Outcome Measures and Variables Affecting Prognosis of Cervical Spondylotic Myelopathy: WFNS Spine Committee Recommendations
Article information
Abstract
This study is conducted to review the literature systematically to determine most reliable outcome measures, important clinical and radiological variables affecting the prognosis in cervical spondylotic myelopathy patients. A literature search was performed for articles published during the last 10 years. As functional outcome measures we recommend to use modified Japanese Orthopaedic Association scale, Nurick’s grade, and Myelopathy Disability Index. Three clinical variables that affect the outcomes are age, duration of symptoms, and severity of the myelopathy. Examination findings require more detailed study to validate their effect on the outcomes. The predictive variables affecting the outcomes are hand atrophy, leg spasticity, clonus, and Babinski’s sign. Among the radiological variables, the curvature of the cervical spine is the most important predictor of prognosis. Patients with instability are expected to have a poor surgical outcome. Spinal cord compression ratio is a critical factor for prognosis. High signal intensity on T2-weighted magnetic resonance images is a negative predictor for prognosis. The most important predictors of outcome are preoperative severity and duration of symptoms. T2 hyperintensity and cord compression ratio can also predict outcomes. New radiological tests may give promising results in the future.
INTRODUCTION
Prognosis of cervical spondylotic myelopathy (CSM) can be affected by many variables. In this review, we wanted to find out the best outcome measures, most reliable clinical and radiological variables of CSM. A literature search was performed for articles published during the last 10 years, using PubMed and Google Scholar database systems. Keywords used for searching were CSM and outcome, and prognosis.
OUTCOME MEASURES FOR CSM
Many measurement tools are used by clinicians for patients with CSM to quantify the disease severity, assist in decision making and to evaluate the outcome of surgical intervention. Moreover, the surgical decisions are made objectively rather than subjectively with the use of these assessment tools. A standard method to define the disease severity can also be established [1].
Presentation of CSM is heterogeneous, as different function (limbs, gait, and bladder/bowel) are affected to different extents in patients and hence multiple outcome measures are required for the assessment tool to be applicable across all the disease population. However, an ideal scale should assess all these aspects.
Singh et al. [2] surveyed doctors in the United Kingdom to determine their use of quantitative assessment scales in the management of CSM. They found that all 117 participating clinicians gave almost equal importance to clinical history, examination, radiological imaging, and quantitative functional assessment. However, only 22 of clinicians (19%) used an assessment scale in the management of CSM patients [3].
1. Ideal Scale for CSM
Before we discuss the various scales used in CSM, it is essential to discuss the qualities of an ideal scale. Singh et al. [3] have reported what an ideal scale should have. An ideal scale should be able to work equally well in all the stages of disease. The neurological examination is normal in the early stages of disease, hence a scale which does not consider the subtle gait disturbance and relies heavily on the power in upper limbs will not adequately assess the disease in early part of its course.
The most commonly used measure in CSM is Nurick grade (cited in 62 studies), followed by modified Japanese Orthopaedic Association Scale (mJOA) (cited in 57 studies), visual analogue scale (VAS) for pain (27 studies), Short Form 36 (SF-36) Health Survey (18 studies), and Neck Disability Index (NDI) (10 studies) [1]. The other less commonly used scales are Myelopathy Disability Index (MDI) (6 studies) and European Myelopathy Scale (EMS) (4 studies). We will discuss the commonly used ones in detail.
1) Japanese Orthopedic Association Scale
JOA is one of the more commonly used outcome measures in the literature, it was developed in 1975 for the global assessment of body motor and sensory functions, urinary autonomic function, and activities of daily living. It is self-administered scale and has 4 sections—upper-limb function (scale range 0–4), lower-limb function (scale range 0–4), sensory function (upper and lower limbs and trunk; scale range 0–2 at each segment), and bladder function (scale range 0–3) [4]. The score ranges from 0 (worst) to 17 (normal). The JOA was revised in 1994 when the sensory and autonomic function scoring was refined and the motor function scoring was enhanced, including the assessment of elbow and shoulder function through testing of manual muscle function [5]. JOA Cervical Myelopathy Evaluation Questionnaire was developed later on and was thought to be better than JOA for medical statistics and more patient-oriented [5].
(1) Modified JOA
The major drawback of the original JOA scale was that it used patient’s ability to use chopsticks to assess the motor dysfunction. Since chopsticks usage is culturally mostly limited to east Asia, JOA could not be used for rest of the world accurately. The 1991, 1993, and 1999 modified JOA scores are more sensitive for use in Western populations [5-7]. One of these “modified JOA scores” (mJOA) is a modification by Benzel et al. [7] which ranges from 0 to 18. It is a clinician-based measure and includes upper and lower extremity motor function, hand sensation, and micturition [1,8].
(2) Psychometric properties of the original (1975) JOA scale
The internal consistency of JOA was studied by Singh and Crockard [8]. In 100 patients before and 6 months after cervical discectomies, they found that JOA has an acceptable Cronbach alpha coefficient for research purposes, but not for clinical purposes [5,8]. The same authors also studied the responsiveness (sensitivity to change) of the original JOA score and found it to be poor (sensitivity to change was 0.21 as compared to 0.42 for Nurick’s grade and 0.52 for the MDI) [5,8]. Moreover, the sensitivity to change was more for the hand function (0.35) as compared to sphincter function (0.04).
The absolute sensitivity of a scale is measured with the coefficient of variation [5,8]. The coefficient of variation for the original JOA score was found by 0.5 and 0.4 respectively, in the preoperative and postoperative period [5,8]. These values are much lower than acceptable limits and hence JOA cannot distinguish absolute levels of CSM severity among individuals in the same group and is not sensitive to changes in function after surgery [5,8].
No studies were found to have assessed the reliability of the original JOA score. Minimum clinically important difference (MCID) of JOA has been estimated to be 2 points by Hirabayashi et al. [9] An expert panel has independently suggested similar MCID of the original JOA [10]. Convergent and divergent construct validities of the original JOA score has been confirmed by various studies [11,12].
(3) Psychometric properties of the modified JOA (1991, Benzel et al. [7])
This version of JOA is the most acceptable version. Psychometric properties of this version are studied better than any other modified JOA version. The internal consistency was studied by Kopjar et al. [13] who reported a Cronbach alpha coefficient of 0.63 (which is below the acceptable values for internal consistency for both research and clinical purposes). Interrater reliability was found to be perfect by Revanappa et al. [14]
Studies have found the 1991 mJOA to be responsive (Cohen effect size of 1) [10,13]. The MCID of this version varied from 1.25 to 3.07 points and the minimal detectable change was 2.08 points [10,15].
Convergent and divergent construct validities of the 1991 JOA score has been studied by many studies. Revanappa et al. [16] found that JOA score and Nurick’s had a good correlation in CSM patients in both preoperative and postoperative period [10]. The correlation was found to be higher between 1991 JOA lower-limb score and Nurick score [10,16]. They have also found the correlation between 1991 JOA and Nurick was more in moderate CSM (1991 JOA scores between 9 and 12) when compared with the groups of patients with mild CSM (1991 JOA score >12) or severe CSM (1991 JOA score <9). Kopjar et al. [13] reported moderate correlation with the Nurick grade, but low correlation between the 1991 JOA and NDI scores, the physical component score (PCS) and mental component score subscores of the SF-36v2, and the 30-m walk test score.
Studies have confirmed convergent construct validity of the mJOA score when compared with data from magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) analyses in mild or moderate CSM [10,17]. The authors found an association between the anteroposterior (AP) spinal cord diameter and the 1991 JOA score, while no association was found between the presence of Intraspinal T2 hyper intensity on MRI scans and the 1991 JOA score [17]. Additionally, the fractional anisotropy (FA) values on DTI data at the site of compression also correlated positively and significantly with this version of mJOA [17]. Same authors in another study reported that intramedullary signal change on T2 was associated with lower JOA score [18]. The same study also reported a statistically significant negative correlation between the 1991 JOA score with maximum fiber tract density at the site of compression [10,18]. They also calculated the ratio of maximum fiber tract density at the level of compression to the average fiber density at the level of C2 with normal-appearing cord tissue. This ratio correlated with the 1991 mJOA in statistically significantly manner (p<0.0001) [10,18].
2) Nurick’s Grade
It was developed for assessment of impairment of gait in CSM patients. It has 6 grades. The disability increases with the increasing grade.
Validity of Nurick’s grade has been tested and proved by many studies. It was compared with JOA [16]; JOA, EMS, and Cooper myelopathy scale [19]; Ranawat, JOA, and SF-36 [8]; and VAS [20]. Singh and Crockard [8] reported good reliability and responsiveness of the Nurick’s grade.
3) Myelopathy Disability Index
It was developed to assess disability due to CSM in patients with pre-existing rheumatoid arthritis. It consists of 10 items. The score ranges from 0.5 to 100. Higher the disability, higher the score. It has been found to be valid, reliable and responsive in studies [8,21]. MDI was reported to have high sensitivity to detect different severity levels of disease and to detect the change that occurs after surgery [8]. However, it is not a commonly used outcome measure.
4) Neck Disability Index
It is a modification of the Oswestry Disability Index. It measures neck pain and consists of 10 items with a maximum score of 100. Higher score indicates greater disability. The NDI has been validated in patients who underwent neck surgery. In a recent study, NDI and JOA have also been validated in patients with cervical myelopathy or radiculopathy with strong test-retest reliability [22].
5) European Myelopathy Scale
It is a scale to assess myelopathy. There are 5 functions (gait, hand function, proprioception, paresthesias, and bladder function) based on which myelopathy severity is assessed. Score ranges from 5 to 18. Higher score indicates greater degree of disability. EMS has been validated against MDI [8]. EMS was found to have poor sensitivity to change [1].
6) SF-36 Health Survey
It is a measure of patient health status. It consists of 8 subscales: vitality, physical functioning, bodily pain, general health, physical role, emotional role, social role, and mental health. The score of each subscale ranges from 0 to 100. The greater the disability, the lower is the score. The SF-36 has been validated against MRI and Roland-Morris scales, NDI, MDI, VAS for neck and arm pain and Nurick scale, and found to be reliable and responsive [23,24].
7) The 30-m walking test
It was developed in 1999 and is a quantitative and objective test to measure gait impairment. Patient has to stand up from a chair, walk for 15 m, turn around, and walk back as quickly as possible [24]. The total time taken and the number of steps denote the disability. Test-retest reliability was found high [24]. Convergent validity was also found to be good with SF-36v2 PCS, mJOA and Nurick’s grade [24].
8) VAS for neck pain
It is a single item assessing the pain level. The patient should mark the pain level on a continuous line between 2 end points. No validity, reliability, or responsiveness studies have been done in a CSM population.
9) QuickDASH
It is used to measure physical function of upper-limb musculoskeletal disorders by creating a shorter version of the Disabilities of the Arm Shoulder and Hand Questionnaire (DASH). The disability and symptom module consist of 11 items ranging from 1 to 5 points. The higher the score, the greater the disability. There are no studies available which have tested validity, reliability, or responsiveness of the QuickDASH in the CSM population [1].
Currently, JOA scale and the Nurick’s grade are the most commonly used measures to quantify CSM. Both scales evaluate limb function, gait and sphincter control. However, both scales are not adequate to evaluate the patients with mild disease as these parameters are unable to assess all aspects of disease.
SF-36 and NDI are commonly used to assess improvement in CSM following an intervention. They provide adequate information regarding self-perceived function and have been validated for use in patients with cervical spine disorders [1].
It is essential to select an appropriate scale as the results might differ depending on the scale used. One study found that when Nurick’s grade is used, many contradicting results were found like the duration of symptoms was not correlated with worse outcome [25]. We believe that these results might have occurred due to the shortcomings in the Nurick’s grade, as it relies too much on mobility and employment of the patient and ignores the rest of the aspects. To prevent such inaccurate assessment, a method using ancillary measures to determine the disease severity is required [1]. Outcome measures with high responsiveness are required to define the patients with mild disease and to determine the predictors of disease progression.
Some authors have recommended the use of Graded Redefined Assessment of Strength Sensibility and Prehension (GRASSP) (partial), Grip strength, 30-m walking test (30MWT), NDI, mJOA, and MDI as screening tools. They recommended GRASSP (complete), Grip strength, QuickDASH, Berg Balance Scale, 30MWT, 10MWT, 6MWT, NDI, mJOA, and MDI for clinical use [1].
No single existing scale is ideal. We recommend using a combination of outcome measures to have a comprehensive assessment of a patient with CSM. We support the proposal that functional measures should be used along with quantitative measures and functional quality life measures [2].
CLINICAL VARIABLES AFFECTING PROGNOSIS
The symptoms of CSM include neck pain, hand numbness and clumsiness, gait difficulties and sphincter dysfunction. Physical examination shows increased tone, exaggerated deep tendon reflexes, clonus, diminished superficial reflexes, and the presence of pathologic reflexes. Spasticity, motor weakness and loss of proprioception contribute to the functional disability of the upper and lower limbs. Severely affected individuals are tetraparetic when first seen. CSM progresses in stepwise fashion and usually has insidious onset of symptoms [26].
The keywords used for the search of clinical variables were JOA score, Nurick, SF-36, prognosis, and CSM. The total number of articles found was 382. Articles which studied the predictors of surgical outcome directly or indirectly were included. A total of 37 studies seemed relevant to our search and were taken into consideration [10,26-61].
There are previously published clinical guidelines for the management of CSM. In most situations, these clinical guidelines have been unable to issue definitive guidance because rigorous, high-quality clinical research comparing treatment approaches is lacking. Several studies have attempted to identify patient characteristics that predict clinical improvement after surgery.
The clinical variables were divided into descriptive variables (self-reported findings such as age, sex, comorbid conditions, severity of current symptoms) and predictive variables (mainly the examination findings like wasting of muscles, hyperreflexia, clonus, sensory involvement, and other relevant examination signs).
1. Descriptive Variables
Three variables that were most commonly related with CSM are age, duration of symptoms and severity of the myelopathy.
1) Age
While most articles in the literature have considered age as an important factor in prognosis of CSM, there are few who have provided a cut off value for age. One study suggests that age less than 60 years is a good prognostic factor and surgical outcomes are better in younger age groups [30,41,53,54]. However, this may be correlated with the duration of the symptoms. Another survey of an international consensus suggests that age more than 65 years is a negative impact factor in the CSM [55].
2) Duration of symptoms
Chronic, long-standing compression of the spinal cord may lead to irreversible damage due to demyelination and necrosis of the gray matter. Numerous studies have shown that longer duration of symptoms have a negative impact on the outcome after surgery [34,56]. However, we did not find any study that specifies the exact length of the duration which negatively impacts outcomes. However, Chagas et al. [30] and Yamazaki et al. [58] have suggested 2 years and 1 year respectively. Karpova et al. [41] suggested that duration of symptoms correlated well with preoperative functional status but did not seem to affect the postoperative outcome.
3) Severity of symptoms at presentation
Most of the studies in the literature assess severity of symptoms at presentation using disability indices, JOA score, and Nurick’s score being the most commonly applied. Although most papers report that more severe baseline scores are associated with worst outcomes, there seems to be no one baseline score index which is considered foolproof. JOA score and its modifications are most commonly used to assess the baseline severity of score at presentation.
Tetreault et al. [55] in their review article emphasized that a modified JOA score of 12 was the threshold preoperative severity above/below which there becomes a negative impact on outcome. Su et al. [53] showed that the postoperative JOA scores were significantly affected by preoperative JOA scores along with age and preoperative increased signal intensity (ISI) on T2-weighted MRI. They concluded that age, preoperative JOA scores, and preoperative ISI were the independent factors that significantly affect disease prognosis and surgical outcomes.
4) Comorbid conditions
There were only handful studies that studied the association of comorbid conditions with CSM. Diabetes was the most commonly studied comorbid condition. Dokai et al. [34] studied surgical outcome of expansive laminoplasty for diabetic patients with CSM and if it differs from that for nondiabetic patients. They suggested that patients with diabetes had poorer recovery of sensory and motor function in the lower extremities following expansive laminoplasty for CSM. They further determined a negative correlation between the recovery rate and the preoperative HbA1c levels and recommended good sugar control preoperatively.
Kim et al. [42] studied 87 patients undergoing expansive laminoplasty and effect of diabetes and smoking on the outcome. Their study suggested that age and duration of symptoms adversely affect the outcome only if the patient had diabetes. Smoking had no effect on the outcomes.
Wang et al. [57] studied the prognostic value of prior history of cerebral infarction in patients of CSM and found it to be a risk factor for predicting poor surgical outcomes. They also suggested that rapid progressive myelopathy with advanced neurological impairment is an indicator for poor surgical outcomes as compared to patients with ordinary symptoms.
In another study, presence of chronic kidney disease was found as predictor of worse outcome [59]. Sakaura et al. [59] have also reported that the aortic arch calcification as a marker of systemic atherosclerosis was a predictive factor for poor outcome.
2. Predictive Variables
These include mainly the examination findings like wasting of muscles, hyperreflexia, clonus, sensory involvement and other relevant examination signs of CSM.
1) Hand atrophy
Alafifi et al. [29] studied 76 patients retrospectively to find the presence of intrinsic hand muscle atrophy. Hand muscle atrophy has a great association with abnormal preoperative MRI signals and a less favorable postoperative outcomes.
2) Leg spasticity and clonus
Patients with high intramedullary signal change on T2-weighted image who do not have clonus or spasticity may experience a better surgical outcome and may have reversal of the MRI abnormality [29].
3) Babinski sign
Although Alafifi et al. [29] found that a positive Babinski sign in association with abnormal preoperative MRI signal change is predictive of a worse outcome, some others have reported presence of a Babinski sign was found to be related with a better outcome [60,61].
In general, hyperreflexia shows the highest sensitivity followed by Hoffmann reflex, Babinski sign, and ankle clonus. For screening myelopathic patients, pyramidal signs and Hoffmann reflex may be useful because of high sensitivity. The prevalence of the pyramidal signs is closely correlated with the increasing severity of myelopathy. There are not many studies concentrating or evaluating these parameters.
In a study by Cook et al. [33] in 2010 evaluated thirteen clinical findings for capacity to diagnosis CSM. Five clinical signs like gait deviation; positive Hoffmann’s test; inverted supinator sign; positive Babinski test; and age >45 years demonstrated the capacity when positive to rule out CSM. They have suggested that instead of one variable, a combination of multiple variables would be more appropriate to prognosticate CSM.
Clinical variables are standard and can be assessed by any person trained in neurological assessment. The clinical variables can be further grouped into 2 parts as descriptive variables and predictive variables. While descriptive variables are patient dependent or are self-explanatory variables, predictive variables are dependent on the person examining the patient. Both variables are more reliable than the radiological and surgical variables.
RADIOLOGICAL VARIABLES AFFECTING PROGNOSIS
There are many radiological factors which were reported affecting postoperative outcomes: cervical curvature, instability signs, angulation and kyphosis, transverse area (TA) of the spinal cord, or AP diameter of the spinal canal, spinal cord, and vertebral body at the maximum compression site, high signal intensity on MR images, positron emission tomography MRI findings.
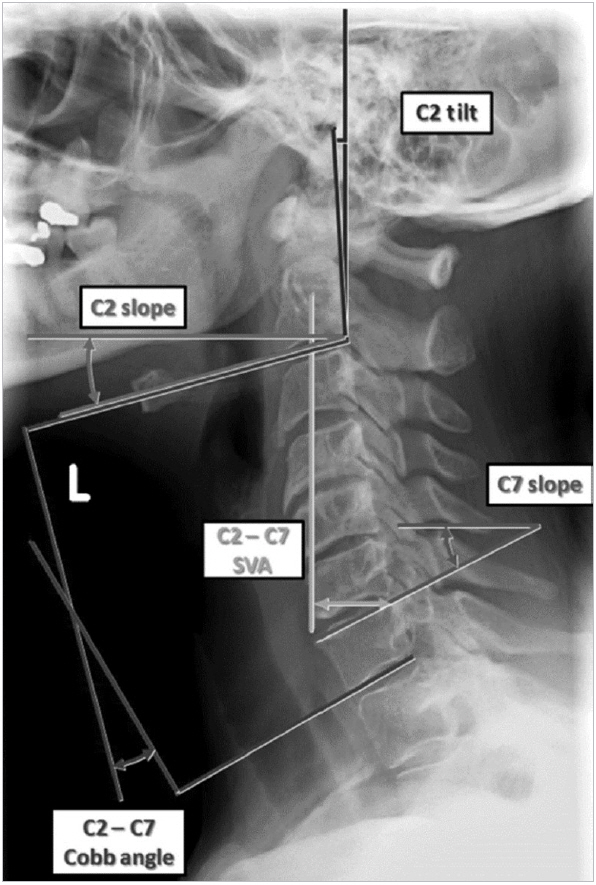
Cervical alignment parameters have been implemented recently. Most important parameters as described by Smith et al. [62] are described below and depicted in Fig. 1.
1. Cervical Sagittal Vertical Axis
Horizontal offset between a chosen plumb-line and the posterosuperior corner of the C7 vertebral body. Measured using plumb-lines from the barycenter of C1 (C1–7 sagittal vertical axis [SVA]), the barycenter of C2 (C2–7 SVA), or the center of gravity of the head (COG), taken as the midpoint of the line between the 2 external auditory canals (COG-C7 SVA).
1) C2 tilt
Angle between the posterior aspect of the C2 vertebral body and the vertical (-) for posterior inclination and (+) for anterior inclination.
2) C2 slope
Angle between the C2 inferior endplate and horizontal reference line.
3) C7 slope
Angle between the C7 superior endplate and horizontal reference line.
4) T1 slope
Angle between the T1 superior endplate and horizontal reference line.
5) C2–7 Cobb angle
Sagittal cervical curvature from C2 to C7, using the Cobb method. (-) for lordosis and (+) for kyphosis.
A post hoc analysis of the prospective, multicenter AOSpine North America Cervical Spondylotic Myelopathy Study have found that mJOA scores correlated negatively with C2–7 SVA, C2 tilt, C2 slope. The mJOA score correlated weakly with T1 slope minus C2–7 Cobb angle [62]. The mJOA score was not found to correlate significantly with COG-C7 SVA, C2–7 Cobb angle, or the posterior or anterior length of the spinal column.
AOSpine North America study group has summary statements about cervical radiographic alignment [63]:
(1) Cervical sagittal alignment (cervical SVA and kyphosis) is related to thoracolumbar spinal pelvic alignment and to T1 slope.
(2) When significant deformity is clinically or radiographically suspected, regional cervical and relative global spinal alignment should be evaluated preoperatively via standing 3-foot scoliosis X-rays for appropriate operative planning.
(3) Cervical sagittal alignment (C2–7 SVA) is correlated to regional disability, general health scores and to myelopathy severity.
(4) When performing decompressive surgery for CSM, consideration should be given to correction of cervical kyphosis and cervical sagittal imbalance (C2–7 SVA) when present.
The effect of the cervical spine curvature to outcomes has also been examined by others. Buell et al. [64] have analyzed neurological improvement in patients both with and without normal cervical curvature. They found that neurological improvement was significant only in patients with normal cervical lordosis.
2. Kyphotic Cervical Spine
In kyphotic alignment of the cervical spine, the spinal cord may be “draped” over the anterior vertebral bodies and disc-osteophyte complexes. Myelopathy develops by (1) increasing the longitudinal cord tension, (2) increasing intramedullary pressures, and (3) reducing blood supply due to loss of small arterial feeding.
Decompression with stabilization of the cervical spine in lordosis of the operated segment allows the posterior shift of the spinal cord [64].
Cervical alignment has significant predictive role in outcomes. If cervical spine sagittal alignment parameters are abnormal, those patients are expected poor outcomes. If the curvature is lordotic, these patients generally have greater clinical improvement after surgery. If patients with kyphotic cervical spine have obtained correction of the curvature by an anterior or a combined anteriorposterior approach, they have improved outcomes.
3. Instability
Instability is measured by neutral and flexion-extension lateral radiograms. Translational instability is defined as more than 3.5-mm horizontal displacement of one vertebra in relation to an adjacent vertebra. Rotational instability is defined as more than 11° rotational difference from that of either adjacent vertebra. Instability further causes spinal cord damage by socalled dynamic compression [65]. In addition to segmental instability, longer duration of symptoms, lower preoperative JOA score, and more preoperative physical signs were found to predict a poor surgical outcome [65].
4. AP Diameter of the Spinal Canal
Although decrease in canal diameter is expected to cause myelopathy, there is no evidence between AP diameter of the spinal canal and neurological outcomes.
5. Magnetic Resonance Imaging
Various parameters on preoperative MRI have been shown to be predictive of neurological status and postoperative outcome [66,67].
6. MRI Transverse Area
One study concluded that transverse area (TA) of spinal cord matches the severity of CSM, and predict surgical outcome as well. Myelopathic signs were also related to TA and signal intensity changes on T1 and T2 images [68].
7. Spinal Cord Compression Ratio
The spinal cord compression ratio is a critical point differentiating worse outcomes. Compression ratio can be calculated from preoperative magnetic resonance (MR) images. The formula for compression ratio is below:
Compression ratio=sagittal cord diameter×100/transverse diameter.
Five papers have compared the outcome with spinal canal compression ratio [69]. They have concluded that compression ratio is a critical factor for prognosis of CSM (class III evidence).
8. Spinal Cord Atrophy
Some authors suggest that outcome would strongly correlate with spinal cord atrophy. This relationship is evident in 7 class III studies in which low transverse cord areas were correlated with poor surgical outcome (range, <30–45 mm) [70]. The degree of canal stenosis is less predictable. Four class III studies indicated that the severity of canal stenosis correlated with the poor outcome while the results of 2 class III studies did not support this relationship. In conclusion, the literature is equivocal and no consensus can be reached.
With regard to canal stenosis and cord atrophy, there is class III evidence that restricted transverse spinal cord area on preoperative MR imaging may portend a poor surgical prognosis [70].
9. T2 Hyperintensity on Axial MR Images
You et al. [71] have classified T2 hyperintensity on MR in 3 types (Fig. 2).

Type of magnetic resonance imaging cord signal intensity. Type 0, normal signal intensity of spinal cord without any intramedullary T2 hyperintensity; type 1, diffuse pattern of intramedullary T2 hyperintensity occupying more than two-thirds of axial dimension of spinal cord with an obscure and faint border; and types 2 and 3, focal patterns of intramedullary T2 hyperintensity occupying less than two-thirds of axial dimension of spinal cord. Reprinted form You et al. Radiology 2015;276:553-61, with permission of RSNA [71].
1) Type 1
A fuzzy margin of intramedullary T2 hyperintensity may reflect acute and currently active injury to the spinal cord, which is related to cord edema or inflammation.
2) Type 2
This is was similar in concept to the previously reported snakeeye appearance, is not associated with a poor prognosis.
3) Type 3
A discrete margin of intramedullary T2 hyperintensity may reflect a chronic and poor prognosis that is related to gliosis or cystic cavitation.
There are many studies examining the relation with MR signal intensity and outcomes. There is class III evidence that multisegmental high signal changes in the cervical cord on T2-weighted MR images predict a poor outcome following surgery [34]. There is also class III evidence that T1 hypointensity when combined with T2 hyperintensity predicts a worse outcome. Conflicting class III data exists on the significance of focal cervical cord T2 hyperintensity. Some studies have shown focal T2 hyperintensity as a negative prognostic indicator while others have not. In general, T1 hypointensity and T2 hyperintensity are indicators of poor prognosis.
10. Dynamic MRI
In some patients, hyperintensity on T2 images is only visible with the neck in flexion [72]. That may explain why hyperintensity is first detected after surgery in some patients. Dynamic MRI is useful to determine more accurately the number of levels where the spinal cord is compromised, and to better evaluate narrowing of the canal and T2 hyperintensity.
Some newer MRI techniques are under evaluation for their role in CSM. Some of them have already been tried in humans while some are still in animal experimental stage [73]. Resting state functional MRI (rs fMRI) of the spinal cord was evaluated in one study on which the severity of myelopathy was found to be associated with increase of amplitude of low-frequency fluctuation on rs fMRI [74]. Other new MRI techniques are double diffusion encoding, spinal cord perfusion MRI, T2-weighted imaging, and magnetic resonance spectroscopy of the spinal cord. Spinal cord perfusion MRI can pick up the degree of ischemia in CSM patients [73].
FA value was found to be a prognostic marker with a preoperative FA <0.55 found to be associated with significantly poorer outcome [68]. Patients who develop spinal cord swelling after decompressive surgery have had intramedullary Gd-DTPA enhancement [75]. They had a poor prognosis.
Patients in the acute-onset phase of symptomatic CSM and also patients with chronic-stable myelopathy and new-onset symptoms exhibit a focally increased glucose hypermetabolism (18F-fluorodeoxyglucose uptake) at precisely the individual level of their stenosis and cord compression [76]. Decompressive surgery during the phase when hypermetabolism is present results in a good clinical recovery and favorable outcome.
During the chronic phase of CSM, the metabolic pattern may change, and a post stenotic glucose hypometabolism occurs. This suggests that the post stenotic hypometabolism represents a condition that involves an irreversibly damaged spinal cord.
SURGICAL VARIABLES AFFECTING PROGNOSIS
Although surgical indications of CSM, various surgical techniques, their success rates, and complications are discussed in another paper, we wish to say a few comments on outcomes of the surgery.
The discussions on which approach should be used in CSM are more resolved than previous years. If the compression is 1 or 2 level, the surgery should better be performed from the site of compression. There is mostly an anterior compression and anterior approach is chosen if CSM only involves 1 to 2 levels. When more than 2 vertebrae are involved, the rate of complications of the anterior approach increases so that the posterior approach may be considered. The decision maker in that circumstance is the curvature of the spine (or cervical SVA) [77] and presence of instability. If there is a significant kyphosis, combined approach can also be considered. In a study by Shamji et al. [77] kyphotic patients experienced better results when handled with an anterior or combined approach.
In a meta-analysis reviewing 10 studies to compare anterior and posterior approaches in multilevel (more than 2 levels) CSM, there were no significant differences in postoperative neurological clinical status between the 2 approaches [78]. But the complications such as the reoperation rate, the intraoperative blood loss and operation time and the length of stay were higher in the anterior approach.
In a 10 years outcome study, Hirai et al. [79] found that the anterior approach with fusion and laminoplasty gave the same outcome at 10-year follow-up. However, the anterior group approach had a higher reoperation rate.
RECOMMENDATIONS
1. Outcome Measures for CSM
• There are a variety of outcome measures used for CSM. As functional measures we recommend mJOA, Nurick’s grade, and MDI.
• Walking tests can be used for quantitative measurements and SF-36 is a good functional quality life measure.
2. Clinical Variables
• Three clinical variables that are most commonly related with CSM are age, duration of symptoms, and severity of the myelopathy at presentation. Greater the age, the longer the duration of symptoms and the more severe symptoms at presentation, the more adverse outcomes can be expected after surgery.
• However, examination findings require more detailed study to validate their effect on the outcomes of surgery. The predictive variables which were studied and seemed to affect the outcomes in CSM are hand atrophy, leg spasticity, clonus, and Babinski’s sign.
3. Radiological Variables
• Cervical alignment parameters are correlated with general health scores and myelopathy severity. The curvature of the cervical spine has been found one of the most important variables.
• Cervical spine kyphosis predicts worse outcomes. Neurological improvement is significant in patients with normal cervical lordosis.
• Instability of the cervical spine is predictive for outcomes. In patients with single segmental CSM with instability, longer duration of symptoms, lower preoperative JOA score, and more preoperative physical signs are highly predictive of a poor surgical outcome.
• Spinal cord compression ratio is a critical factor for prognosis of CSM. However, AP diameter of the spinal canal has no clinical significance.
• Spinal cord atrophy cannot predict outcomes.
• High signal intensity on T2-weighted MR images is a negative predictor for prognosis.
4. Surgical Variables
• Surgery should be done from anterior or posterior if the disease is focal (1 or 2 levels).
• If the anterior compression is more than 2 levels or if it is a diffuse narrowing, posterior decompression should better be chosen.
• The most important factor on decision making in cases with multilevel (more than 2) CSM is cervical SVA.
• The complication rates of anterior surgery, although mostly graft and fusion related are more often.
Notes
The authors have nothing to disclose.