Acceptance of Early Surgery for Treatment of Spinal Cord Cavernous Malformation in Contemporary Japan
Article information
Abstract
Objective
Spinal cord cavernous malformation (CM) is an intramedullary vascular lesion that may present with progressive symptoms. Surgery is recommended for symptomatic patients, but optimal timing of surgery is debatable. Some advocate waiting until plateau of neurological recovery and others support emergency surgery. There is no statistic on how commonly these strategies are utilized. We aimed to find contemporary practice pattern among neurosurgical spine centers in Japan.
Methods
A database of intramedullary spinal cord tumors assembled by Neurospinal Society of Japan was surveyed and 160 patients with spinal cord CM were identified. Neurological function, disease duration, and number of days between presentation to hospitals and surgery were analyzed.
Results
Duration of disease before presentation to hospitals ranged from 0 to 336 months (median, 4 months). Number of days between patients’ presentation and surgery ranged from 0 to 6,011 days (median, 32 days). Time from symptom onset to surgery ranged from 0 to 336.9 months (median, 6.6 months). Patients with severe preoperative neurological dysfunction had shorter duration of disease, fewer days between presentation and surgery, and shorter time between symptom onset and surgery. Patients with paraplegia or quadriplegia were more likely to improve when operated on within 3 months from onset.
Conclusion
Timing of surgery for spinal cord CM in Japanese neurosurgical spine centers generally was early, with 50% of patients undergoing surgery within 32 days after presentation. Further study is needed to clarify optimal timing of surgery.
INTRODUCTION
Spinal cord cavernous malformations (CM), also referred to as cavernoma [1], cavernous angioma [2], or cavernous hemangioma [3], are vascular lesions characterized by thin, sinusoidal vascular channels without intervening neural tissue. Three major patterns of clinical presentation may be observed: (1) multiple episodes of discrete neurological deterioration with varying degrees of recovery between the acute insults; (2) slow progression of neurological deterioration; (3) sudden onset of symptoms with rapid decline over hours or days or gradual worsening lasting weeks to months [3,4].
Surgery is indicated in patients with significant or progressive neurological deficits, for whom complete excision should be attempted. Patients with mild or spontaneously resolving symptoms may be followed expectantly, but surgery should be considered if the lesion is exophytic [4-6]. Annual hemorrhage rates range from 0% to 4.5% and should be taken into consideration [4].
While there is a consensus on indication for surgery of spinal cord CM, the optimal timing of surgery is less clear [7]. A literature review found that symptom duration less than 3 years is associated with higher percentage of patients improving after surgery [3]. Imagama et al. [8] reviewed 41 patients and reported that patients who had stable gait preoperatively had shorter preoperative disease duration and had better chance of retaining stable gait at the follow-up. He recommended early surgery for patients who have stable gait, and delayed surgery after initial rehabilitation until the plateau stage of recovery for patients with motor paresis. During this interval for recovery, a gliotic plane develops between the lesion and the spinal cord, allowing for relatively safe removal [9]. On the other hand, Duan et al. [10] reviewed 52 patients and found that emergency rescue surgery (within 3 days from onset in patients with acute symptom onset with rapid decline or within 7 days from onset in patients with repeating deterioration of neurological symptoms with acute onset) resulted in higher chance of neurofunctional improvement at long-term follow-up. There is no data on how commonly each of these strategies (delayed or emergency surgery) are utilized among spine surgeons. We reviewed a database of intramedullary spinal cord tumors to find the practice pattern in contemporary Japan.
MATERIALS AND METHODS
1. Ethics
This study was performed as a part of multicenter cohort study by Endo et al. [11] with recognition from Neurospinal Society of Japan, an affiliate of the Japan Neurosurgical Society. The study protocol was approved by the Institutional Review Board of Tohoku University Hospital (2021-1-130) and participating centers. As this was a retrospective and noninvasive study, the requirement for written informed consent from patients was waived. Instead, a public notice that provided information on this study was given on websites of participating centers.
2. Patient Selection
A questionnaire was sent to 58 participating neurosurgical spine centers across Japan and data on patients with intramedullary spinal cord tumors treated surgically between 2009 and 2020 were collected. Patients with spinal lipoma or myxopapillary ependymoma were excluded. Patients who had previously undergone surgery for the same lesions were also excluded.
3. Baseline Characteristics
Data were collected on patients’ age, sex, histological diagnosis, types of surgery, presence of recurrence at the follow-up, duration of the disease before presenting to the hospital, and dates of presentation to the hospital and surgery. Neurological functions preoperatively and at follow-ups were graded according to modified McCormick scale (mMS) (grade I: intact neurologically, normal ambulation, minimal dysesthesia; grade II: mild motor or sensory deficit, functional independence; grade III: moderate deficit, limitation of function, independent with external aid; grade IV: severe motor or sensory deficit, limited function, dependent; grade V: paraplegia or quadriplegia, even with flickering movement) [12,13]. All data were anonymized.
4. Statistical Analysis
The database was stored in Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Duration of the disease before presentation to the hospital, time between hospital presentation and surgery, and time between symptom onset and surgery (sum of duration of disease before presentation and time between presentation and surgery) were compared among patients with different mMS using Kruskal-Wallis test. Difference in number of patients with each mMS in preoperative and final follow-up states were analyzed using Fisher exact test. Statistical analysis was performed using a free statistical software EZR, which runs on R ver. 4.2.2 (R Foundation, Vienna, Austria) [14].
RESULTS
There were 160 patients (70 women and 90 men) with spinal cord CM, whose age ranged from 7 to 85 years (mean, 52.01 years; median, 52 years) (Table 1).
Duration of disease before presenting to the treating hospital ranged from 0 to 336 months (mean, 27.88 months; median [interquartile range], 4 [1–32]; n = 158) (Fig. 1).

Disease duration before presentation to the hospital, stratified by preoperative modified McCormick scale (mMS).
Number of days between patients’ presentation to the hospital and the surgery ranged from 0 to 6,011 days (mean, 143.8; median [interquartile range], 32 [9.5–75.5]; n = 159) (Fig. 2).
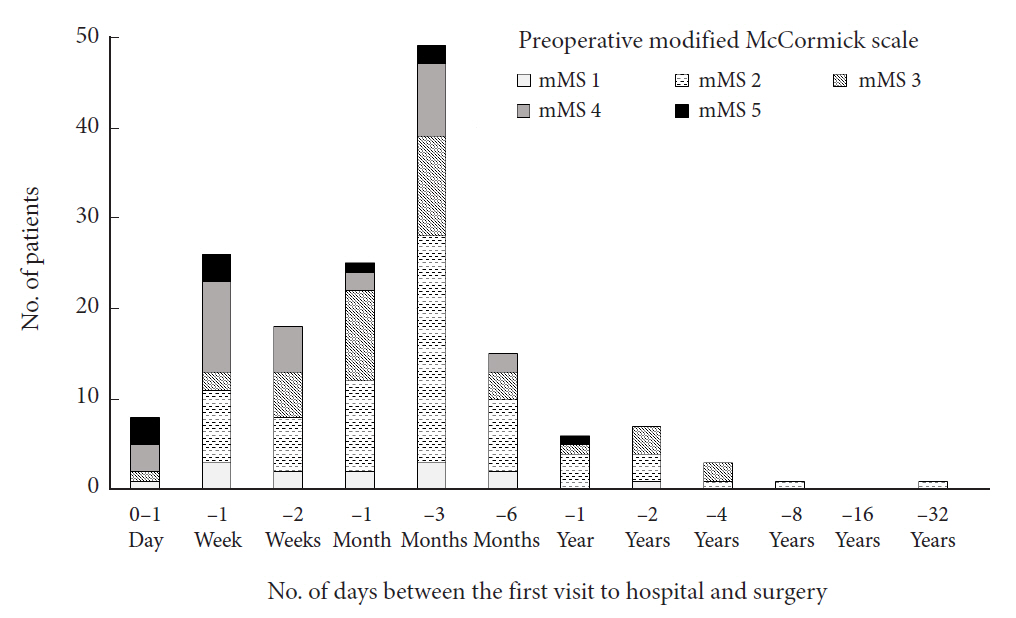
Number of days between the first visit to hospital and surgery, stratified by preoperative modified McCormick scale (mMS).
Correlation between the duration of disease before presentation and the days from presentation to surgery was not statistically significant (correlation coefficient, 0.109; 95% confidence interval, -0.0478 to 0.261).
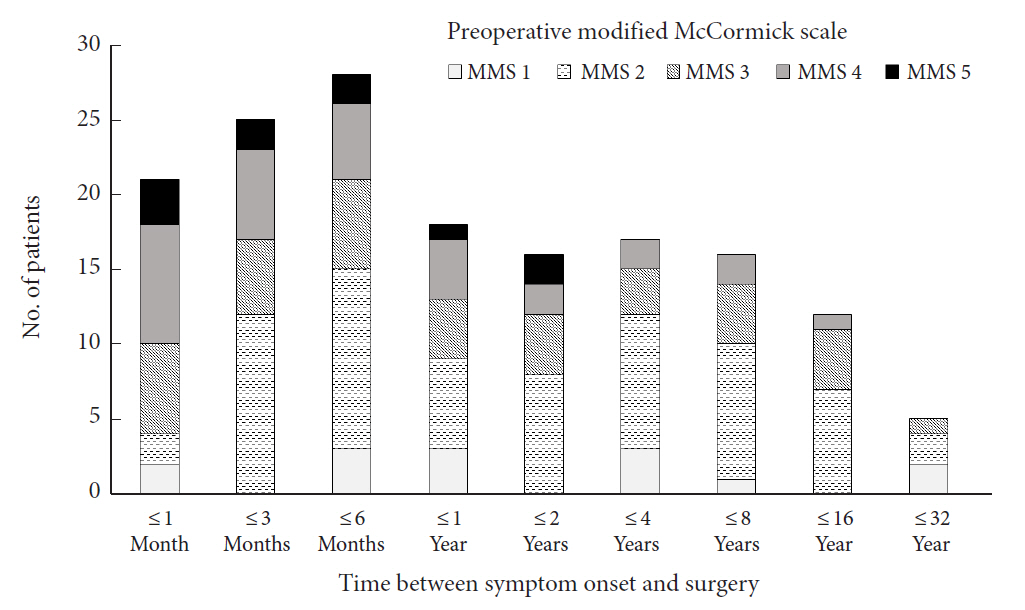
Time from symptom onset to surgery ranged from 0 to 336.9 months (mean, 32.6; median [interquartile range], 6.6 [2–34.8]; n = 158) (Fig. 3).
Neurological function before surgery, classified in mMS, was grade I in 14, grade II in 67, grade III in 39, grade IV in 30, and grade V in 10 patients. Patients with severe preoperative neurological dysfunction (mMS grades IV and V) had spent shorter time in duration of disease before presentation to the hospital (p = 0.009, Kruskal-Wallis rank-sum test), from presentation to the hospital to surgery (p = 0.001, Kruskal-Wallis rank-sum test), and from symptom onset to surgery (p = 0.002, Kruskal-Wallis rank-sum test) (Table 1). No significant between-group differences were found in preoperative mMS according to age, sex, or level of the lesion. No statistically significant difference between patients with cervical and thoracic lesions were found for duration of symptoms before presenting to the hospital, time between presentation and surgery, and time between symptom onset and surgery.
Surgical procedures performed were biopsy in 1 patient, partial or subtotal resection in 17 patients, and total resection in 142 patients. Four patients underwent surgery via anterior approach and 156 via posterior approach.
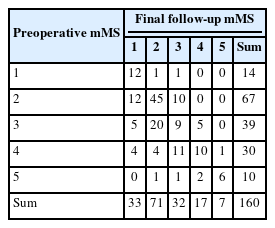
Patients were followed up for average of 45.5 months (median, 39; range, 0–142) after surgery. At the last follow-up evaluation, mMS was grade I in 33, grade II in 71, grade III in 32, grade IV in 17, and grade V in 7 patients (Table 2). Difference in the distribution of mMS grades before surgery and at last follow-up was statistically significant (p = 0.013, Fisher exact test). Patients whose preoperative mMS was grade V were more likely to improve at the follow-up if they were operated on within 3 months from symptom onset compared with after 3 months (p = 0.048, Fisher exact test) (Table 3). Such difference was not found in patients with preoperative mMS of I to IV.
Preoperative neurological function and outcome at final follow-up in modified McCormick scale (mMS) (all cases, n=160)

Preoperative and final follow-up neurological function graded using modified McCormick scale (mMS) in patients who underwent surgery within 3 months (n=46) and more than 3 months (n=112) after symptom onset
The mMS grades at follow-up worsened in 18 out of 160 patients (11.3%). The risk of neurological worsening was not significantly different for those who underwent surgery within 3 months from onset (5 of 46, 10.9%) and after 3 months (13 of 112, 11.6%). There was no statistically significant difference in the distribution of patients with each mMS at the follow-up between patients with cervical and thoracic lesions. Similarly, there was no significant difference in the proportion of patients whose mMS worsened at the follow-up compared to their preoperative level between these 2 groups.
Recurrences occurred in 7 patients: 3 after partial or subtotal resection (17.6%, 3 of 17) and 4 after total resection (2.8%, 4 of 142). The recurrence rate was significantly lower after total resection (p = 0.027, Fisher exact test). All 7 patients with recurrences underwent surgery within 60 days after presentation. But there was no statistically significant difference in rate of recurrence between patients who underwent surgery within 60 days from presentation and those whose surgeries were more than 60 days after presentation (7 of 107 vs. 0 of 52, p = 0.095, Fisher exact test).
There were 53 patients who presented to the hospital within 1 month after symptom onset. Fifteen of them (28.3%, 15 of 53) underwent surgery within 7 days after presentation, which may have been emergency surgery, and 38 had surgery more than 7 days after presentation (range, 8–534 days). In the 15 patients who underwent emergency surgery, preoperative neurological dysfunction in mMS were grade I in 2 patients, grade II in one, grade III in one, grade IV in 9, and grade V in 2; At the last follow-up, mMS were grade I in 3 patients, grade II in 3, grade III in 4, grade IV in 3, and grade V in 2. In the 38 patients who underwent delayed surgery, patients with preoperative mMS grades I to V were 0, 12, 15, 8, and 3, respectively, and at the last follow-up, 9, 17, 8, 4, and 0, respectively. The difference between preoperative and last follow-up mMS grade distribution was not statistically significant in emergency surgery group but was significant in delayed surgery group (p = 0.213 and p = 0.001, respectively, Fisher exact test).
DISCUSSION
This is the first multicenter survey that showed a contemporary practice pattern in Japan regarding the timing of surgery for spinal cord CM. This study revealed that patients with spinal cord CM had a wide variation in duration of disease before presentation to the hospital, from 0 to 336 months. These patients were operated on after a wide range of waiting periods, from 0 to 6,011 days after presentation with a median of 32 days. In total, patients underwent surgery from 0 to 336.9 months after symptom onset, with 50% undergoing surgery within 6.6 months. We found that patients with preoperative mMS grade V neurological dysfunction were more likely to improve if they had surgery within 3 months from symptom onset. Favorable neurological outcome of patients who underwent surgery within 3 months of symptoms was also noted in a previous systematic review [15].
Overall rate of functional deterioration at the final follow-up was 11.3% (18 of 160). Seventeen of these worsening had one-level change from the baseline. This retrospective study did not contain the details of symptomatic response from patients, and any symptomatic changes that occurred within the borders of each mMS scales are not recognizable. There is always a risk of functional deterioration after surgery of spinal cord CM. In case series studies from high-volume centers, neurological worsening occurred in approximately 10% of patients immediately after surgery and remained in 3%–7% of patients at the follow-up [16,17]. Reasons for somewhat higher rate of worsening in our series is unclear, but one of the reasons may be short follow-up in some patients.
Spinal cord CM may manifest with slowly progressive symptoms or acute neurologic deterioration. Acute episodes may be followed by spontaneous recovery or deterioration over hours to months [3,4]. There is no known prognostic factor to distinguish patients who will spontaneously recover from those who will deteriorate. One study reported that patients at risk of not recovering stable gait were those with large lesions and those with lesion in the thoracic spinal cord [8]. Our study did not find statistically significant difference in outcome with lesion involving different levels, but we think it is reasonable that a lesion in the thoracic spinal cord is more detrimental than that in the cervical cord because the thoracic spinal cord has smaller cross-section area with a circular contour compared with the cervical cord [18]. It is commonly known in geometry that for the same perimeter, a circle, not ellipses, has the greatest area. Therefore, when a spinal cord lined by a rigid pia mater becomes swollen, an elliptical cervical cord can increase cross-section area without stretching the pia, while in a circular thoracic cord a small increase in the cross-section area would stretch the pia and result in increased parenchymal pressure. Additionally, a certain amount of bleeding within the cord would damage larger portion of the cross-section area in the smaller thoracic cord. Because the pia mater is a rigid material [19], increased spinal cord parenchymal pressure results in shifting of the spinal cord parenchyma cranially and caudally. This shift of parenchyma would obstruct the arterioles supplying the spinal cord, because they are fixed to the rigid pia at the penetration point. With a concomitant decrease in perfusion pressure (difference between the blood pressure and the parenchymal pressure), ischemia and infarction would develop in the adjacent segments of the spinal cord, which in turn, result in edema and further longitudinal progression of necrosis, a phenomenon called pencil-shaped softening of the spinal cord [20]. This progressive expansion of the lesion may be more pronounced in the conus medullaris, where the cross-section area is small and circular, and the pial tube is closed distally. We think that progressive neurological deteriorations in patients with spinal cord CM are caused by repeated hemorrhage and/or pencil-shaped softening of the spinal cord. Emergency surgery theoretically would relieve spinal cord parenchymal pressure and prevent secondary neural injury. However, our data did not show benefits of emergency surgery due to lack of statistical power.
Emergency surgery should not be employed in all patients presenting with acute neurological decline because many of them will spontaneously recover. Many authors recommend several weeks of waiting period to facilitate resection [8,9]. We agree that resection of CM is difficult in the acute phase because the lesion is often collapsed due to compression by hematoma, gliotic plane is absent between the lesion and the spinal cord, and the spinal cord tissue is edematous and fragile. However, we have seen patients who rapidly deteriorated to complete spinal cord injury and did not recover after rehabilitation and lost the opportunity to have surgery. These patients could have benefited from emergency surgery.
This study was a retrospective analysis of patients who were surgically treated. There is a selection bias because it lacks information on patients who did not have surgery after recovering normal function or deteriorating to complete paraplegia or quadriplegia. In our database, the neurologic dysfunction graded in mMS are recorded before operation, not at the times of onset and presentation. This limits our ability to tell whether the surgery was performed at acute stage or as an elective case. A data of a patient with preoperative neurological dysfunction of mMS grade V who underwent surgery 60 days after onset could mean that the patient had only a mild sensory disturbance for 59 days and suddenly deteriorated to paraplegia on the 60th day and had an emergency surgery, but it also could mean that the patient initially presented with paraplegia and did not improve after 60 days of rehabilitation and had an elective surgery. Another limitation of our study is a lack of data on detailed anatomical location of the lesions within the spinal cord, such as anterior, posterior, subpial, or deeply embedded. Such anatomical feature is important in surgical decision making and should be included in future studies of spinal cord CM.
CONCLUSION
In Japanese neurosurgical spine centers, patients with spinal cord CM underwent surgery generally in early phase. Half of patients underwent surgery within 32 days after presentation to hospital, and 75% of patients within 75.5 days. Emergency surgery within 7 days were applied to approximately 28% of patients who presented within 1 months of onset.
Patients with paraplegia were more likely to improve at the last follow-up if they underwent surgery within 3 months from symptom onset. Although this retrospective study did not seek to determine the optimal timing of surgery, it may be acceptable that patients with severe neurological symptoms or progressive worsening should be operated on early to prevent secondary neural injury from increased intraparenchymal pressure. Whether emergency surgery is beneficial cannot be determined in this retrospective study. Future studies should investigate details of clinical course to identify risk factors for progressive neurological deterioration. Such studies would enable us to correctly select patients for emergency surgery or initial rehabilitation.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: RK, TE; Data curation: RK, TE, TT; Formal analysis: RK, TE, TT; Methodology: RK, TE; Project administration: RK; Visualization: RK; Writing - original draft: RK; Writing - review & editing: RK, TE, TT.
Acknowledgements
Investigators of intramedullary spinal cord tumors in the Neurospinal Society of Japan -- Masahito Hara and Masahiro Aoyama; Aichi Medical University. Taku Sugawara; Akita Cerebrospinal and Cardiovascular Center. Hiroaki Shimizu; Akita University. Kotaro Ogihara; Iwakuni Clinical Center. Atsushi Sugawara; Iwate Medical University. Phyo Kim and Kazushige Itoki; Utsunomiya Brain and spinal cord center. Seiji Matsui and Seiji Shigekawa; Ehime University. Noritsugu Kunihiro; Osaka City General Hospital. Kentaro Naito; Osaka City University. Shinji Yamamoto; Ohnishi Neurological Center. Takao Yasuhara; Okayama University. Motoyuki Iwasaki; Otaru City Hospital. Yasuyuki Miyoshi; Kawasaki Medical School. Hideki Hayashi; Kitano Hospital. Nakayama Noriyuki and Toru Iwama; Gifu University. Daisuke Umebayashi; Kyoto Prefectural University of Medicine. Hiroshi Nakagawa and Manabu Sumiyoshi; Kushiro Kojinkai Memorial Hospital. Yasukazu Hijikata: Spine and Low Back Pain Center, Kitasuma Hospital. Hisaaki Uchikado; Kurume University. Hitoshi Fukuda; Kochi University. Tomoaki Nakai and Takashi Sasayama; Kobe University. Kazuhiko Mishima; Saitama Medical University, International Medical Center. Tomoo Inoue; Saitama Red Cross Hospital. Shunsuke Yano, Kazutoshi Hida, and Toru Sasamori; Sapporo Azabu Neurosurgical Hospital. Nobuhiro Mikuni and Yukinori Akiyama; Sapporo Medical University. Tsuyoshi Hara; Juntendo University. Gakuji Gondo; Shonan Kamakura General Hospital. Mitsuhiro Yoshida; Yokkaichi Municipal Hospital. Hideki Komatani and Yuichi Takahashi; Shin Komonji Hospital. Minoru Hoshimaru and Shigeo Ueda; Katano Hospital ShinAikai Spine center. Kiyoshi Ito; Shinshu University. Hisaharu Goto and Node Yasuhiro; Shin-yurigaoka General Hospital. Mizuki Watanabe; Seirei Hamamatsu General Hospital. Yasunobu Ito; Tokyo General Hospital. Yoshitaka Hirano; Southern Tohoku Research Institute for Neuroscience. Teiji Tominaga; Tohoku University. Hirokazu Takami; Tokyo University. Jun Karakama; Tokyo Medical and Dental University. Hiroki Ohashi; The Jikei University School of Medicine. Naoyuki Harada; Toho University. Tetsuro Shingo and Satoshi Kawajiri; Dokkyo Medical University. Tomohiro Yamauchi; Tomakomai City Hospital. Tetsuji Uno; Tottori University. Keisuke Takai and So Fujimoto; Tokyo Metropolitan Neurological Hospital. Yasufumi Otake; Nakamura Memorial Hospital. Yasuhiro Takeshima and Hiroyuki Nakase; Nara Medical University. Akihiko Saito; Niigata City hospital. Daijiro Morimoto and Kyongsong Kim; Nippon Medical School. Tatsuya Ohtonari; Brain Attack Center, Ota Memorial Hospital. Hiroto Kageyama; Hyogo Medical University. Takafumi Mitsuhara; Hiroshima University. Yosuke Kuromi; Fukushima Medical University. Toshiyuki Takahashi and Ryo Kanematsu; Fujieda Heisei Memorial Hospital. Tatsushi Inoue; Fujita Health University. Toshitaka Seki and Kazuyoshi Yamazaki; Hokkaido University. Izumi Koyanagi; Hokkaido Neurosurgical Memorial Hospital. Kazuhisa Yoshifuji; Hokkaido Medical Center for Child Health and Rehabilitation. Masashi Fujimoto and Masaki Mizuno; Mie University. Misao Nishikawa; Moriguchi-Ikuno Memorial Hospital. Takashi Yagi and Hiroyuki Kinouchi; University of Yamanashi. Hidetoshi Murata; Yokohama City University. Mari Kitayama; Wakayama Medical University.