Concepts and Techniques to Prevent Cervical Spine Deformity After Spine Surgery: A Narrative Review
Article information
Abstract
Adult cervical spine deformity is associated with decreased health-related quality of life, disability, and myelopathy. A number of radiographic parameters help to characterize cervical deformity and aid in the diagnosis and treatment. There are several etiologies for cervical spine deformity, the most common being iatrogenic. Additionally, spine surgery can accelerate adjacent segment degeneration which may lead to deformity. It is therefore important for all spine surgeons to be aware of the potential to cause iatrogenic cervical deformity. The aim of this review is to highlight concepts and techniques to prevent cervical deformity after spine surgery.
INTRODUCTION
The cervical spine functions to position the head over the body in space and to maintain horizontal gaze [1]. Under physiologic conditions, these functions occur without excess recruitment of soft tissue structures or fatiguing of cervical musculature [2]. As cervical alignment deviates from normal, increased energy is required to maintain horizontal gaze [2]. Cervical spine deformity (CSD) is associated with increased disability, decreased health-related quality of life (HRQoL), and myelopathy [3-6], and cervical alignment is closely related to global sagittal alignment [7].
Causes of cervical deformity are numerous, and are broadly categorized as congenital, traumatic, inflammatory, infectious, degenerative, and iatrogenic [8-10]. The most common cause of deformity is iatrogenic, and relates to patient positioning, hardware placement, size and quantity of bone graft used, and technical error [8]. The natural history of cervical degenerative disc disease may also be accelerated after cervical spine surgery, leading to increased adjacent segment degeneration and deformity [11,12]. More recent literature has highlighted the concept of reciprocal change, when unfused spinal regions adapt after primary deformity fusion in other regions such as the thoracolumbar spine [13,14]. Applications of techniques to prevent cervical deformity therefore apply to a wide range of procedures to treat different spine pathology.
Several radiographic alignment parameters aid in measuring and defining CSD. Cervical lordosis (CL) is the cobb angle from the anterior and posterior tubercles of C1 or the inferior endplate of C2 and inferior endplate of C7, with normal C1–7 lordosis measuring approximately 40° and normal C2–7 measuring approximately 10° (Fig. 1) [7]. Translation of the head in the sagittal plane refers to the cervical sagittal vertical axis (cSVA), the distance of a plumbline from the center of C2 to the posterosuperior corner of C7 (Fig. 1). The average cSVA in healthy individuals is about 1.5 cm, and values greater than 4 cm are associated with disability and negative HRQoL [15,16]. The chin-brow vertical angle (CBVA) is the angle between a line from the chin to the brow and the vertical axis and helps infer horizontal gaze (Fig. 1). The CBVA is positive when the head is facing down and negative when the head is facing up. The CBVA is a primary target for cervical deformity correction, as restoration to physiologic values between +10° and -10° correlates with improved outcomes [17,18]. The last major angle defining cervical alignment is T1 slope, a line parallel to the superior endplate of T1 and the horizontal. The T1 slope closely relates to the overall CL and is similar to the association of lumbar lordosis and pelvic incidence [1]. Staub et al.19 proposed that normative CL may be predicted using the formula CL = T1 slope – 16.5° ± 2°. Table 1 depicts normative values for the discussed radiographic parameters [15,20,21].

Pictorial representations of commonly used angular measurements to describe cervical alignment. CBVA, chinbrow vertical angle; cSVA, cervical sagittal vertical axis; CL, cervical lordosis. Adapted from Passias et al. Neurosurgery 2018;83:651-9, with permission of Wolters Kluwer Health, Inc. [7]
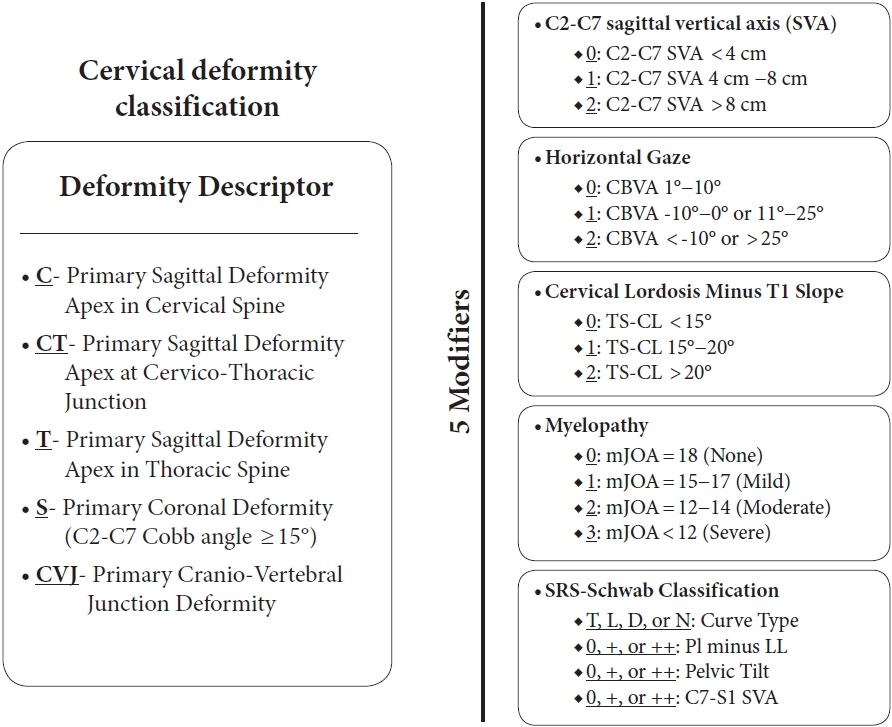
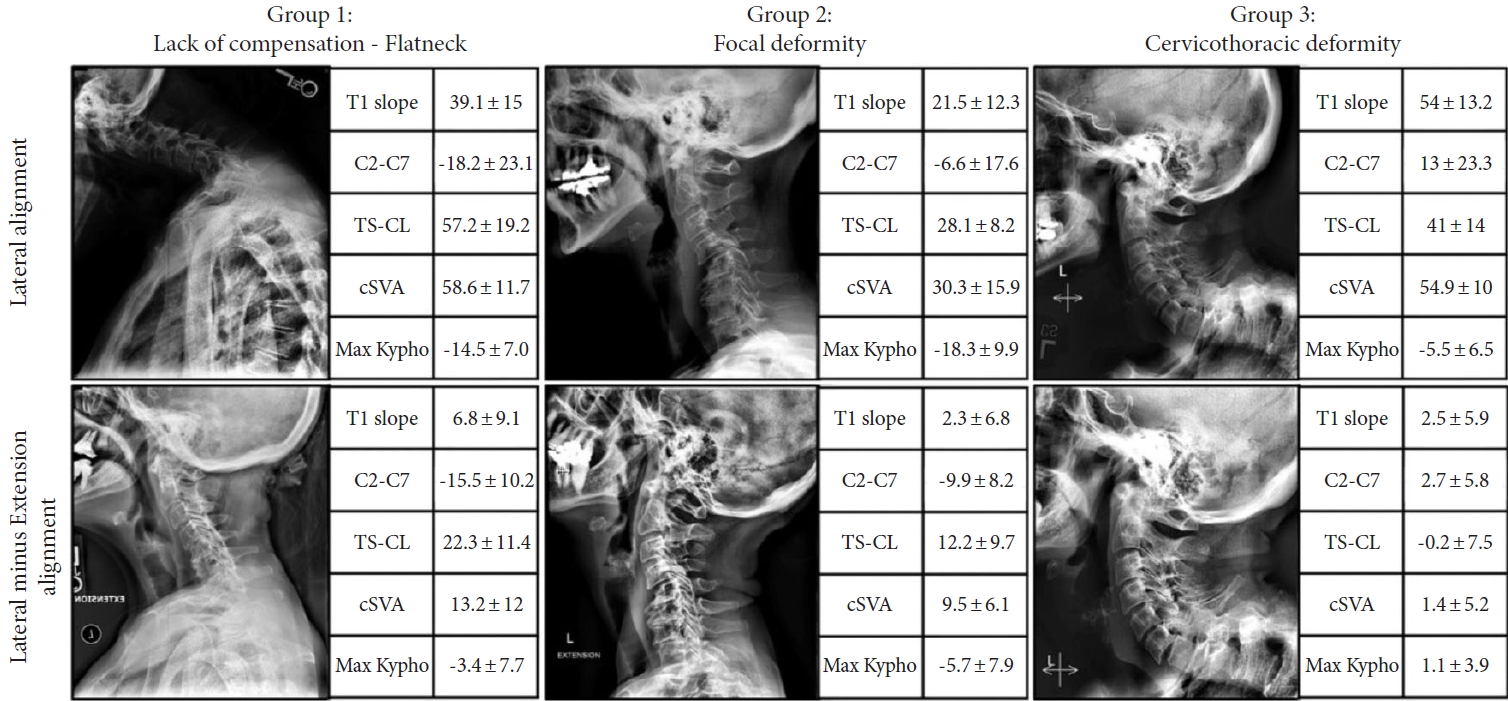
Recent efforts to classify CSD have provided systems that may improve communication, research, and treatment algorithms. The Ames-Misclassification includes a “Deformity Descriptor” based on the location of the deformity with 5 modifiers: C2–7 SVA, CBVA, T1 slope minus CL (T1–CL) mismatch, myelopathy (modified Japanese Orthopedic Association score), and thoracolumbar deformity (Scoliosis Research SocietySchwab classification) (Fig. 2) [22]. The Kim-International Spine Study Group classification uses dynamic radiographs to define 3 distinct groups: “flat neck,” “focal deformity,” and “cervicothoracic deformity,” each with unique drivers of CSD and surgical treatment strategies (Fig. 3) [23]. The Cervical Spine Research Society (CSRS)-Europe system classifies CSD into 4 groups based on cervical alignment, regional balance, and global balance; this system has practical implications for myelopathy, osteoporosis, and treatment approach [24].
Cervical deformity based on abnormal radiograph parameters correlates with disability and negative HRQoL [3,4,25-27]. The strongest predictors of disability and poor HRQoL are decreasing T1–CL, high C2–7 SVA, and high CBVA [3,25,26,28]. As a kyphotic deformity ensues, the spinal cord drapes over the posterior aspect of the vertebral bodies, leading to compression of the spinal cord and its ventral blood supply [29,30]. This process can lead to neuronal loss, demyelination, and ultimately development of myelopathy. Two important considerations about the cervical deformity literature, though, are the radiographic correlations to outcome are not as strong as those in the lumbar spine, and additionally the majority of studies are on primary, rather than iatrogenic deformity. Therefore, the relationship between iatrogenic deformity and HRQoL is not as clear. Nonetheless, considering the most common cause of CSD is iatrogenic, it is paramount for spine surgeons to recognize pitfalls in surgical techniques that may lead to postoperative CSD. Furthermore, surgical correction of iatrogenic CSD has a high incidence of complications [31,32]. Overall complication rate has been cited as high as 56.5% if a 3-column osteotomy is involved, with neurologic deficits and postop mortality cited as high as 17.4% and 9.2%, respectively [31]. These findings further emphasize the importance of preventing iatrogenic cervical deformity after spine surgery. The aim of this review is to discuss current concepts and techniques to prevent postoperative CSD.
PREVENTING CERVICAL DEFORMITY AFTER ANTERIOR CERVICAL PROCEDURES
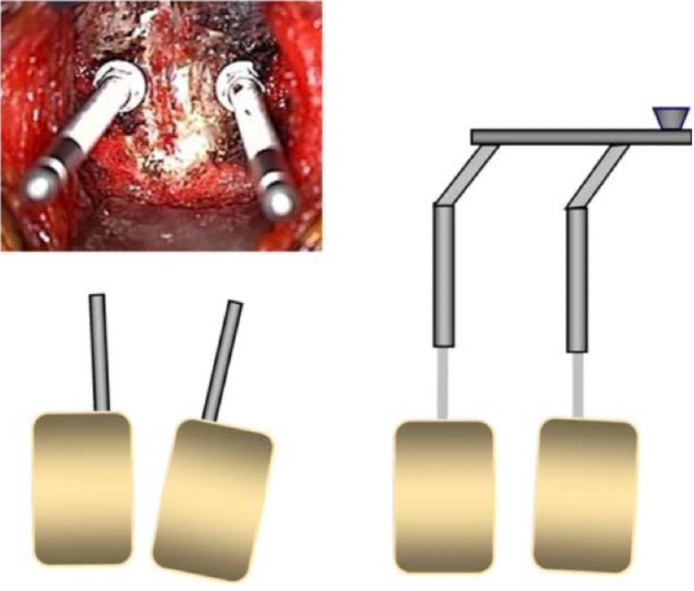
Lack of preoperative radiographic assessment/planning and positioning are the first parts of anterior cervical procedures that may contribute to iatrogenic sagittal, coronal, and axial deformity. Obtaining appropriate preoperative flexion/extension films and entire spine films in addition to advanced imaging (computed tomography/magnetic resonance imaging) will mitigate against missing instability or thoracolumbar issues that may affect outcome. For positioning, a bump is often placed under the patient’s neck to drop the scapulae and head to increase exposure. In doing so, hyperlordosis may occur. Fusion in excessive lordosis may cause posterior neck and interscapular pain in addition to nerve root symptoms from narrowing of the neuroforamen [33]. It is imperative to adequately inspect the patient both clinically and radiographically prior to beginning a fusion procedure. Adjusting the bump to create neutral to slightly lordotic alignment is desirable. If excess lordosis is noted intraoperatively, the technique of placing converging Caspar pins may help reduce the lordosis once the pins are parallelized and distracted (Fig. 4) [33]. Coronal malalignment may occur from over tensioning of one shoulder when the shoulders are taped down. Extra caution and inspection must be done to ensure the head is not tilted towards one side. Axial malalignment can also occur and may be difficult to detect, because it usually occurs during retraction after the patient has been draped. Several methods can mitigate this, such as taping the forehead to stabilize head rotation prior to draping, use of Gardner-Wells tongs, or using commercially available head holders that utilize a chin strap to maintain rotation [33]. Positioning is a seemingly benign event that can lead to poor outcomes, and it is critical for the entire surgical team to be cognizant of the patient’s position both clinically and radiographically prior to fusing any motion segments.
Asymmetric exposure of the disc and vertebral bodies may lead to subsequent asymmetric discectomy/corpectomy. Placing a graft eccentrically may then induce a coronal deformity. It is therefore recommended that complete anterior exposure of the disc to the margins of the transverse foramina and to the uncovertebral joints be performed [33]. These osseous structures provide reliable landmarks in each patient to aid in execution of a symmetric discectomy. If Caspar pins and distraction are used to aid in decompression, misplaced pins may lead to sagittal, coronal, and axial malalignment as demonstrated in Fig. 5 [33]. Lastly, performing multiple corpectomies with a single, straight graft will leave the patient in neutral alignment. Therefore, depending on the pattern of compression, performing skip decompressions instead will produce multiple points of fixation where lordosis can be restored [33]. Table 1 summarizes several tips and techniques for preventing iatrogenic cervical deformity after various spine surgeries.
PREVENTING CERVICAL DEFORMITY AFTER POSTERIOR CERVICAL PROCEDURES
Like anterior procedures, positioning is crucial for posterior procedures. The head is often fixed in a tong or halo device which can be adjusted to flex or extend the neck. Ensuring proper position of the neck clinically and radiographically, particularly if a fusion procedure is to be performed, is essential. Not only can the neck be fixed with improper sagittal alignment from the head positioner, but axial and coronal malalignment can also occur [33]. In heavier patients who have redundant skin folds on the posterior neck, the skin should be manually retracted to inspect the actual position of the posterior neck prior to final positioning, as these redundant skin folds can often make the neck appear to be in neutral alignment when it is in fact too flexed or extended. As with anterior procedures, the shoulders are usually taped during posterior procedures to aid in radiographic imaging of the cervical spine. Asymmetric taping of may lead to a coronal malalignment. We recommend that prior to beginning the procedure the entire surgical team agree that the neck is positioned adequately both clinically and radiographically. The above nuances are particularly essential when performing an occipital-cervical fusion where no compensation can occur postoperatively. Choice of fixation device, such as Mayfield vs. Gardner Wells tongs becomes an important choice. Mayfield tongs can aid in locking in the exact alignment, however if any adjustments need to be made this can prove challenging intraoperatively. Additionally, the position afforded by Mayfield tongs is generally one of extension and lordosis, which may make other aspects of the surgery challenging. A mitigating strategy is to use Gardner-Wells tongs and bivector traction to be able to perform decompression and osteotomies in flexion and fix the patient in adequate extension. This also allows for mobility of the spine during the procedure to ensure good motion post laminoplasty.
The facet joints and posterior tension band are critical for stability, particularly in the sagittal plane [34,35]. Foraminotomies may destabilize the spine if more than 50% of the facet is resected [34]. Surgeons must be cognizant of the entire margin of the facet, particularly if the foraminotomy is performed through a minimally invasive retractor that may obscure visualization. Laminectomies remove the tension band, and postlaminectomy kyphosis can occur in up to 21% of patients [36,37]. Kaptain et al. [36] performed a study of 46 patients undergoing laminectomy alone for cervical spondylotic myelopathy, and found that patients with a straight spine preoperatively (within 4° of neutral) had the highest incidence of postoperative kyphosis (30%). Outcomes, however, were not found to correlate with development of postoperative kyphosis. Instrumented fusion may reduce loss of lordosis and improve neurologic and functional outcomes after laminectomy [38]. Even if instrumented fusion accompanies a laminectomy, patients can still experience postoperative kyphotic deformity. If patients have a high preoperative cervical kyphosis, fusion in a lordotic position may lead to hardware failure and recurrence of the kyphotic alignment. These patients must therefore be considered for anterior-posterior procedures [33].
Laminoplasty is a motion preserving technique that has become widely popular for posterior decompression of the cervical spine. Postlaminoplasty kyphosis is a complication that occurs in about 10% of cases and is very technique dependent [39]. During posterior cervical dissection, care must be taken not to violate the facet capsules as the dissection is taken out laterally. The semispinalis cervicis muscle is a strong, dynamic stabilizer that inserts on the spinous process of C2. It has been clearly shown that disruption of this muscular attachment contributes strongly to postlaminoplasty kyphosis [40-42]. When performing laminoplasty, it is essential to preserve, or repair, the C2 muscular attachments. Preoperative cervical kyphosis and increased T1 slope correlate with increased postlaminoplasty loss of lordosis [43]. Lee et al. [44] performed a radiographic analysis and determined that there was a weak but statistically significant correlation (r= 0.3) between increasing T1 slope and loss of CL. They also determined that preoperative T1 slope > 29° was the most sensitive and specific threshold value for predicting a postoperative kyphotic change ≥ 5, though the sensitivity and specificity were only 63% and 69%, respectively. It is very important to understand though, that there were no clinical outcomes measured, and additional studies have found no correlation between clinical outcomes and loss of lordosis after laminoplasty. Laminoplasty should not be performed if preoperative kyphosis is > 13° [45].
For any posterior cervical procedure, not only are the muscular attachments of C2 important, but the entire posterior soft tissue envelope serves as a tension band. The impetus for laminoplasty versus laminectomy alone is to leave a osseous shell for reattachment or scarring of the soft tissue envelope which provides the benefit of a soft tissue tension structure. Closure of cervical procedures should therefore be performed meticulously and in several layers to fortify the soft tissue tension band.
CERVICAL DEFORMITY AFTER ADULT THORACOLUMBAR SPINAL DEFORMITY CORRECTION
Patients with positive sagittal imbalance secondary to adult thoracolumbar spinal deformity may use compensatory mechanisms to maintain horizontal gaze. These include pelvic retroversion, hip extension, knee flexion, ankle dorsiflexion, a loss of thoracic kyphosis, and cervical spine hyperextension [7]. Studies demonstrate how surgical correction of thoracolumbar deformity affects unfused cervical alignment [13,46,47]. Smith et al. [13] studied 75 patients with a positive sagittal imbalance (SVA> 5 cm) who underwent a lumbar pedicle subtraction osteotomy and found that there was a statistically significant reduction in cervical hyperlordosis from 30.8° to 21.6°. The authors also demonstrated that a correction of the SVA to less than 5 cm was associated with the greatest correction of CL. Passias et al. [46] expanded on this concept by studying the incidence of cervical deformity after adult spinal deformity correction. The authors observed a 63% incidence of postoperative cervical deformity in the 215 patients studied. Independent predictors of postoperative cervical deformity included diabetes and increased preoperative T1 slope minus cervical lordosis (T1–CL). An upper instrumented vertebra lower than T4 protected against postoperative cervical deformity. Despite the high incidence of postoperative cervical deformity, clinical outcomes were similar between those who developed cervical deformity and those who did not.46 Whether the postoperative cervical deformity requires revision surgery has yet to be determined. Additional studies have demonstrated that high preop C2–T3 cobb angle and increased number of Smith-Peterson osteotomies (SPOs) are risk factors for postoperative cervical deformity after thoracolumbar deformity correction [48]. While the concept of cervical cervical deformity after thoracolumbar ASD deformity correction has been well studied, optimal strategies to prevent development of cervical deformity have yet to be identified. Future work should aim to elucidate such strategies. Future studies must also determine the impact of cervical deformity after adult thoracolumbar deformity correction on clinical outcomes. The current knowledge of the interplay between cervical reciprocal changes and potential for cervical deformity development after ASD surgery is nonetheless useful when counseling patients undergoing ASD corrective surgery.
AVOIDING DISTAL MALALIGNMENT AFTER CERVICAL DEFORMITY CORRECTION
A relevant complication following cervical deformity correction is distal junctional kyphosis (DJK), defined as kyphosis > 10° between the superior endplate of the most caudal level included in the correction, and the inferior endplate of the next caudal vertebrae. While the radiographic incidence of DJK can be up to 32.2% after adult cervical deformity correction, a much smaller percentage (as low as 6%) of patients are actually symptomatic [49,50]. Preoperative T1–CL > 36.4°, thoracic kyphosis < 50.6°, and CL < 12° are all predictors of postoperative DJK after cervical deformity correction, and future studies may investigate these as targets to minimize postoperative DJK [49]. An important technical aspect of deformity correction that requires clarification is the effect of lower instrumented vertebra on incidence of DJK. One study found that patients with a primary thoracic driver of cervical deformity without inclusion of the thoracic apex in the correction tended to have higher incidence of DJK and worse clinical outcomes [51]. Further studies should expand on these findings as that may be an important technique for prevention of postop DJK.
Lafage et al. [52] recently developed a cervical score based on the difference between postoperative alignment and age adjusted targets. The score consisted of T1–CL, T1 slope, and SVA, therefore incorporating both cervical and overall global sagittal alignment. Points are assigned to each measurement based on how much they differ from age adjusted targets, and the total score is the sum of the points for each measurement. Importantly, positive scores suggest under correction, while negative scores suggest overcorrection. Of the patients studied, 21% experienced mechanical failure, defined as either requiring a revision for mechanical failure or developing radiographic DJK. The authors found that the mean cervical score in those experiencing mechanical failure was 4 (under correction), compared to a score of 1 for those who did not experience mechanical failure. This differs from the thoracolumbar literature on PJK that suggests that overcorrection of thoracolumbar deformity contributes to PJK [53]. The cervical score not only takes into account cervical alignment, but also global alignment through the SVA. Surgeons must therefore consider global alignment during deformity correction, and how compensatory mechanisms after cervical deformity correction may affect global alignment. Additionally cervical deformity must be corrected to within the range of age adjusted targets to best prevent DJK [52].
To summarize, the current literature suggests the optimal strategies to prevent postoperative cervical deformity after cervical deformity correction include restoring horizontal gaze, including the primary driver apex in the construct, and achieving adequate correction and global sagittal alignment.
PREVENTING CERVICAL DEFORMITY AFTER CERVICAL TUMOR RESECTION
CSD after cervical spine tumor resection occurs for many of the same reasons as after posterior approach to the cervical spine for laminectomy or laminoplasty, namely large resection of the posterior elements including the facets and detachment of the semispinalis cervicis from C2 spinous process. Tumor surgery also faces the additional challenges of possible weakness from compression of the tumor, and postoperative radiation, both of which contribute to a high incidence of postoperative deformity, particularly in adolescents [54,55]. The incidence of CSD after cervical spinal cord tumor (CSCT) resection ranges from 0%–41% according to a meta-analysis by Noh et al. [56]. The authors identified younger age, C2/C3/laminectomy at the cervicothoracic junction, and increasing number of laminectomy levels as 3 main risk factors for postoperative deformity after CSCT resection. Young, skeletally immature patients may be at increased risk due to soft tissue laxity, and abnormalities that may develop from resection of elements of the spine that may still be growing [54]. Instrumented fusion is not required in all CSCT patients, and overutilization of instrumented fusion may add additional time, costs, and complications. Young patients (age< 33) with preoperative cervical deformity who undergo a C2 laminectomy, ≥ 3 level laminectomy, cervicothoracic junction (CTJ) laminectomy should undergo a concomitant instrumented fusion [57,58].
CONCLUDING REMARKS
CSD is a debilitating problem that often occurs iatrogenically. Surgical intervention to treat CSD can lead to high morbidity and complications, and therefore surgeons must implement every technique possible to try to mitigate or prevent iatrogenic cervical deformity. During anterior procedures performed for degenerative cervical conditions, the clinical and radiographic cervical alignment must be optimized prior to fusing any segments. This requires proper positioning, adequate exposure, symmetric discectomy, and placement of pin distractors if used. Posterior cervical procedures similarly rely on adequate positioning to avoid iatrogenic deformity. Resecting greater than 50% of a facet joint and violating the posterior tension band should be avoided if possible. If multilevel laminectomies must be performed, then instrumented fusion should be considered. If nonfusion procedure like laminectomy is chosen, surgical dissection must preserve the C2 muscular attachments and must also not be performed on any patient with preoperative kyphosis of 13°. Surgeons must be aware of the concept of reciprocal change and compensatory cervical response to adult thoracolumbar or lumbar deformity correction. Increased preoperative T1–CL, high preoperative C2-T3 cobb angle, and increased number of SPOs are all risk factors for developing cervical deformity after thoracolumbar deformity correction. Future studies should aim to identify strategies that protect against developing iatrogenic cervical deformity after thoracolumbar deformity correction. After cervical deformity correction, horizontal gaze should be restored with adequate correction of global sagittal balance and inclusion of the primary driver of the cervical deformity in the correction construct. Tumor surgery resection from a posterior approach follows similar principles, but cervical deformity occurs at an increased incidence compared to when the posterior approach is performed for degenerative conditions. Instrumented fusion may be warranted in younger patients due to the risk of post laminectomy kyphosis, particularly if C2 laminectomy, CTJ laminectomy, or ≥ 3 level laminectomy is performed.
Further research is required to elucidate factors which may contribute to iatrogenic cervical deformity and to develop strategies to minimize these complications. Additionally, the relationship between radiographic iatrogenic deformity and impact on clinical outcome must be clarified.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-forprofit sectors.
Author Contribution
Conceptualization: RM, JC, TA, SQ; Writing - original draft: RM, JC; Writing - review & editing: TA, SQ.