bipolar cautery, and surgical scissors, the areas of screw placement in the C2 pedicle were exposed in the space between the OCI and the SSC muscles, thus completely sparing the C2 muscle attachments [12]. The rods were passed under the preserved SSC muscles into C2 bilaterally. Lateral mass screws at C4 to C6, or C5 pedicle screws, were used as mid-cervical anchors in the LPF group. Local bone graft was placed in the LPF group but not in the LP group. Postoperatively, no cervical collar was applied in either group. The postoperative exercise was started within 2 days in both groups, and patients were permitted to sit up or walk within 1 week after surgery.
4. Measurements of O–C7 Angle, O–C2 ROM, and Rotation ROM
Pre- and postoperative O–C7 angles in flexion and extension were measured using the McGregor line and the posterior tangents of the C7 vertebral body on flexion and extension lateral radiographs, respectively, of the cervical spine (Fig. 4) [10]. O–C2 ROM was also measured using the McGregor line and the Cobb line of the C2 vertebral body on flexion and extension lateral radiographs of the cervical spine (Fig. 5). All radiographs were measured using XTREX VIEW (J-Mac System, Inc., Sapporo, Japan), which was accurate to 0.01°. XTREX VIEW is a highly flexible graphical user interface that enables measurements to be easily and accurately performed simply by moving a cursor.

Measurements of O–C7 angle. Measurement lines obtained using McGregor line and the posterior tangents of the C7 vertebral body on flexion (A) and extension lateral (B) radiographs of the cervical spine. O, occiput.
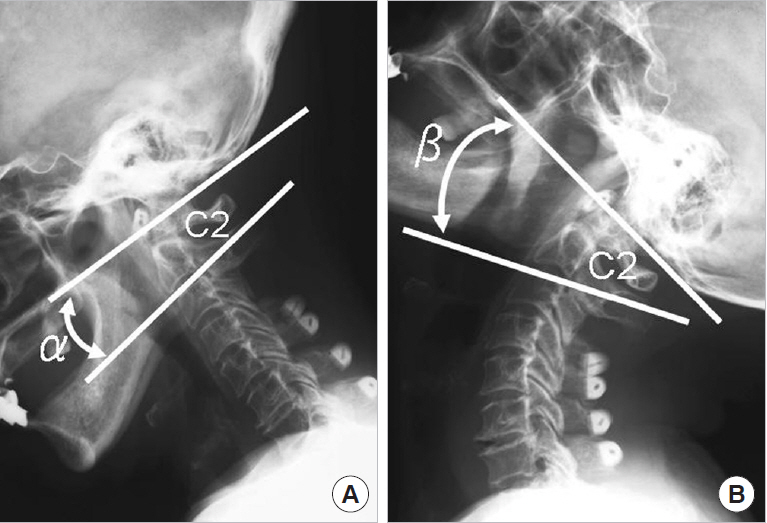
Measurements of O–C2 range of motion (ROM). Measurement lines obtained using the McGregor line and the Cobb line of the C2 vertebral body in lateral radiographs taken with the cervical spine in flexion (A) and extension (B). O– C2 ROM=β–α. O, occiput.
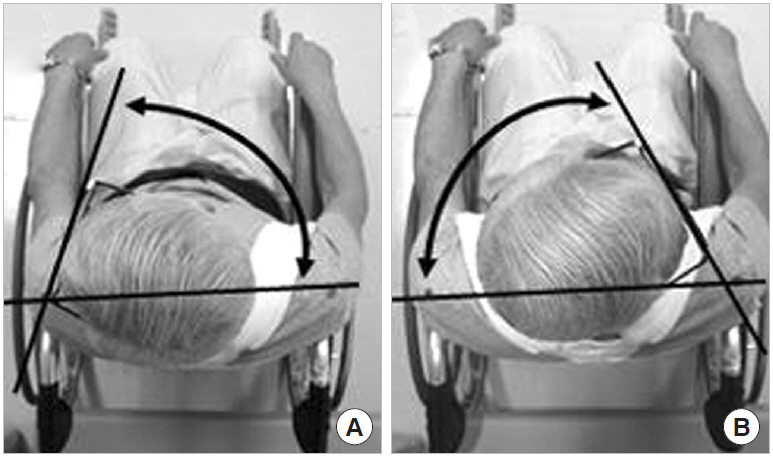
Pre- and postoperative rotation ROMs were measured on photographs. Patients were placed in a special wheelchair and secured in the sitting position with belts, and cranial-view digital photographs were taken with the patient wearing eyeglasses (Fig. 6) [9,10]. The photographs were scanned into a computer and were measured using image processing software (Image J, US National Institutes of Health, Bethesda, MD, USA), which is accurate to 0.01°.
5. Evaluation of Axial Symptoms
Axial symptoms were evaluated in both groups using a 100-mm visual analogue scale (VAS). Patients were classified as having axial symptoms if they fulfilled 3 of the following 4 diagnostic criteria: (1) pain with minimal motion, (2) no tenderness with palpation, (3) pain improved with warming and worsened with cooling, and (4) pain improved by lying down, regarding pain or stiffness around the posterior neck or suprascapular area [7]. Pre- and postoperative axial symptoms were compared within and between groups.
6. Evaluation of ADLs Associated With Postoperative Reduced Neck Mobility
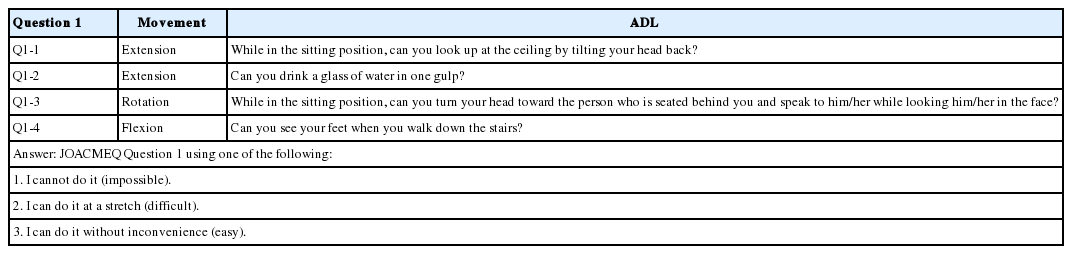
The frequencies of pre- and postoperative limitations of ADLs associated with each of the following neck movements in both groups were determined using Question 1 (cervical spine function) of the Japanese Orthopedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ) (Table 3) [18]: extension (Q1-1 and Q1-2), rotation (Q1-3), and flexion (Q1-4). The severity of the limitation of each ADL was assessed preand postoperatively via a JOACMEQ completed by the patient.
7. Statistical Analysis
O–C7 angle, O–C2 ROM, and rotation ROM were analyzed using Student’s t-test. Axial symptoms were analyzed using Student t-test, chi-square test, and Wilcoxon signed-rank test. Limitations in ADLs were analyzed using the chi-square test. Differences with p-values less than 0.05 were considered statistically significant.
RESULTS
1. Measurements of O–C7 Angle, O–C2 ROM, and Rotation ROM
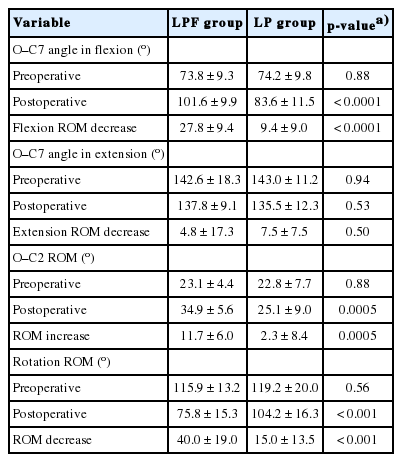
Results of radiographic parameters and rotation ROM are shown in Table 4.
Mean O–C7 angle in flexion was similar between groups preoperatively, but was significantly larger in the LPF group postoperatively (101.6° vs. 83.6°, p<0.0001). The mean postoperative increase in O–C7 angle in flexion was also significantly larger in the LPF group (27.8° vs. 9.4°, p<0.0001). Objectively, therefore, it became hard to turn to the bottom 27.8° postoperatively in the patients in the LPF group. However, mean preand postoperative O–C7 angles in extension and mean postoperative decrease in O–C7 angle in extension were similar between groups.
Although mean preoperative O–C2 ROM was similar between groups, mean postoperative O–C2 ROM was significantly larger in the LPF group (34.9° vs. 25.1°, p=0.0005). Mean postoperative increase in O–C2 ROM was also significantly larger in the LPF group (11.7° vs. 2.3°, p=0.0005).
The mean preoperative rotation ROM was similar between groups, but mean postoperative rotation ROM was significantly smaller in the LPF group (75.8° vs. 104.2°, p<0.0001). The mean postoperative decrease in rotation ROM was significantly larger in the LPF group (40.0° vs. 15.0°, p<0.0001).
2. Axial Symptoms
The mean preoperative and postoperative VAS scores for axial symptoms was similar between groups as shown in Table 5. The frequency with which patients experienced postoperative worsening of axial symptoms was similar between groups (LPF group: 12 cases, LP group: 12 cases, p=0.7513, chi-square test). The intragroup comparison demonstrated no significant aggravation of axial symptoms postoperatively in either group (LPF group: p=0.17, LP group: p=0.35, Wilcoxon signed-rank test) (Fig. 7). Furthermore, the proportion of patients experiencing new-onset axial symptoms after surgery was also similar between groups (LPF group: 7 cases, LP group: 9 cases, p=0.5170, chi-square test).
3. Frequency and Severity of Limitations in ADLs Associated With Each Neck Movement
The numbers of patients in each group who found pre- and postoperative ADLs involving neck movements impossible, difficult, or easy are shown in Table 6. The frequency and severity of limitations in ADLs for both groups are shown in Fig. 8. There were no significant between-group differences in the number of patients with postoperative worsening of their ability to perform neck extension movements as assessed by JOACMEQ Question 1-1 (LPF group: 9 cases, LP group: 16 cases, p=0.07, chi-square test), neck extension as assessed by JOACMEQ Question 1-2 (LPF group: 7 cases, LP group: 7 cases, p=0.19, chi-square test), neck rotation as assessed by JOACMEQ Question 1-3 (LPF group: 7 cases, LP group: 6 cases; p=0.11, chi-square test), or neck flexion as assessed by JOACMEQ Question 1-4 (LPF group: 7 cases, LP group: 6 cases; p=0.11, chi-square test). In other words, the objective loss of motion did not affect the ability of the patients in either group to perform their ADLs.

Severity and distribution of limitations in activity of daily livings associated with reduced neck mobility in both groups. LPF, laminoplasty with C2 to T1 fusion; LP, laminoplasty; postop, postoperative; preop, preoperative; JOACMEQ, Japanese Orthopedic Association Cervical Myelopathy Evaluation Questionnaire; Impossible, I cannot do it; Difficulty, I can do it intermittently; Easy, I can do it without inconvenience.
DISCUSSION
A few reports have reported poor neurologic improvement in cervical myelopathy due to large OPLL or K-line (-) OPLL after cervical LP alone [1,2], and that good postoperative neurologic improvement could be achieved by treating these OPLLs with posterior decompression and fusion [3]. However, there have been few reports on axial symptoms or limitations in ADLs associated with reduced neck mobility after posterior decompression and fusion [12].
In the present study, postoperative VAS score measuring axial symptoms, and the proportion of patients whose symptoms worsened after surgery, were similar between groups. Takeuchi et al. [7] changed the LP procedure from conventional C3–7 LP, with reattachment of the SSC at C2, to C4–7 LP with C3 laminectomy, with preservation of the SSC at C2; their report demonstrated that modified LP with preservation of the SSC significantly reduced postoperative axial symptoms and maintained whole-cervical posterior muscle volume on magnetic resonance images compared with conventional LP with muscle reattachment. The SSC, most of which inserts on C2 [19,20], acts as a dynamic stabilizer and extensor of the cervical spine [21-25]. In the present study, there is a possibility that, in the LPF group, the preserved SSC insertion at C2 may act not as a dynamic extensor, but as a muscle that maintains the cervical spine in extension, and that preservation of C2 muscle attachments prevents atrophy of all posterior cervical muscles and, thus, postoperative axial symptoms.
Although the objective data showed that the postoperative loss of O–C7 angle in flexion and the rotation ROM in the LPF group were significantly larger than in the LP group, the objective loss was not clinically important; i.e., it did not affect the ability to perform ADLs in either group. In this regard, our null hypothesis was accepted. The postoperative increase in O–C2 ROM was significantly larger in the LPF group. In our LPF group, the RCPM and OCI, as well as the SSC, were preserved at the time of insertion of the C2 pedicle screws. The RCPM and OCI extend the cervical spine, therefore, patients in the LPF group, in whom all muscle attachments to C2 were preserved, compensated for the loss of C2–7 ROM by increasing the O–C2 ROM. Moreover, because the preserved RCPM and OCI act as neck rotators [23], preserving these muscles may prevent loss of rotation ROM postoperatively and may, therefore, prevent worsening of limitations in ADLs requiring neck rotation at a minimum.
This study had several limitations. We did not compare posterior decompression and fusion with and without preservation of the C2 muscle attachments. In other words, the usefulness of the muscle-sparing in posterior decompression and fusion was not directly demonstrated. Furthermore, patients with K-line (+) OPLL underwent the LP procedure, while the LPF procedure was used for patients with K-line (-) OPLL. This was done because previous reports have suggested that K-line (-) OPLL is a risk factor for poor results after LP [1]. Therefore, there is the possibility that the different K-line characteristics of each group may have influenced their postoperative axial symptoms and ability to perform ADLs. However, since the preoperative axial symptoms and limitations to ADLs were similar between groups, as were the magnitudes of the postoperative changes in these parameters, we feel that this between-group comparison is still worthwhile. Furthermore, our findings that both procedures provide similar postoperative axial symptoms and ADL outcomes, albeit in different patient groups, will be of great interest to surgeons dealing with patients suffering from OPLL. Further studies are required to investigate the outcomes after LP and LPF in patients with the same K-line characteristics. In addition, the 1-year postoperative follow-up period was relatively short. OPLL has been reported to elongate after LP. Hori et al. [26] examined 55 patients with OPLL more than 5 years after LP and reported that 12 patients (21.8%) had the progression of OPLL thickening, and many authors have reported the elongation or thickening of OPLL after LP [27-31]. Therefore, the long-term outcome is unclear.
CONCLUSION
The postoperative losses of O–C7 angle in flexion and rotation ROM and the postoperative increase in O–C2 ROM in the LPF group were greater than those in the LP group. The pre- to postoperative changes in axial symptoms were almost the same in both groups. Although the frequency of patients with postoperative worsening of limitations in ADLs accompanying reduced neck mobility was greater for all neck movements in the LPF group than in the LP group, the differences were not statistically significant. With regard to axial pain and limitations of ADLs associated with reduced neck mobility after surgery, LP and C2 to T1 fusion with preservation of all C2 muscle attachments and muscle-sparing LP are equally recommended.
Notes
The authors have nothing to disclose.