History and Evolution of the Minimally Invasive Transforaminal Lumbar Interbody Fusion
Article information
Abstract
The minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) is a popular surgical technique for lumbar arthrodesis, widely considered to hold great efficacy while conferring an impressive safety profile through the minimization of soft tissue damage. This elegant approach to lumbar stabilization is the byproduct of several innovations throughout the past century. In 1934, Mixter and Barr’s paper in the New England Journal of Medicine elucidated the role of disc herniation in spinal instability and radiculopathy, prompting surgeons to explore new approaches and instruments to access the disc space. In 1944, Briggs and Milligan published their novel technique, the posterior lumbar interbody fusion (PLIF), involving continuous removal of vertebral bone chips and replacement of the disc with a round bone peg. The following decades witnessed several PLIF modifications, including the addition of long pedicle screws. In 1982, Harms and Rolinger sought to redefine the posterior corridor by approaching the disc space through the intervertebral foramen, establishing the transforaminal lumbar interbody fusion (TLIF). In the 1990s, lumbar spine surgery experienced a paradigm shift, with surgeons placing increased emphasis on tissue-sparing minimally invasive techniques. Spurred by this revolution, Foley and Lefkowitz published the novel MIS-TLIF technique in 2002. The MIS-TLIF has demonstrated comparable surgical outcomes to the TLIF, with an improved safety profile. Here, we present a view into the history of the posterior-approach treatment of the discogenic radiculopathy, culminating in the MIS-TLIF. Additionally, we evaluate the hallmark characteristics, technical variability, and reported outcomes of the modern MIS-TLIF and take a brief look at technologies that may define the future MIS-TLIF.
INTRODUCTION
Degenerative disc disease of the lumbar spine is a common disabling condition, often resulting in low back pain, radicular leg pain, and spinal deformity. In the case of conservative treatment failure, the discogenic radiculopathy is often treated surgically in the form of a lumbar interbody fusion. Several approaches are used for this procedure, differing in their strategic access route to the disc space [1].
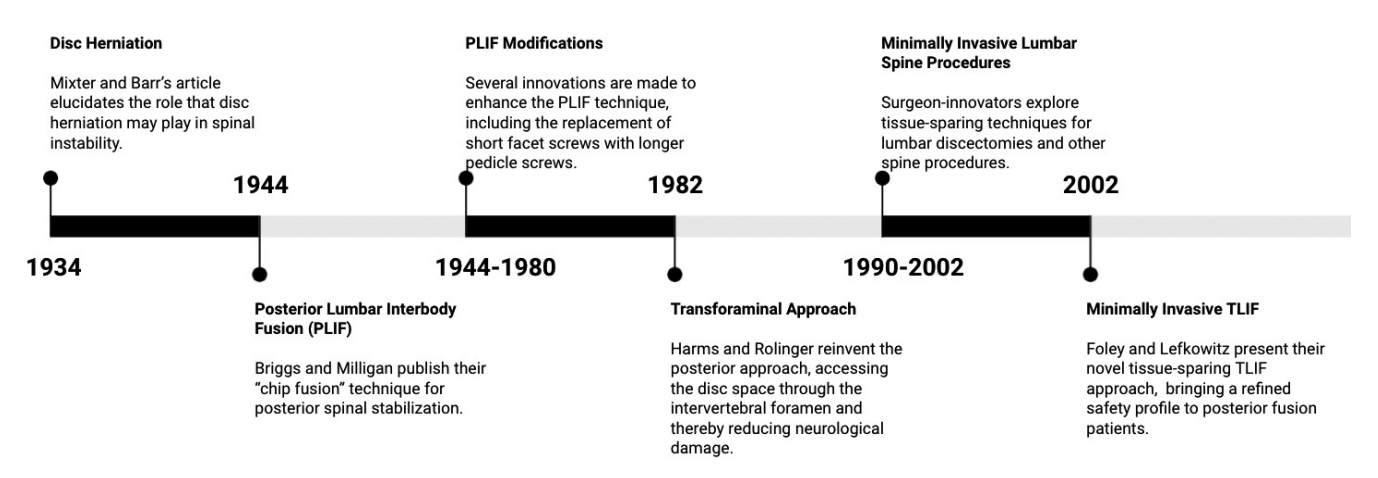
In the mid-1900s, surgeons began exploring posterior access corridors for lumbar arthrodesis. One such technique, the transforaminal lumbar interbody fusion (TLIF), was developed in 1982 and thereafter gained traction among the surgical community due to its innovative respect for neurological elements that can be harmed while approaching the disc space. In 2002, the TLIF was modified to incorporate tissue-sparing retraction, and the minimally invasive TLIF (MIS-TLIF) was born. In the present article, we deliver a historical perspective on the development of the MIS-TLIF, as well as an evaluation of its current outcomes and a look at further innovations on the horizon.
A LOOK TO THE PAST: SETTING THE STAGE FOR THE MIS-TLIF
The history of the MIS-TLIF begins when clinicians reached a consensus on the focal significance of lumbar disc herniation in radiculopathy after decades spent poised at the brink of clarification. This pathophysiological understanding was imperative before surgeons could make purposeful strides toward a technical solution. Thus, the story of the MIS-TLIF begins nearly 70 years before its inception in 2002.
1. Understanding Discogenic Radiculopathy: 1896–1934
In August of 1934, the New England Journal of Medicine published an article from 2 Boston physicians that would change the landscape of lumbar spine surgery. William J. Mixter and Joseph S. Barr [2], working out of Massachusetts General Hospital, wrote of the phenomenon of intervertebral disc herniation, and its critical role in lumbar instability and sciatica. They suggested nerve root decompression and spinal fusion as the preferred treatment, prompting surgeons to develop novel access methods and specific tools to accomplish these goals.
Though Mixter and Barr’s connection has had a profound impact, notable advancements had previously been made. One 2013 historical investigation led by Stienen et al. [3] has elucidated the early progress in the treatment of disc herniation, preceding the work of Mixter and Barr, by physicians published in German. As early as 1896, spinal trauma related to disc rupture had been noted by Swiss surgeon Emil Theodor Kocher [4], and in 1909 a report of degenerative disc resection was delivered by German physicians Hermann Oppenheim and Fedor Krause [5]. At that time, however, no correlation was drawn between disc herniation and sciatica. A 1927 case report [6] from Zurich neurologist Otto Veraguth and his surgeon colleague Hans Brun detailed successful transdural resection of herniated L4–5 disc fragments. Veraguth accurately correlated clinical and radiographic findings with a lesion of the lower lumbar segment (though he was unable to identify its true origin in the disc space), and Brun’s operation successfully relieved the patient of radicular pain.
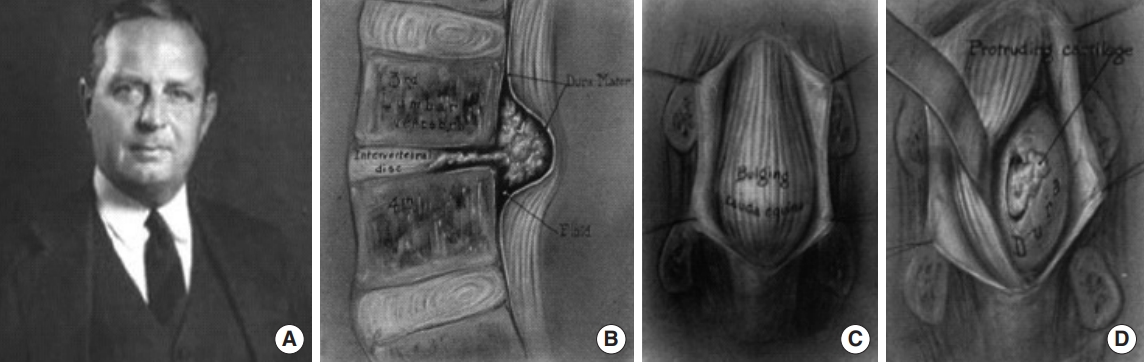
Interestingly, another Boston duo demonstrated insight regarding the discogenic radiculopathy before Mixter and Barr. In 1911, Joel Goldthwait [7] uncovered a correlation between annulus fibrosus rupture, neurologic signs, and symptomatic sciatica after anatomical studies on a patient following treatment by neurosurgeon Harvey Cushing (a former student of Emil Theodor Kocher), who performed a laminectomy for cauda equina decompression. In 1929, Baltimore neurosurgeon Walter Dandy (Fig. 1) published his surgical experience [8] removing loose cartilaginous fragments transdurally, which he postoperatively concluded to be consistent with disc material and traumatic in nature [9,10]. Dandy described the disc material as bulging out like a tumor into the spinal canal, compressing the nerve roots of the cauda equina and causing radicular motor and sensory paralysis.
Despite these advances in identification, pathophysiological understanding, and surgical treatment of intervertebral disc herniation, it was not until Mixter and Barr’s seminal 1934 paper (Fig. 2) that a consensus was reached. However, it is thanks to the work of those that came before that the stage was set for Mixter and Barr to uncover the phenomenon of the discogenic sciatica and usher in a new era of surgical disc repair [11].
2. Posterior Approaches to Lumbar Fusion: 1944–1998
Spurred by the new information of the discogenic sciatica as well as Mixter and Barr’s surgical advice, in 1944 Briggs and Milligan [12] published a landmark paper describing their novel surgical technique, the posterior lumbar interbody fusion (PLIF). Using the term “chip fusion,” their technique consisted of exposing the spine posteriorly and removing chips of bone from the spinous processes, as well as partially excising the lamina and facet joints. These excisions exposed the disc space, and the disc was removed and replaced with a round bone peg. The bone chips initially obtained were placed over the dura and facet joints after insertion of the bone peg. Though Briggs and Milligan saw promising results in their PLIF patients, several issues remained: patients faced a long and arduous recovery in the hospital, the procedure carried an imposing complication profile particularly with neurological complications and pseudarthrosis, and it proved difficult for surgeons to adopt. Briggs and Milligan may have achieved some success in postoperative fusion, though they did not report any [13]. As such, it failed to gain complete acceptance throughout the spine surgical community [14].
Despite the early work of Briggs and Milligan, later investigation has revealed that a neurosurgeon from Hawaii named Ralph Cloward [15] actually demonstrated the earliest PLIF attempt [13]. After noticing an opportunity to insert spinous bone fragments into the disc space during a discectomy in 1940, Cloward essentially attempted the first PLIF, though the patient died and he did not pursue the technique further [16]. In 1943, however, he reattempted the procedure and noted fusion success, after which he devoted much of his career to continually enhancing the PLIF [17].
Over the next 40 years, the PLIF underwent several modifications, including replacement of the bone peg with bone grafts from the ilium or cadavers [15], addition of Harrington rod instrumentation [18], and preservation of certain structures like the proximal facet joint and cortical plate [19]. One concept that would prove particularly valuable was the alteration of facet screws to form longer screws to be placed through the vertebral pedicles [20-22]. This innovation emerged from a keen insight that pedicle screws can provide three-column fixation and thereby maximize lumbar stability during fusion [21]. Pedicle screw instrumentation was brought to the PLIF in the late 1980s by a team led by orthopaedic surgeon Art Steffee [23], who intuitively recognized that placement of pedicle screws through the anterior spinal column delivered an optimization of stabilization and facilitation of fusion that had eluded facet screws.
Interestingly, the work of 2 innovators during this time foreshadowed future spine surgical techniques. In 1968, American surgeon Leon Wiltse wrote of a novel technique [24] for posterior lumbar spinal access. Later referred to as the Wiltse paraspinal approach, this technique called for dissection of the natural cleavage between the multifidus and longissimus paraspinal muscles as a pathway to the vertebral column. Wiltse’s insightful conceptualization of a muscle-splitting route to the spine served as a unique glimpse into the later field of minimally invasive spine surgery.
In 1973, Philadelphia orthopaedic surgeon Parviz Kambin [25,26] introduced a transforaminal route to the disc space, exploiting an access corridor free of significant vascular and neural structures. Kambin initially explored this pathway in percutaneous posterolateral resection of herniated L3–4 and L4–5 discs, using fluoroscopic guidance and an incision 8–9 cm from the midline [27]. “Kambin’s triangle” (recently described three-dimensionally as “Kambin’s prism” by Fanous et al. [28]) is enclosed anteriorly by the exiting nerve root, inferiorly by the proximal endplate of the lower vertebral body, posteriorly by the superior articular process of the lower vertebra, and medially by the traversing nerve root and thecal sac. Without necessitating bone removal, this anatomical prism enabled Kambin to perform endoscopic discectomy procedures while avoiding neural retraction [29].
In 1982, 2 German surgeons sought to entirely rethink the posterior approach to lumbar fusion, via a transforaminal access route similar to that explored by Kambin. Harms and Rolinger [30] published their new technique, the TLIF, which employed a unilateral corridor through the intervertebral foramen to directly access the anterior disc space and implant titanium mesh packed with bone graft [14]. With this new procedure, and with Harms and Jeszenszky’s [31] subsequent refinement of the technique in 1998 to incorporate a complete removal of the facet joint [32], surgeons could now posteriorly access the disc space with impressively reduced damage to nerve roots and other key anatomic complexes [1]. Though reminiscent of Kambin’s transforaminal insight, the TLIF called for excision of the facet joint, pars interarticularis, and hemilamina, and as such moved beyond Kambin’s prism to create an expanded transforaminal corridor which facilitated insertion of an interbody device [28].
The improvements delivered by the TLIF were intuitive. The unilateral approach inherently demanded less soft tissue damage than the PLIF’s bilateral approach. The PLIF required substantial neural retraction, and subsequently held a particularly dangerous risk of injury to nerve roots and dura mater, among other structures [1]. Incorporating a complete facetectomy, the TLIF dramatically reduced thecal sac traction, protecting patients from much of these potential neurological injuries [14]. Accordingly, the TLIF steadily gained recognition as an effective and safer surgical option. Studies conducted in 2017 [33], 2018 [34], and 2021 [35] comparing PLIFs and TLIFs in 2,825 total patients found TLIFs to exhibit impressive reductions in operative time, blood loss, dural tears, and nerve root injuries. An additional advantage of the TLIF is the superior restoration of lordosis [36,37], a critical aim when surgically correcting spondylolisthesis. A 2022 study [38] reporting an 8-year dual-center comparison of single-level PLIFs and TLIFs noted markedly similar clinical and radiographic outcomes, while PLIFs held a 5-time greater risk of dural tears.
3. Inception of the MIS-TLIF: Turn of the 21st Century
While Harms and Rolinger’s TLIF demonstrated significant improvement in the search for optimal lumbar stabilization, surgeons would remain discontent with its efficacy. The success of posterior-approach lumbar arthrodesis was curtailed by exposure-related adverse events that would garner the general term of “fusion disease.” [39] Extensive retraction and muscle stripping resulted in an imposing amount of injury to soft tissues, paving the way for postoperative issues including prolonged back pain and poor long-term outcomes [40]. In light of these unwanted issues of paraspinal iatrogenic injury associated with the open TLIF [1], further innovations to posterior-approach lumbar stabilization were inevitable.
In 2002, Kevin Foley and Michael Lefkowitz [41] of the University of Tennessee published an article in Clinical Neurosurgery entitled “Advances in minimally invasive spine surgery” detailing their adaptation of the TLIF to employ tissue-sparing mechanisms. Though certainly not the first change to posterior-approach lumbar interbody fusion, the proposed MIS-TLIF brought a new type of change.
By the early 2000s, minimally invasive techniques had found their way into several spinal procedures. Notably, lumbar discectomies had seen successful applications of laparoscopic [42] and microendoscopic [43-45] techniques. Issues of lengthy hospital stay, significant costs, and increased morbidity associated with open procedures had aroused increasing concern for spine surgeons of the time. The danger of substantial muscle retraction especially revealed itself. Studies of this time reported significant damage to lumbar musculature and subsequently increased incidence of low back pain [46], increased levels of ischemia [47], increased weakness [48], and persistent pathological alterations of paraspinal muscles [49], all in direct association with the extensive use of muscle retractors in open spine surgery. Dr. Foley, a pioneer of the microendoscopic lumbar discectomy, recognized the innovative significance of minimally invasive surgery far different from the innovations that lumbar procedures had seen in the past. The TLIF improved the PLIF by altering it conceptually, changing the access window to the disc space. In contrast, the MIS-TLIF improved upon the open TLIF in a more nuanced manner, keeping it conceptually unaltered but refining it to further enhance its safety profile for patients, with a reduced “surgical footprint.” [40]
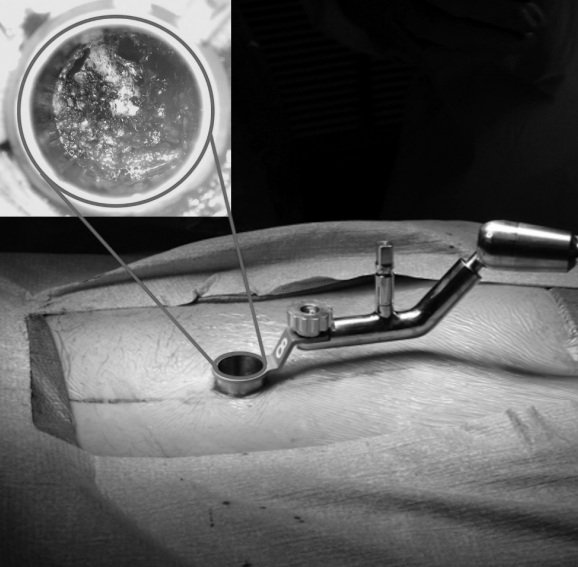
Foley and Lefkowitz’s MIS-TLIF incorporated 2 mirroring 1-inch paramedian incisions. The first incision was used for the insertion of a tubular retractor to the facet joint for facetectomy, discectomy, and placement of an interbody implant and bone graft. Upon tubular retractor removal, both contralateral paramedian incisions were then used for pedicle screw fixation using a percutaneous screw-rod system [50]. The use of a tubular retractor enabled the surgeon to reach the disc space and perform the TLIF through a circumferentially defined surgical window, with impressive mitigation of soft tissue disruption along the way. Preservation of soft tissue was the innovative hallmark of the MIS-TLIF, aimed at improving the patient experience perioperatively (Fig. 3).
IN THE PRESENT: CHARACTERIZING THE MIS-TLIF
1. Technological Advancements of the MIS-TLIF
Technological innovations in surgery have paved the way for procedures like the MIS-TLIF. Perhaps the quintessential piece of equipment enabling this procedure is the tubular retractor (Fig. 4), introduced by Foley and Smith in 1994 [50,51]. The first tubular retractor system allowed surgeons to split muscles, rather than cut them, on the path to the spine [52]. This was accomplished using a series of sequential tubular dilators with consecutively increasing diameters, and placement of a tubular retractor over the final dilator [45]. Surgeons could therefore access the spine via a tissue-sparing pathway, and still incorporate previously developed microsurgical instruments including Kerrison rongeurs and nerve root retractors [27]. The resulting decrease in muscle damage was the pivotal change necessary to temper the chronic back pain and other postoperative symptoms faced by open TLIF patients.
Though tubular retractors yielded a beneficial preservation of soft tissue and subsequent reduction of access-related back pain, the use of this technology came at a certain cost. Tubular retractors dramatically limit operative visualization and subsequent understanding of the spatial positioning of anatomical structures. In an open TLIF, a large surgical incision maximizes the view of anatomical landmarks to direct operative steps. The small operative window of the MIS-TLIF restricts the ability to place a large interbody spacer, threatening the potential to adequately restore lordosis. The insufficient ability for a discectomy and large cage insertion, as well as the incorporation of only a unilateral facetectomy, may put the MIS-TLIF at further risk regarding postoperative pseudarthrosis [53].
To account for restricted visualization, MIS-TLIF surgeons rely on diagnostic imaging modalities such as intraoperative fluoroscopic guidance and neuromonitoring [54]. Diverging from the open technique, surgeons must continuously interpret these modalities and integrate them fluidly within the operation. Additionally, surgeons must be prudent in managing intraoperative fluoroscopy so as to minimize radiation exposure to patients and staff. Thus, to perform the MIS-TLIF successfully, operating surgeons must conduct diligent preoperative planning to overcome decreased visualization, as successful patient outcomes rely on fluid integration of imaging with MIS techniques. In addition, MIS techniques carry a considerable surgical learning curve, rendering it imperative that surgeons take appropriate training measures and hold in high regard the importance of preoperative planning and the anatomical makeup of the operative field which loses direct visibility [55].
2. Modern Variability of the MIS-TLIF
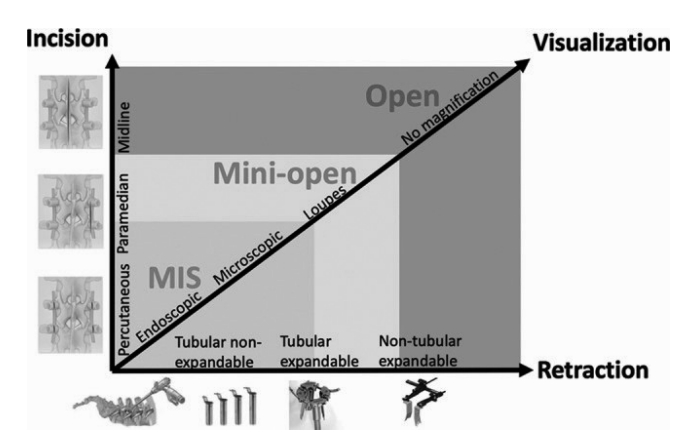
In a 2020 effort to define the modern MIS-TLIF, Lener et al. [56] evaluated the technical and procedural aspects reported in 75 MIS-TLIF-related articles published between 2010–2018. Their comprehensive analysis of 4,920 total patients yielded 3 criteria most characteristic of MIS-TLIFs, related to the processes of retraction, incision, and visualization. The first of these is the use of tubular retractors in approaching the facet joint for cage insertion. The second is the use of paramedian incisions, thus excluding procedures using midline incisions from MIS standing. The third is that the considerable reductions in operative visualization are mitigated by the use of a microscope or endoscope. The intersection of these 3 hallmarks of the MIS-TLIF is illustrated in Fig. 5.
Though Lener’s team has admirably differentiated MIS-TLIFs from mini-open or traditional open techniques, the very need for their analysis is related to one interesting quality of the MIS-TLIF: that it contains within itself much variability. For while the 3 aforementioned criteria set a broad framework for the procedure to take form, there exist within that framework different variations of the technique. Fortunately, Lener and colleagues’ comprehensive and systematic investigation of the MIS-TLIF yielded some definition to its variability.
The first point of diversion among MIS-TLIFs involves the type of retractors used to access the facet joint. Though 81% of their included studies reported the use of tubular retractors, there remained variability as to which type of tubular retractors were used. 35% of studies discussed nonexpandable tubular retractors, 21% discussed expandable, and 25% discussed both types. Despite this slight variability, the general use of tubular retractors appears to be invariable in MIS-TLIFs, excluding procedures that use nontubular retractors or endoscopic access.
The primary point of MIS-TLIF diversity pertains to the properties of the interbody cage implanted into the affected disc space. Cages can vary in 3 distinct qualities: shape, material composition, and dynamic ability. Regarding cage shape, the 2 most common designs are banana-shaped and straight-shaped. Of the studies in Lener and colleagues’ analysis that reported the type of interbody cage used, 65.2% disclosed straight-shaped, 15.2% disclosed banana-shaped, and 15.2% mentioned either. A 2018 randomized controlled trial by Choi et al. [57] assigned 44 patients to receive banana-shaped cages and 40 patients to receive straight-shaped. Their results indicated similarly favorable fusion rates and clinical improvements in disability and pain, though straight-shaped cages ended up in concluding favor based on the discovery that banana-shaped cages were associated with significantly higher rates of postoperative cage subsidence.
The material of interbody cage composition takes 2 primary forms: titanium and polyetheretherketone (PEEK). Lener’s team found that 82.8% of studies reporting cage material used PEEK, while 10.3% used titanium. Fusion rates reported in the literature are comparably favorable between the 2 cage materials [58], though a 2017 systematic review [59] evaluating 410 patients did identify a significantly higher rate of postoperative cage subsidence from titanium cages. Based on these findings, the general favorability of PEEK cages over titanium may be related to a greater chance for successful fusion, similar to the general favorability of straight-shaped cages over banana-shaped.
The final area of cage variation concerns its dynamic capabilities. Interbody cages can be either static or expandable. The traditional use of static cages rendered the TLIF’s ability to adequately restore sagittal alignment controversial, due to its small window of disc space access relative to anterior-approach procedures. To mitigate this, expandable cages were developed such that they may be inserted into the disc space and then expanded to achieve the desired restoration of disc height and lordosis [60,61]. Since their inception, expandable cages have become commonplace in MIS-TLIFs, used in 88.1% of studies in Lener’s review that reported type of interbody cage, in contrast to the 11.9% reporting use of static cages. Despite the intuitive benefits of expandable cages regarding lordosis restoration, the comparative effects between static and expandable cages remain unclear [62]. A recent study [60] comparing long-term radiographic outcomes between these cage types revealed that while expandable cages do appear to produce an environment more suitable to restoration of sagittal alignment and disc height, they also showed higher rates of cage subsidence, which may threaten their true advantage. Such subsidence may be related to the force of expansion required in the considerably small disc space, potentially placing unwanted stress against vertebral endplates and leading to subsequent cage migration [61]. In contrast, Li et al.’s analysis [64] of 284 osteoporotic MIS-TLIF patients spoke to the fusion-related advantages of expandable cages, finding similar lordotic angles but significantly higher rates of intraoperative subsidence, postoperative subsidence, and cage migration with static cages. Teams led by Hawasli [64] and Russo [65] found expandable cages to provide significantly greater and longer-lasting restoration of disc height, foraminal height, and segmental lordosis following MIS-TLIF than static cages, as well as improved postoperative disability scores. More comparative investigation is required, particularly in fusion rates.
Along with the cage implanted into the disc space, a bone graft is typically used to induce successful fusion. Though several variations exist, including bone morphogenetic protein and allograft transplants which continue to be explored, nearly 80% of studies in Lener’s analysis reported the use of autograft, which typically comes in the form of iliac crest bone graft (ICBG). ICBG, taken from the hip of the patient receiving the MIS-TLIF, has been shown to lend itself kindly to successful fusion [66].
After interbody implant placement, the surgeon is left with the insertion of percutaneous pedicle screws for increased stabilization of the fused vertebrae. This step contains further diversity among MIS-TLIFs. Foley and Lefkowitz’s initial concept employed a bilateral approach for pedicle screw fixation over 2 contralateral paramedian incisions. In the years since, however, surgeons have explored the idea of unilateral fixation. The unilateral technique utilizes the same incision that was made for cage insertion to insert pedicle screws, without necessitating a mirroring incision. In a prospective trial exploring the operative and clinical results of these 2 techniques, Choi et al. [67] randomized 26 patients to undergo unilateral fixation and 27 patients to undergo bilateral fixation. While their unilateral fixation cohort demonstrated significantly lower operative time and blood loss, the perceived benefit of this technique stopped with these perioperative improvements. Both cohorts saw notable clinical improvements in disability and pain through 2 years. The most significant finding of this trial, however, is the observed difference in fusion rates. 84.6% of unilateral fixation patients showed radiographic evidence of successful fusion at 2 years, while 96.3% of bilateral fixation patients fused at 2 years. Choi et al. provide rationale for the unrivaled importance of fusion rates in this comparison, identifying this as the key measure of successful pedicle screw fixation, the aim of which is simply to facilitate fusion. Reflecting this, the investigators concluded that bilateral fixation is a superior option, providing more impressive lumbar stabilization and thereby delivering an environment more likely to promote successful fusion following MIS-TLIF.
Throughout the entirety of the MIS-TLIF, there remains the challenge of restricted operative visualization. While Lener’s team did find that invariably some sort of magnification is used, the vast majority of procedures additionally incorporate some type of intraoperative imaging to guide their surgical effort. 79% of the 75 reviewed articles reported the use of standard fluoroscopy, while 3-dimensional (3D) fluoroscopy was noted in 11% of articles and intraoperative computed tomography (CT) imaging in 3% of articles. The demand for intraoperative guidance due to reduced visualization has shown to result in significantly greater fluoroscopy times for MIS-TLIFs relative to open TLIFs [68], posing a threat of extended radiation exposure to both patients and operative staff. Though cone-beam CT imaging systems provide reductions in surgeon radiation exposure relative to traditional fluoroscopy [69], standard fluoroscopy remains most prominent in MIS-TLIF procedures. Furthermore, it has been suggested that cone-beam CT imaging may provide simply a nonsignificant reduction in surgeon exposure while unnecessarily increasing radiation exposure to the patient [70].
3. Current MIS-TLIF Outcomes
Recent studies have explored MIS-TLIF outcomes, often pursuing a comparison to the open TLIF. Due to the characteristic reduction in muscle disruption, the relative superiority of the MIS-TLIF is primarily perioperative. Operative time, blood loss, hospital stay, and narcotic administration have seen encouraging reductions in MIS-TLIF populations. In addition, short and long-term outcomes in disability, back pain, and leg pain have favored MIS-TLIF patients relative to their open TLIF counterparts [71]. Seng et al. [72] compared MIS-TLIF to open TLIF with 5-year follow-up, observing similar midterm and long-term outcomes for disability, neurogenic symptom score, back/leg pain, and physical function as well as similar 1-year fusion rates. While long-term clinical trajectory was similar, the authors observed important MIS-TLIF benefits, specifically noting improved initial postoperative pain, decreased bleeding, earlier rehabilitation times, and shorter hospitilization [72]. These findings have been echoed by teams led by Lau et al. [73] and Wang et al. [74] in comparative studies of open versus MIS-TLIF in obese populations, where authors reported significant perioperative benefits, reduced complications, and improved postoperative back pain for MIS-TLIF patients. A 2016 systematic review by Hu et al. [75] noted that MIS-TLIF held significant advantages over open TLIF in blood loss, length of hospital stay, and complication rates, but it was also discovered that MIS-TLIF patients were faced with significantly greater radiation exposure during surgery, suggesting that heightened fluoroscopy use may threaten long-term success. These findings were echoed by a 2020 systematic review from Miller et al. [76]. The widely demonstrated perioperative advantages of the MIS-TLIF over the open TLIF also appear to translate to financial benefits, with authors reporting reduced hospital costs and considerable preservation of hospital resources due to reductions in operative time and length of hospital stay [77,78]. Cost-effectiveness of the MIS-TLIF may be further increased given recent trends of transitioning cases to the outpatient setting, where intuitively the imposing costs of hospitalization can be avoided [79].
Similar to its relationship with the open TLIF, the MIS-TLIF is evolutionary linked to the PLIF, and the inherent differences between the 2 procedures are well represented in comparisons of their outcomes. In a 2016 systematic review evaluating 856 MIS-TLIF patients and 806 open PLIF patients, Goldstein et al. [80] found the 2 procedures undifferentiated by way of patient-reported clinical outcomes, though MIS-TLIFs demonstrated significant favorability in blood loss, time to ambulation, length of hospital stay, and perhaps most notably adverse events. These advantages of the MIS-TLIF are intuitive; with reduced muscle disruption through the use of muscle-splitting tubular retractors, it follows that the MIS-TLIF can deliver great perioperative benefit over its open procedural counterparts. Another systematic review by Goldstein et al. [81] in the same year revealed both direct and indirect cost-savings associated with MIS-TLIFs relative to open TLIFs and PLIFs, further suggesting that the advantages of the MIS-TLIF lay primarily in the perioperative realm. The MIS-TLIF yields similar clinical outcomes to the TLIF and PLIF [82] while improving the safety and economic considerations of lumbar fusion surgery.
One region at which the MIS-TLIF may remain limited is the L5–S1 vertebral level. The biomechanical significance of L5–S1 demands a strong effort in sagittal restoration, and the 2 surgical techniques most commonly used to achieve this are the TLIF and the anterior lumbar interbody fusion (ALIF), both of which have demonstrated successful fusion induction [83]. A recent study [84] by our team comparing MIS-TLIF to ALIF at L5–S1 discovered that ALIF patients on average experienced more favorable postoperative clinical outcomes in physical function, back pain, and leg pain, as well as significantly fewer incidences of postoperative fever. The ALIF is widely considered to be particularly suitable for lordosis restoration at L5–S1 [85], due to its increased and direct vertebral access window, allowing for implantation of a larger interbody cage. Open TLIFs also have potential for considerable lordotic restoration, accomplishing comprehensive bilateral anterior column stabilization through a unilateral approach [86]. In particular, surgical methods such as the cantilever technique (c-TLIF) incorporate bilateral facetectomy and posterior column compression in conjunction with expansion of the anterior column through release of the anterior longitudinal ligament (ALL), mechanically inducing further sagittal correction [87-89]. In this regard the open TLIF can approach the lordotic success of the ALIF, though this potential evades the MIS-TLIF as the restricted operative view eliminates the ability for contralateral facetectomy or safe ALL release. Current data regarding lordosis restoration following MIS-TLIF is variable, highlighting the need for further investigation [90]. Nonetheless, the MIS-TLIF may be well-equipped to restore disc height and sagittal alignment at this vertebral level through the use of expandable cages. As previously mentioned, long-term radiographic and clinical data will be required to confirm the lordotic potential of expandable cages in MIS-TLIF.
LOOKING AHEAD: CONTINUED EVOLUTION OF THE MIS-TLIF
In the evolution of the TLIF, innovations have been characterized by refinement. Through a series of minor improvements, the MIS-TLIF has emerged as a technique capable of granting patients significant clinical and radiographic improvement while minimizing soft tissue damage and associated adverse events. Recent trials suggest that this trend will continue, with surgeons around the world conceptualizing robotic-assisted pedicle screw fixation, augmented reality-enhanced intraoperative navigation, and more.
1. Robotics
The experience of Cui et al. [91] with robotic-assisted pedicle screw placement in 23 MIS-TLIF patients revealed significant favorability in pedicle screw accuracy, intraoperative blood loss, postoperative pain, postoperative drainage, recovery time, and paraspinal muscle atrophy relative to 25 open TLIF patients. Lin et al. [92] compared 75 MIS-TLIF patients who underwent robotic-assisted pedicle screw fixation to 149 patients receiving freehand fluoroscopy-assisted fixation, noting similar postoperative outcomes between cohorts, with robotic-assisted patients experiencing significantly reduced blood loss, as well as reduced operative time for procedures on more than 3 vertebral levels. Vo et al. [93] recently commented on the current state of robotic applications in spine surgery, indicating that robotics are most highly investigated in the capacity of instrumentation guidance. Their analysis highlights the infant nature of spinal robotics, citing one early randomized controlled trial [94] that demonstrated less accuracy from robotic-assisted pedicle screw fixation relative to freehand. Though aforementioned later trials have shown more promise, this remains an area requiring comprehensive investigation and long-term data.
2. Augmented Reality
Jamshidi et al. [95] recently published a video and abstract of their experience with augmented reality visualization to improve pedicle screw accuracy following endoscopic TLIF surgery. The innovative head-mounted display, integrated with a tracking camera, enables the surgeon to view navigation assistance in the same field as the operative site. After demonstrating successful and accurate pedicle screw placement in cadavers [96], this technology underwent a recent first-in-human trial [97], showing clinical accuracy and technical precision. Though such technologies currently exist in infancy and have yet to prove their true efficacy, the future appears open to further refinements of the MIS-TLIF. A recent systematic review [98] noted pedicle screw malpositioning among the most frequent of MIS-TLIF complications, second only to radiculitis, suggesting that pedicle screw fixation may be the area of the MIS-TLIF most vulnerable to change.
3. Interbody Implant Alternatives
A 2021 detailed review has shown that MIS-TLIF interbody implants may also be subject to change. Lo et al. [99] indicate that the commonplace usage of ICBG may fade given concerns regarding the difficulty of autologous harvesting as well as potential for infection development, and suggest that bone grafting may shift towards materials such as ceramics and cell-based regenerative therapeutics (including stem cells, cellular bone matrices, and platelet-derived biomaterials). In addition, 3D printing may find a profound role in designing interbody cages of optimal characteristics.
CONCLUSION
The story of the MIS-TLIF reveals a consistent commitment to innovation, ultimately yielding the tissue-sparing refinement of posterior-approach lumbar arthrodesis. Through the use of muscle-splitting tubular retractors in conjunction with fluoroscopic guidance, surgeons can perform the TLIF, as designed by Harms and Rolinger in 1982, in a manner that minimizes tissue trauma and perioperative morbidity, all while maintaining or improving the clinical outcomes of the open technique.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-forprofit sectors.
Author Contribution
Conceptualization: MP, KJ, MP, HP, NV, KS; Project administration: KS; Writing - original draft: MP, KJ, MP, HP, NV, KS; Writing - review & editing: MP, KJ, MP, HP, NV, KS.