Commentary on “Different Ways to Die: Cell Death Pathways and Their Association With Spinal Cord Injury”
Article information

Although there have been decades of research on spinal cord injury (SCI), it is still one of the most challenging medical conditions to treat in developed countries worldwide [1-6]. Clinical manifestations of SCI (e.g., paralysis, autonomic nervous system dysregulation) can severely impact the quality of life and burden social security systems [1-6]. The primary mechanism of disease and cause of clinical symptoms is the death of neurons and supporting glial cells in the spinal cord [1-6]. Thus, to achieve a lasting and successful SCI treatment, it is imperative to prevent the death of cells post-injury (Fig. 1). Previous attempts to broadly suppress well-known SCI pathophysiological processes have been controversial. In a high-profile example, methylprednisolone (a glucocorticoid with broad anti-inflammatory action) was investigated to limit the neurological and functional damage in SCI patients [5]. Unfortunately, the broad immunosuppressive and side effects of methylprednisolone have been found to be potentially deleterious in large human clinical trials [5,6]. A more nuanced approach to regulating specific cellular pathways may be warranted to prevent side effects during clinical use (Fig. 1).
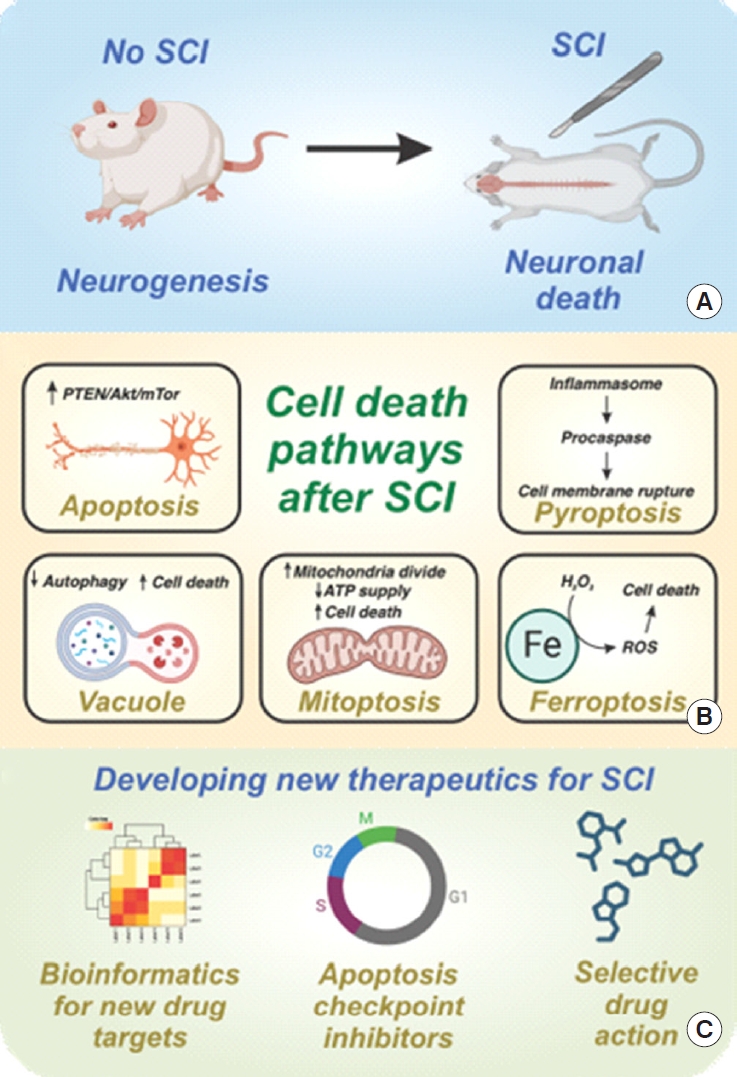
Overview of cell death in spinal cord injury (SCI). (A) SCI can induce substantial neuronal death, which inhibits neurogenesis and functional recovery. (B) A variety of cell death pathways have been identified in Guha et al. recent review paper [7], each with associated cell signaling pathways and defined cellular events. (C) This publication is well suited to inform subsequent work aimed at developing new therapeutics for SCI, including but not limited to bioinformatics, apoptosis/nonapoptosis checkpoint inhibitors, and drug development for enhanced specificity and reduced side effects. PTEN, phosphatase and tensin homolog; ATP, adenosine triphosphate; ROS, reactive oxygen species.
In the June 2023 issue of Neurospine, Guha et al. [7] have compiled the most pertinent literature on cell death mechanisms in SCI. In this manuscript, the authors investigated the key cellular pathways related to SCI pathogenesis, the key signaling proteins involved, and the current treatment options (Fig. 1). Here, readers are encouraged to use this manuscript as a stepping stone toward the next step in targeted SCI treatment research and development. For example, the systematic review of key cellular death pathways laid in the manuscript of Guha et al. [7] can be readily integrated into a variety of studies. Mechanistic investigations into novel SCI treatment options, such as systemic hypothermia, can be accelerated with the manuscript of Guha et al. [6,7]. For example, future studies into the antiapoptotic effects of hypothermia can be distinguished from confounding (non-)programmed and nonapoptotic pathways of cell death [6]. Stem cell therapies can benefit by anticipating and counteracting key death mechanisms that would cause transplanted cells to die within the patient [2]. Alternatively, stem cell-secreted antiapoptosis/necrosis growth factors can be identified as a low immunogenicity substitute for allogeneic stem cell transplantation [2]. More efficient treatment options can be determined by further examination into which pathway contains the most “druggable” target. Protein-ligand simulations with critical apoptosis and necrosis checkpoints can give rise to more specific therapeutics with fewer off-target effects [4]. Novel drug combinations that have either synergistic effects, such as targeting multiple checkpoints within the same pathway, or complementary effects, like targeting different pathways leading to the same physiological result, may be possible through key theorized crosstalk junctions [3]. In summary, this evaluation of cell death mechanisms in SCI can provide interested researchers with the ability to accelerate their research and draw further connections to other manuscripts in the field.
Notes
Conflict of Interest
The authors have nothing to disclose.
