Synthetic Cages Associated With Increased Rates of Revision Surgery and Higher Costs Compared to Allograft in ACDF in the Nonelderly Patient
Article information
Abstract
Objective
The aim of this study was to compare all-cause reoperation rates and costs in nonelderly patients treated with anterior cervical discectomy and fusion (ACDF) with structural allograft versus synthetic cages for degenerative pathology.
Methods
We queried a private claims database to identify adult patients (≤ 65 years) who underwent single-level ACDF in a hospital setting using either structural allograft or a synthetic cage (polyetheretherketone, metal, or hybrid device), from 2010 to 2016. The rate of all-cause reoperations at 2 years were compared between the 2 groups. Index hospitalization costs and 90-day complication rates were also compared. Significance was set at p < 0.05.
Results
A total of 26,754 patients were included in the study. 11,514 patients (43%) underwent ACDF with structural allograft and 15,240 (57%) underwent ACDF with a synthetic cage. The patients in the allograft group were younger and more likely to be male. There was no significant difference between the 2 groups with respect to 90-day complications including: wound dehiscence, dysphagia, dysphonia, and hematoma/seroma. In the 2-year postoperative period, the synthetic cage group had a significantly higher rate of allcause reoperation compared to the allograft group (9.1% vs. 8.0%, p = 0.002). Index hospitalization costs were significantly higher in the synthetic cage group compared to those in the allograft group ($23,475 vs. $20,836, p < 0.001).
Conclusion
Structural allograft is associated with lower all-cause reoperation rates and lower index costs in nonelderly patients undergoing ACDF surgery for degenerative pathology. It is important to understand this data as we transition toward value-based care.
INTRODUCTION
The anterior cervical discectomy and fusion (ACDF) procedure, first described in 1958 by Smith and Robinson [1], has risen to the forefront of treatment for cervical degenerative pathology. The number of cervical spine procedures performed annually in the United States continues to rise [2], with ACDF constituting the majority of these procedures [3]. The concerns of morbidity associated with iliac crest allograft have resulted in widespread adoption of structural allograft and synthetic cages [4,5]. While allografts demonstrate osteoconduction,4 they may lack osteoinduction [6]. Synthetic cages have gained popularity in recent years due to concerns regarding accessibility, sterility, and storage of allograft [7,8].
While clinical outcomes following ACDF are generally excellent, the procedure still presents risk of complication, one of the most challenging of which is nonunion or pseudarthrosis [9-11]. Systematic reviews show the rate of pseudarthrosis ranges from 0.9% to 4.8% following ACDF [12], with similar rates of fusion in ACDFs utilizing allograft and synthetic cages [13]. Patients with nonunion may report decreased satisfaction, particularly related to neck pain and associated disability [14]. To date, there is a scarcity of literature investigating the relationship between interbody technique (i.e., allograft vs. synthetic cages) and symptomatic pseudarthrosis or nonunion requiring reoperation. Furthermore, there is little data comparing the relative costs of these techniques, which has become a distinct area of focus as the United States moves toward a value-based model of healthcare.
The purpose of this study is to compare the all-cause reoperation rate following ACDF for degenerative pathology in the nonelderly patient performed with structural allograft versus synthetic cages. The secondary aim of the study was to compare costs and 90-day postoperative complication rates of the 2 graft techniques. The primary hypothesis of the study was that ACDF performed using synthetic cages would have higher rates of reoperation, and would have higher associated costs than ACDF procedures performed using structural allograft.
MATERIALS AND METHODS
1. Data Source
The MarketScan Commercial Claims and Encounters Database (Truven Health Analytics, Ann Arbor, MI, USA) contains private health insurance claims data for approximately 45 million patients younger than 65 years in all 50 US states. Data, including surgery date, are stored in a Health Insurance Portability and Accountability Act-compliant format, with unique patient identifiers to allow individual claims to be linked across outpatient, inpatient, and prescription drug services. We queried the inpatient claims database to identify our cohort and we leveraged the inpatient and outpatient database to identify reoperations and cervical spine specific complications. Data were available from January 2010 through December 2017.
This study was exempt from Institutional Review Board approval.
2. Study Population
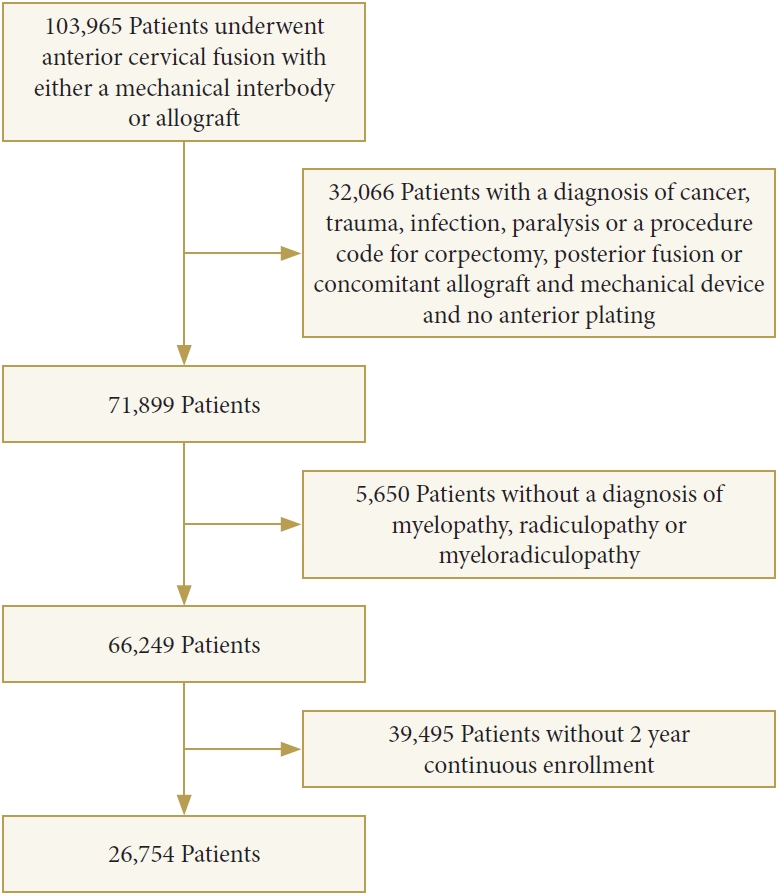
By using current procedural terminology (CPT) codes (Supplementary Table 1), we identified 103,965 patients greater than 21 years old and younger than 65 years old who underwent first-time single-level ACDF (CPT codes: 22551, 22554) in a hospital setting for a diagnosis of myelopathy, radiculopathy or myeloradiculopathy with the use of structural allograft (CPT code: 20931) or synthetic cages (polyetheretherketone [PEEK], metal, or combination) (CPT code: 22851). 29,525 patients were excluded. Excluded patients included those who had a concomitant corpectomy, a posterior fusion, or who had both allograft and a synthetic cage placed. 2,541 patients were excluded due to lack of anterior plating (CPT code: 22845, 22846, 22847). Patients with a diagnosis of cancer, trauma, infection, and spinal cord injury were also excluded. In order to allow for longitudinal analysis, patients were required to have continuous insurance enrollment for 2 years postoperatively to be included in the study (Fig. 1).
3. Outcomes of Interest
The primary outcome of interest was all-cause reoperation rate in the 2-year period following surgery. The secondary outcomes were costs and 90-day complication rates.
Surgical reoperations assessed within the first 2 years postoperatively included revision anterior fusion, posterior cervical fusion, and additional cervical surgery. Additional cervical surgery included posterior decompression laminectomy or laminoplasty, irrigation and debridement, and removal of hardware. 90-day cervical spine specific complications, including wound dehiscence, dysphagia, dysphonia, and hematoma/seroma formation, were also assessed (Supplementary Table 1).
Index hospitalization costs and surgeon professional fee reimbursement by payer were calculated and compared between the 2 groups.
4. Statistical Analysis
Mean± standard deviation was used to describe continuous variables and count (percentage) was used to describe categorical variables. Demographic data assessed included patient’s age, sex, region, and Elixhauser comorbidity index (ECI). The ECI was calculated for each patient as described by Quan et al. [15]. Univariate analysis using chi-square test was performed to identify significant differences in complication rates between the 2 groups. A Mann-Whitney U-test was used to compare index hospitalization payments and surgeon professional fee reimbursement between the 2 groups. Multivariate logistic regression analysis, adjusting for age, sex, region, and ECI was utilized to determine adjusted odds ratio (OR) and confidence intervals (CIs) for each complication with a rate > 1%. Significance was set at p < 0.05. All statistical analysis was conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
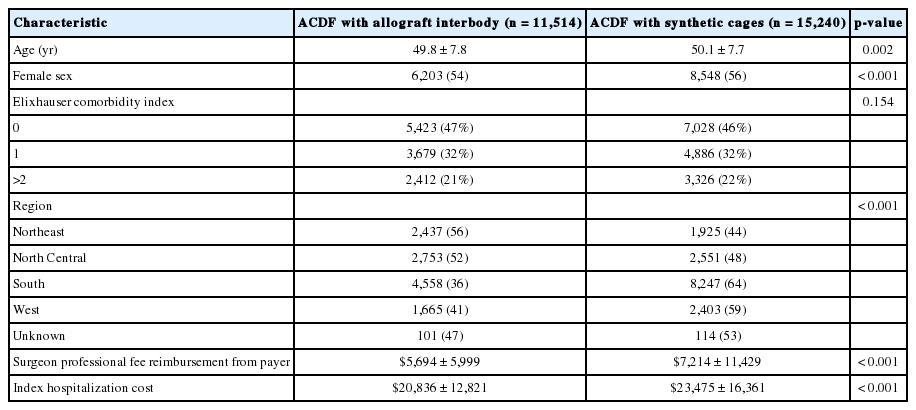
A total of 26,754 patients were included in the study. 11,514 patients (43%) underwent one-level ACDF with an allograft interbody and 15,240 (57%) underwent ACDF with a synthetic cage. Patients in the allograft group were significantly younger and more likely to be male compared to those in the synthetic cage group (both p = 0.002) (Table 1).
1. Reoperation
Within 2 years following the index procedure, all-cause reoperation was significantly higher in the synthetic cage group (9.1%) compared to the allograft group (8.0%) (p = 0.002) (Table 2). Eight hundred 7 patients (5.3%) underwent revision anterior fusion in the synthetic cage group versus 514 (4.5%) in the allograft group (p = 0.002). Similarly, 525 patients (3.4%) underwent additional posterior cervical fusion in the synthetic cage group versus 353 (2.8%) in the allograft cage group (p = 0.004).
In a multivariate model, after adjusting for patient age, sex, and comorbidities, patients in the synthetic cage group had 19% increased odds of requiring a revision anterior fusion (OR, 1.19; 95% CI, 1.06–1.33) and 21% increased odds of requiring a posterior fusion (OR, 1.21; 95% CI, 1.05–1.39) compared to the allograft ACDF group (Table 2).
2. Cervical Spine Specific Complications
Within 90 days of surgery, there was no statistically significant difference in the rate of wound dehiscence, dysphagia, dysphonia, or hematoma/seroma formation between patients in the synthetic cage group and those in the allograft group (p > 0.05) (Table 3).
3. Cost Analysis
The index hospitalization costs ($23,475 vs. $20,836, p < 0.001) were significantly higher in patients treated with synthetic cages compared to those in the allograft group (Table 1). Further, the surgeon professional fee reimbursement from payer was significantly higher in patients with synthetic cages compared to allograft group ($7,214 vs. $5,694, p < 0.001).
DISCUSSION
Both allograft and synthetic cages are used widely [5] for interbody structural in the nonelderly patients undergoing ACDF surgery for degenerative pathology. However, the literature is limited with respect to comparison of rates of revision surgery between the 2 techniques [4,16,17]. In the current analysis, we found that while there was no difference observed in rates of cervical spine related 90-day complications, patients who underwent ACDF with synthetic cages had an increased all-cause reoperation rate at 2-year follow-up compared to patients who underwent ACDF with structural allograft. Additionally, we found increased costs associated with synthetic cages for the index hospitalization.
There have been several studies dedicated to comparing rates of nonunion and reoperation rate in patients undergoing ACDF with allograft compared with synthetic cages. The early studies, which were small in scale, could not consistently show a difference in the rates of nonunion [18-20]. Our results are consistent with studies by Krause et al. [16] and Yang et al. [17], who found that ACDF with synthetic cages had significantly higher rates of pseudarthrosis, complications, and need for revision surgery compared with ACDF with allograft. Similarly, a large, claimsbased study including elderly patients, found higher rates of nonunion in the synthetic cage versus allograft groups (5.32% vs. 1.97%) [21]. Additionally, Goz et al. [4] used the same claims database to demonstrate a statistically different rate of revision between cage and allograft groups (0.56% vs. 0.50%). Our study showed a significantly higher rate of revision following ACDF with allograft and synthetic cages than the previous study performed by Goz et al. [4]. This study utilized a different data source with a younger patient population; young patients are more likely to undergo revision surgery, as their activity demands are generally increased compared with older patients [22].
Additionally, we found that ACDF using synthetic cages were associated with significantly higher hospitalization costs. This result is in line with the cost-effectiveness analysis conducted by Virk et al. [23], who found allograft to be the most cost-effective graft option, costing $2,492 per quality-adjusted life year (QALY), in contrast to PEEK cages, which cost $3,328 per QALY. Further, we found that use of synthetic cages was associated with higher surgeon payments. Most payers cover surgeon professional fees for placement of synthetic cages on a per level basis, while the surgeon is reimbursed for placement of structural allograft only once regardless of the number of levels involved. The additional work performed using structural allografts is uncompensated for the surgeon if greater than 1-level ACDF is performed. The reasons for this financial disincentive are presently unclear.
Our study has several limitations. First, we are not able to accurately capture the clinical cause of reoperation. While the data indicate that synthetic cages are associated with higher rates of all-cause reoperation, the clinical reason is not indicated. This is a limitation inherent to database research. Second, we are not able to control for additional interventions performed during surgery such as use of osteobiologics or local bone graft which may potentially impact fusion rates. Third, we are not able to perform subanalyses comparing various types of synthetic cages that may have been used: PEEK-based devices, metal interbody devices, or hybrid devices. There may be inherent differences among device categories which may alter pseudarthrosis and reoperation rates. Fourth, the cost data that was utilized is specific to the United States, and therefore may not be generalizable internationally, as the cost of each implant likely varies in each country. Finally, the database does not contain any radiographic data or clinical data such as patient alignment and information regarding the use of bracing postoperatively, which may also impact fusion and reoperation rates.
Our study has several strengths. First, since the analysis is built on claims data, we are able to follow patients longitudinally across various inpatient and outpatient clinical contexts and accurately capture reoperations. Second, the large number of patients allows us to have enough statistical power to detect differences that may otherwise be missed in smaller clinical series. Finally, we can assess costs associated with the interventions in the United States, which allows for a more comprehensive analysis.
CONCLUSIONS
In summary, compared to synthetic cages, structural allograft is associated with lower all-cause reoperation rates and lower index costs in nonelderly patients undergoing ACDF surgery for degenerative pathology. It is important to understand this data as we transition toward value-based care.
Notes
The authors have nothing to disclose.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 can be found via https://doi.org/ns.2040216.108.
Anterior cervical decompression and fusion reoperation and complications International classification of diseases, 9th and 10th revisions codes