Outcomes of Intramedullary Spinal Cord Tumor Surgery in Older Versus Younger Adults: A Multicenter Subanalysis Study by the Neurospinal Society of Japan
Article information
Abstract
Objective
Intramedullary spinal cord tumors (IMSCTs) are uncommon and difficult to treat. Studies examining the efficacy of rare IMSCT surgery in the elderly are limited. We conducted a subanalysis using multicenter retrospective-historical data provided by the Japan Neurospinal Society to compare surgical outcomes between older and younger adults with IMSCTs.
Methods
We classified patients with IMSCTs into younger (aged 18–64 years) or older ( ≥ 65 years) groups. The primary outcomes of “improved” or “worsened” from the preoperative period to 6 months after surgery were evaluated using the modified McCormick scale (mMCs). A favorable outcome was defined as an mMCs grade of I/II at 6 months.
Results
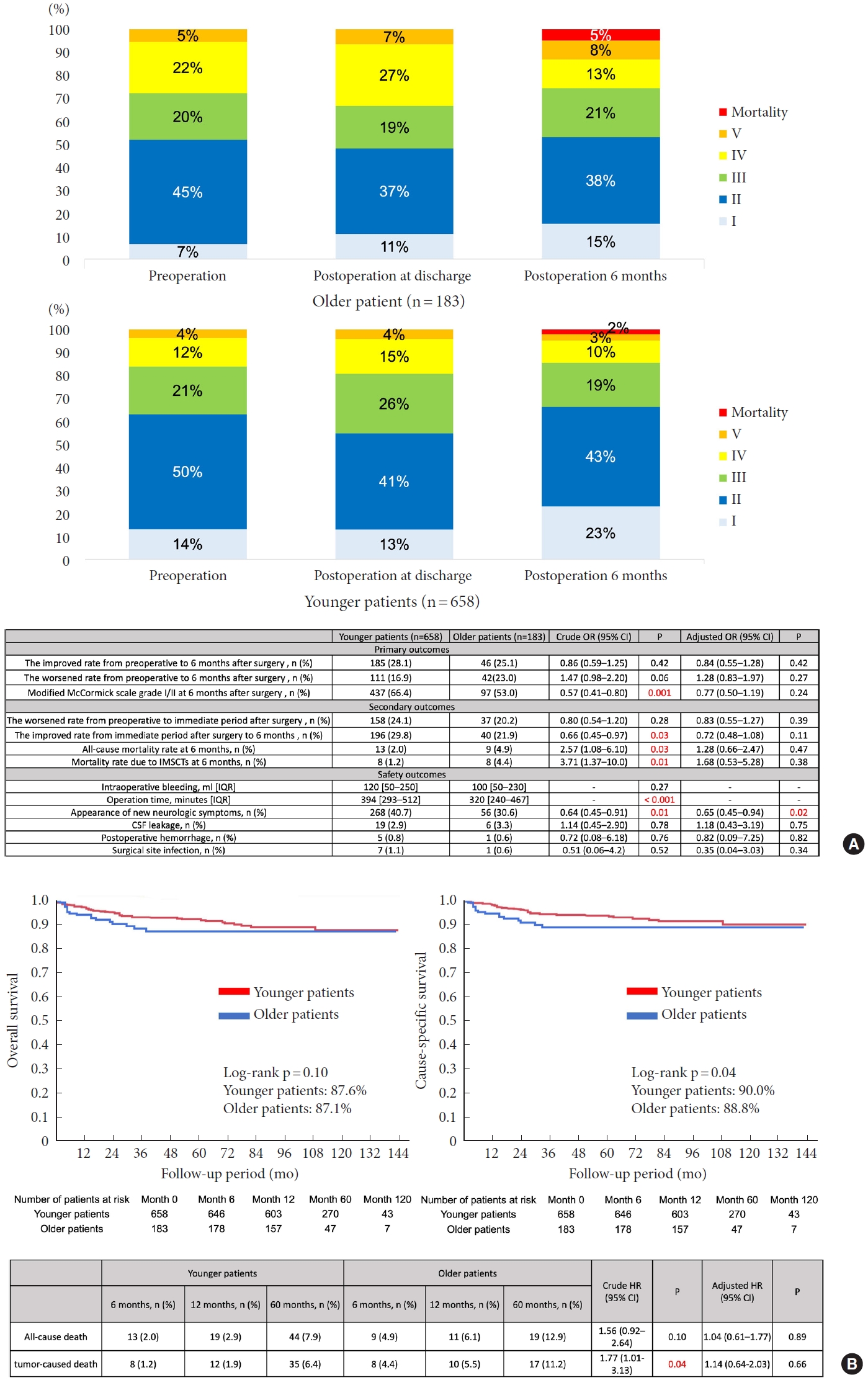
Among 841 patients registered, there were 658 younger (78.2%) and 183 older patients (21.8%) evaluated using mMCs at 6 months. Median preoperative mMCs grades were significantly worse in older patients than in younger patients. Neither the “improved” nor “worsened” rate differed significantly between the groups (28.1% vs. 25.1%; crude odds ratio [cOR], 0.86; 95% confidence interval [CI], 0.59–1.25; adjusted OR [aOR], 0.84; 95% CI, 0.55–1.28; 16.9% vs. 23.0%; cOR, 1.47; 95% CI, 0.98–2.20; aOR, 1.28; 95% CI, 0.83–1.97). Favorable outcomes were significantly less common among older adults in the univariate analysis but were not significant in the multivariate analysis (66.4% vs. 53.0%; cOR, 0.57; 95% CI, 0.41–0.80; aOR, 0.77; 95% CI, 0.50–1.19). In both younger and older patients, preoperative mMCs accurately predicted favorable outcomes.
Conclusion
Age alone is not a sufficient reason to prohibit surgery for IMSCTs.
INTRODUCTION
The elderly population is increasing rapidly worldwide, and a significant percentage of older adults undergo surgical procedures. There is an urgent issue to examine the surgical criteria that sustainably support the extension of healthy life expectancy. According to the World Population Prospects Report 2022, the global population aged ≥ 65 years is projected to rise from 10% in 2022 to 16% in 2050 [1]. Japan has the most rapidly aging population, with 28.9% Japanese aged > 65 years in 2022 [2]. Japan is expected to create a role model for the elderly, including healthcare for patients with cancer. Although age is not a primary consideration for surgical risk generally, older patients have higher perioperative complications and mortality compared to younger patients, despite the advances in surgical and anesthetic techniques. All older adults undergoing surgery should undergo an assessment of frailty risk, including comorbidities, mobility, functional status, and nutrition [3].
Primary intramedullary spinal cord tumors (IMSCTs) represent a small fraction (2%–4%) of central nervous system tumors, with unique challenges posed by the surrounding tissues [4,5]. IMSCTs can potentially lead to severe neurologic deterioration, decreased living function, poor quality of life, death, and spinal cord injury from thoughtless surgical intervention, which may directly lead to a poor functional prognosis [6]. Studies examining the efficacy of rare IMSCT surgery in the elderly are limited; some papers have suggested patients aged > 65 years with McCormick (modified McCormick scale, mMCs) grade IV or V where imaging suggests a high grade lesion may be better served with a biopsy/chemo/radiation [7]. We tried to clarify what should be noted in IMSCT surgical management of the elderly, by comparing the characteristics and prognosis of IMSCTs in younger and older patients. Furthermore, to confirm whether age is an independent factor in determining the indication for IMSCT surgery and to validate the above suggestion, we conducted a subanalysis to compare surgical outcomes between older and younger adults with IMSCTs.
MATERIALS AND METHODS
We used real-world data derived from a multicenter historical cohort study authorized by the Neurospinal Society of Japan. This retrospective, noninvasive study consecutively enrolled patients with IMSCTs at 58 centers in Japan who were surgically treated between 2009 and 2020. The study protocol and demographic, clinical, and outcome data have been described [8]. The institutional review boards of all 58 participating centers approved the study protocol. The requirement for written informed consent was waived because of the retrospective nature of the study. Instead, a public notice that provided information on this study was given to individual center websites.
1. Patients and Measurements
We excluded nonsurgical cases, spinal lipomas and myxopapillary ependymomas, cases that underwent external decompression only, and those that underwent surgery before 2008. Cavernous malformations are classified as vascular malformations and thus not assigned the World Health Organization classification [9]. However, these IMSCTs may require surgical intervention, so they were included. We classified pediatric patients (<18 years), younger patients (aged 18–64 years), and older patients (≥ 65 years) based on the definition used by many countries, including Japan [10,11]. Pediatric cases were excluded. We measured the level of disability using the mMCs [12] and Karnofsky Performance Scale (KPS) [13] and recorded death. For functional and performance status changes, when a patient remained at the same grade, we termed the pattern “stable” by using mMCs and mortality data. Changes in at least one mMCs grade or death, when compared to the preoperative status, were evaluated as “improved” or “worsened” as appropriate [8].
2. Outcomes
The primary outcome was the rate of an “improved” or “worsened” status from baseline to 6 months postoperatively [8] and the rate of a favorable outcome, defined by mMCs grade I/II, which was evaluated 6 months after surgery. Secondary outcomes were the rates of a “worsened” status from the preoperative period to the immediate period after surgery and an “improved” status from the immediate period after surgery to 6 months, as well as the all-cause/tumor-related mortality rate at 6 months postoperatively and during follow-up. We also recorded intraoperative bleeding, operation time, the appearance of new neurologic symptoms, cerebrospinal fluid (CSF) leakage, postoperative hemorrhage, and surgical site infection.
3. Statistical Analysis
Categorical variables are presented as number (%) and were compared using the chi-square test. If the minimum expected count (or frequency) was < 5, we used Fisher exact test. To compare multiple factors of categorical variables, multiple comparisons were performed after factor analysis using the chi-square test. Continuous variables are expressed as median (interquartile range [IQR]) and were compared using the Wilcoxon ranksum test. Primary and secondary outcomes were compared between younger and older patient groups. We constructed multivariable conditional logistic regression models adjusted for the following variables: preoperative mMCs grade (grade I/II or grade III/IV/V), degree of removal (biopsy, partial resection, subtotal resection, total resection), and pathological findings (ependymoma, hemangioblastoma, astrocytoma, cavernous malformations, undiagnosed, or others). The most important factor in determining the neurological and functional outcomes of IMSCT surgery is the preoperative neurologic status [14-17], and pathological type and removal rate are important prognostic factors [18,19]. The thresholds for the mMCs grade before surgery were determined using the same threshold as that for the primary outcome. The effects of age group (young vs. old) were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). The survival period, defined as the number of months from surgery to death, was censored at the last available follow-up or cutoff study date (December 31, 2020) for survivors. Kaplan-Meier curves were created to estimate survival in groups classified by age. After evaluation of the proportional hazards assumption, between-group differences were assessed using the log-rank test for 10 years. The effects of the patient group (younger vs. older) for all-cause/cause-specific death were estimated using Cox proportional hazard models and expressed as hazard ratios (HRs) with 95% CIs. We adjusted for the following clinically relevant variables to estimate the adjusted HR in the multivariable Cox proportional hazard models: preoperative mMCs grade (grade I/II or grade III/IV/V), degree of removal (biopsy, partial resection, subtotal resection, or total resection), and pathological findings (ependymoma, hemangioblastoma, astrocytoma, cavernous malformations, undiagnosed, or other). Additionally, a multivariate analysis incorporating comorbidities as covariates into the aforementioned adjustment factors was conducted to identify novel factors affecting patient overall survival. The subgroups for primary outcomes were as follows: preoperative mMCs grade (grade I/II or grade III/IV/V), tumor location (cervical level or below the thoracic level), degree of removal (total removal or nontotal removal), pathological findings (astrocytoma or nonastrocytoma), and postoperative treatment (observation or intervention). The subgroups for overall survival were as follows: history of cancer, heart desease, preoperative mMCs grade (grade I/II or grade III/IV/V), degree of removal (total removal or nontotal removal), pathological findings (astrocytoma or nonastrocytoma). In the subgroup analysis, these variables were dichotomized into 2 categories, as shown in the parentheses. For tumor location, we dichotomized the boundary spinal cord level that had potential to induce tetraplegia or paraplegia based on anatomical knowledge. The threshold of the degree of removal was described previously, implying that gross total removal was associated with a favorable prognosis [19]. The threshold of pathological findings was based on a previous report that astrocytomas and other intramedullary tumors have different prognoses [20]. Some studies have defined the elderly as those aged ≥ 75 years [11]; therefore, we dichotomized the adults’ cohort at age 75 years and performed sensitivity analyses for the primary outcomes. We used the Spearman rank correlation coefficient (rho) to assess the association between preoperative mMCs and mMCs at 6 months after surgery. We also used Fisher z-transformation for comparison of correlation coefficients. The diagnostic accuracies of preoperative mMCs in predicting favorable outcomes were investigated via receiver operating characteristic curve analyses and the corresponding area under the curves (AUCs). The AUCs of preoperative mMCs in younger or older patients were compared with each other. To visualize the impact of preoperative mMCs on mMCs at 6 months after surgery, the complete results of the distribution of preoperative and postoperative mMCs at 6 months in both groups were investigated, as well as stratified by lesion site and tumor type. All statistical analyses were conducted by a physician using JMP 16.0 software (SAS Institute Inc., Cary, NC, USA). All reported p-values were two-tailed, and statistical significance was set at p-values < 0.05.
RESULTS
1. Patient Characteristics
Among the 1,080 initially enrolled patients, 841 who were evaluated using mMCs at 6 months were analyzed. There were 658 younger (78.2%) and 183 older patients (21.8%) (Supplementary Fig. 1) with median ages of 44 years (IQR, 35–54 years) and 72 years (IQR, 68–75 years) at the time of surgery, respectively (Table 1).
There were no significant between-group differences in sex (men: 52.6% vs. 53.0%, p= 0.92), body mass index (22.1 vs. 22.3 kg/m2, p= 0.79), and time from symptom onset to the date of the first visit (7 vs. 8, p= 0.20). Although there was no difference in smoking history (29.6% vs. 28.3%, p= 0.74), the rates of hypertension, diabetes mellitus, hyperlipidemia, cancer history, and heart disease were significantly higher in older patients (14.1% vs. 47.0%, 4.7% vs. 14.2%, 8.2% vs. 25.1%, 5.5% vs. 16.4%, and 1.5% vs. 7.7%, respectively; p< 0.001 for all). Preoperative steroids were administered more frequently to older than to younger patients (3.2% vs. 7.2%, p= 0.02). There were no significant between-group differences in previous spinal surgery or anticancer drug use (8.2% vs. 10.4%, p = 0.36 and 1.7% vs. 2.2%, p= 0.64, respectively). With regard to preoperative symptoms, walking disturbances and bladder rectal disorders were more frequently observed in older than in younger patients (52.0% vs. 67.8%, p< 0.001 and 30.3% vs. 40.4%, p= 0.01, respectively). The median preoperative mMCs grade and KPS scores were significantly worse for older than for younger patients (II [IQR: II–III] vs. II [IQR: II–IV], p< 0.001; and 80 vs. 70, p< 0.001, respectively). Patients with a preoperative mMCs grade of ≤ II were significantly fewer among older than among younger patients (63.2% vs. 51.9%, p= 0.006). With regard to radiological findings, although there was no significant between-group difference with respect to the tumor length (26 mm vs. 27 mm, p= 0.95) and tumor characteristics (p= 0.60), there were significantly fewer cervical lesions and more thoracolumbar lesions in older than in younger patients (47.7% vs. 39.3%, p= 0.04 and 7.5% vs. 20.2%, p< 0.001, respectively). With regard to the degree of removal, the percentage of biopsy was significantly higher and gross total removal was less common in older than in younger patients (6.4% vs. 13.7%, p= 0.001 and 69.9% vs. 55.7%, p<0.001, respectively). Pathological findings showed fewer hemangioblastomas and a significantly higher proportion of astrocytomas in older than in younger patients (20.8% vs. 10.4%, p= 0.001 and 13.4% vs. 19.7%, p= 0.003, respectively). With regard to cervical lesions, there were significantly more hemangioblastomas in younger than in older patients (22.0% vs. 8.3%, p= 0.008). The use of chemotherapy was significantly more common in older than in younger patients undergoing postoperative treatment (1.0% vs. 4.1%, p= 0.004). The duration to the last follow-up was significantly shorter for older patients (48 vs. 30 months, p< 0.001).
2. Outcomes
The rates of “improved” and “worsened” status from before to 6 months after surgery were similar between groups (28.1% vs. 25.1%; crude OR [cOR], 0.86; 95% CI, 0.59–1.25; adjusted OR, aOR, 0.84; 95% CI, 0.55–1.28; 16.9% vs. 23.0%; cOR, 1.47; 95% CI, 0.98–2.20; aOR, 1.28; 95% CI, 0.83–1.97; respectively) (Fig. 1A). In the univariate analysis, the rate of mMCs grade I/II at 6 months after surgery was significantly lower for older than for younger patients (66.4% vs. 53.0%; cOR, 0.57; 95% CI, 0.41–0.80). Nevertheless, multivariate analysis showed no significant between-group difference (aOR, 0.77; 95% CI, 0.50–1.19). The rate of a “worsened” status from the preoperative period to the immediate postoperative period was not significantly different (24.1% vs. 20.2%; cOR, 0.80; 95% CI, 0.54–1.20; aOR, 0.83; 95% CI, 0.55–1.27). In the univariate analysis, the rate of “improved” status from the immediate postoperative period to postoperative 6 months was significantly lower for older than in younger patients (29.8% vs. 21.9%; cOR, 0.66; 95% CI, 0.45–0.97). Nevertheless, multivariate analysis showed no significant between-group difference (aOR, 0.72; 95% CI, 0.48–1.08). Both all-cause and tumor-related mortality rates at 6 months were higher for older than for younger patients (2.0% vs. 4.9%; cOR, 2.57; 95% CI, 1.08–6.10; 1.2% vs. 4.4%; cOR, 3.71; 95% CI, 1.37–10.0; respectively), but these effects did not remain after statistical adjustment (aOR, 1.28; 95% CI, 0.66–2.47; aOR, 1.68; 95% CI, 0.53–5.28; respectively). Overall survival and cause-specific survival are shown in Kaplan-Meier curves (Fig. 1B). The 10-year overall survival rate did not differ between groups (87.6% vs. 87.1%; crude HR [cHR], 1.56; 95% CI, 0.92–2.64; adjusted HR [aHR], 1.04; 95% CI, 0.61–1.77). The 10-year cause-specific survival rate was significantly lower in older than in younger patients (90.0% vs. 88.8%; cHR, 1.77; 95% CI, 1.01–3.13), with no significant difference after adjustment (aHR, 1.14; 95% CI, 0.64–2.03). In order to elucidate novel factors influencing overall survival, a multivariate analysis was performed. The findings demonstrated that the presence of a cancer history and heart disease independently served as prognostic factors (aHR, 3.04; 95% CI: 1.59–5.81 for cancer history and 4.76; 95% CI, 1.79–12.6 for heart disease) (Supplementary Table 1).
3. Safety Outcomes
There was no significant difference in intraoperative bleeding volumes between the groups (120 mL vs. 100 mL, p= 0.27) (Fig. 1A). The operation time was significantly shorter for older patients (394 minutes vs. 320 minutes, p< 0.001) while the incidence of new neurological symptoms was significantly higher for younger patients (40.7% vs. 30.6%; cOR, 0.64; 95% CI, 0.45–0.91; aOR, 0.65; 95% CI, 0.45–0.94). There were no significant differences in the occurrence of CSF leakage (2.9% vs. 3.3%, p= 0.78), postoperative hemorrhage (0.8% vs. 0.6%, p= 0.76), or surgical site infection (1.1% vs. 0.6%, p= 0.52).
4. Subgroup Analyses
Subgroup analyses of mMCs grade I/II at 6 months suggested that younger patients had better outcomes in almost all subgroups (Fig. 2A), excluding those with lesions below the thoracic spine level. Among the prognostic factors already reported and the dichotomized variables set up in this study, tumor localization at the cervical spine level (aOR, 0.51; 95% CI, 0.27–0.96) and pathological finding of astrocytoma (aOR, 0.19; 95% CI, 0.05–0.70) caused a difference in prognosis between younger and older patients. Furthermore, there was a tendency toward differences in tumor localization (interaction effect, p = 0.06) and pathological findings (interaction effect, p= 0.05), although the differences were not statistically significant.
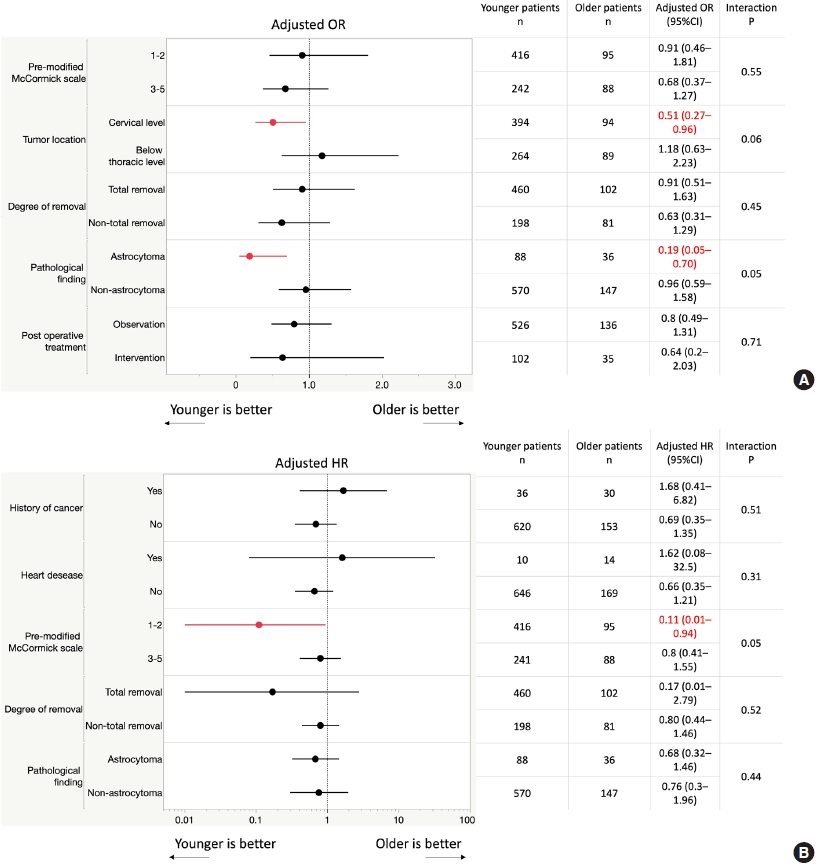
(A) Subgroups for primary outcomes. (B) Subgroups for overall survival. OR, odds ratio; CI, confidence interval; HR, hazard ratio.
Subgroup analyses of overall survival suggested that younger patients had better outcomes in almost all subgroups (Fig. 2B), excluding those with history of cancer and heart disease. Among the prognostic factors already reported and the dichotomized variables set up in this study, preoperative mMCs of I/II (aHR, 0.11; 95% CI, 0.01–0.94) caused a difference in overall survival between younger and older patients. Furthermore, there was a tendency toward differences in preoperative mMCs (interaction effect, p= 0.05), although the differences were not statistically significant.
5. Sensitivity Analyses
In sensitivity analyses, we dichotomized the adults’ cohort at age 75 years (Supplementary Table 2). Sensitivity analyses for the primary outcomes were similar to the main results.
6. The Relationship Between Preoperative mMCs and mMCs at 6 Months After Surgery
In both younger and older patients, preoperative mMCs were significantly associated with mMCs at 6 months after surgery (younger patients: rho= 0.59, 95% CI, 0.55–0.63; older patients: rho= 0.68, 95% CI, 0.61–0.75) (Fig. 3A). There was no significant difference in the correlation coefficient between younger and older patients (p= 0.07). In both younger and older patients, preoperative mMCs accurately predicted favorable outcomes (younger patients: AUC= 0.81, 95% CI, 0.77–0.84; older patients: AUC= 0.84, 95% CI, 0.78–0.89) (Fig. 3B). There was no significant difference in the AUC between younger and older patients (p= 0.26). In older patients, there were no favorable outcomes if their preoperative mMCs were V (Fig. 3C).

Relationship between preoperative modified McCormick scale (Pre-mMCs) and mMCs at 6 months after surgery. (A) The association between Pre-mMCs and mMCs at 6 months after surgery. (B) The diagnostic accuracies of preoperative mMCs in predicting favorable outcomes. (C) The distribution of Pre-mMCs and mMCs at 6 months after surgery. CI, confidence interval; AUC, area under the curve.
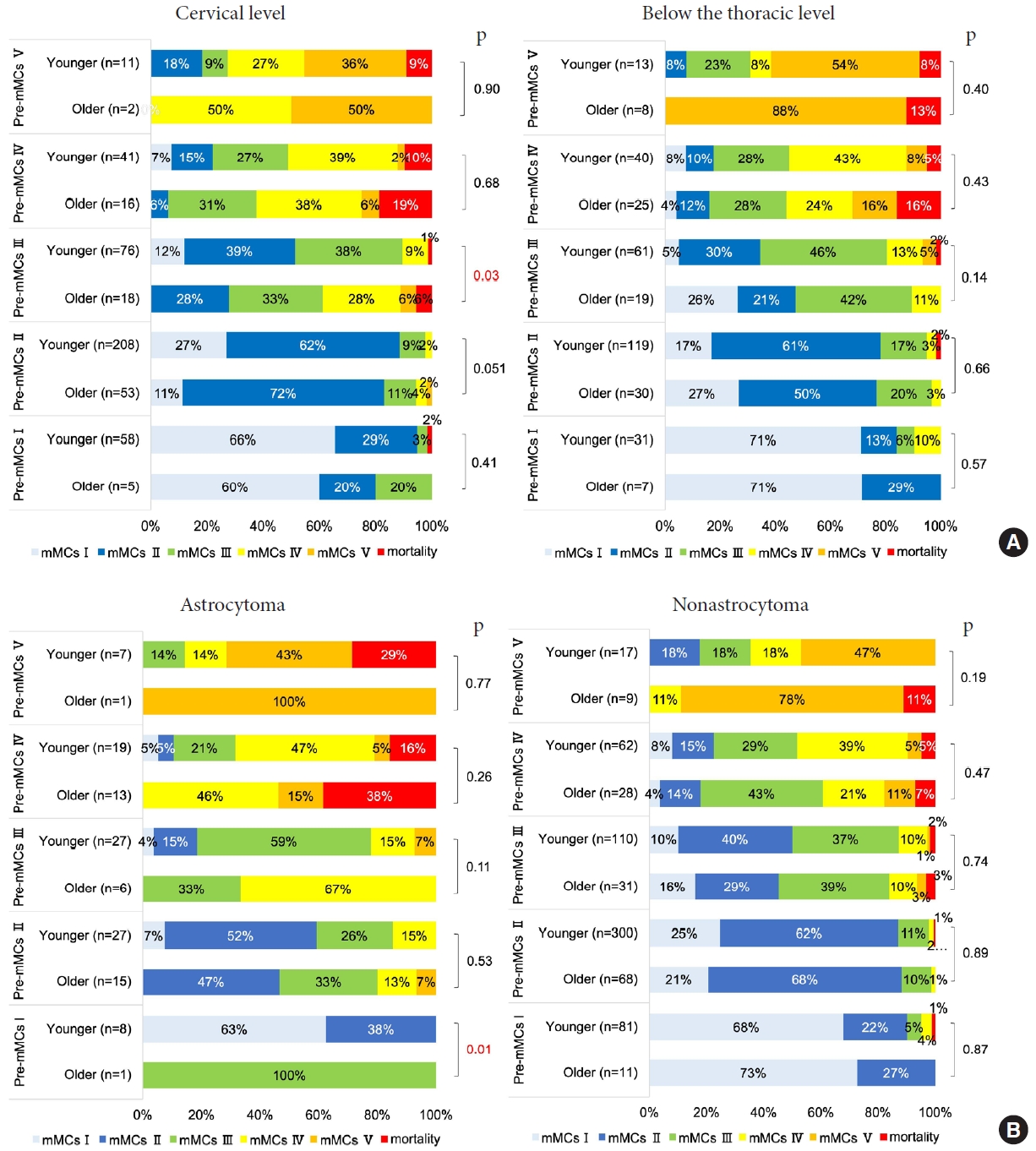
There were significant outcome differences between younger and older patients with the cervical lesion who had preoperative mMCs of III (p= 0.03) (Fig. 4A). In the astrocytoma group, no older patients had favorable outcomes if their preoperative mMCs were III or higher and no younger patients had favorable outcomes if their preoperative mMCs were V (Fig. 4B). There were significant outcome differences between younger and older patients with astrocytomas who had preoperative mMCs of I, although there was only one case in the older group (p= 0.01) (Fig. 4B).
DISCUSSION
1. Older and Younger IMSCTs Patients’ Characteristics
To our knowledge, this retrospective study is the largest to examine IMSCTs. The distribution of IMSCTs in our data was almost even across all age groups (unpublished data); this was consistent with the Japanese population pyramid. We adopted 65 years of age as the threshold for the elderly group, a definition used in many countries and the threshold level of the previous treatment policy suggestion [7,11].
It is reportedly difficult to diagnose IMSCTs in the elderly because symptoms often mimic the features of other diseases such as diabetes mellitus [21]. In this study, while the proportion of patients with diabetes mellitus was higher among older patients, with more patients taking steroids that could mask symptoms, there was no difference in the time from onset to presentation between groups. Primary glial tumors are reportedly more common in the elderly population (80%) [22]. Most adult IMSCTs are ependymomas, followed by astrocytomas [22-24]. Hemangioblastomas are less common, with an incidence of approximately 2%–15% [25], consistent with the high incidence of astrocytomas and low incidence of hemangioblastomas in older patients in the present study. While cavernous hemangiomas are uncommon in the elderly and reportedly present in < 10% patients aged ≥ 65 years [26,27], in our cohort, they accounted for 20% cases in the older patient group, suggesting that surgery for older cavernous hemangiomas may have been aggressively performed in Japan. Interestingly, in our cohort, the rate of thoracolumbar lesions was significantly higher for older patients, with fewer cases of cervical lesions. Depending on age, there may be differences in the localization of surgically treated IMSCTs, partly because of the higher incidence of hemangioblastomas in younger patients, who commonly exhibit cervical lesions [28]. With regard to the extent of tumor removal, biopsies were more common while total tumor removal was significantly less common in older patients. Partly because of this, the operating time was significantly shorter in older patients, and the occurrence of new neurological symptoms, including worsening symptoms, to the extent that they did not appear on mMCs grade change, was significantly higher in younger patients. Furthermore, the between-group difference in new symptom occurrence remained significant after adjustment, suggesting that being older or younger is an independent factor for new symptoms. These results suggest that surgeons may intentionally choose less invasive treatments for older patients.
2. Outcomes After IMSCT Surgery
The change in neurological findings due to surgery was not significantly different between groups. The rate of mMCs grade I/II at 6 months after surgery, which indicates a favorable outcome, was lower for older patients, but the difference was not significant after adjustment. These results are consistent and robust, even though the definition of the older group was changed. The 10-year cause-specific survival rate was also significantly lower for older than for younger patients, with no significant difference after adjustment. The fact that significant differences disappeared after adjustment suggested that preoperative neurological findings were a major confounding factor for determining postoperative outcomes, as observed in previous studies [14-17]. Actually, in our cohort, the preoperative mMCs grade was significantly worse in older patients. Furthermore, preoperative mMCs and mMCs at 6 months after surgery were significantly correlated in both groups, and preoperative mMCs were found to be a good predictor of favorable outcomes at 6 months in both groups.
Previous studies described the risk of postoperative neurological deterioration [29]. Changes in functional status from the preoperative to the immediate postoperative period tended to be worse in younger patients, although the difference was not statistically significant. However, the recovery of functional status from the immediate postoperative period to postoperative 6 months was better in younger patients; this suggests better recovery in younger patients. There was no significant difference in the overall change in status after surgery up to 6 months postoperatively. In summary, although transient neurological deterioration was observed in younger patients because they were often selected for aggressive removal, their recovery was better than that of older patients. It remained unclear whether better neurological recovery after 6 months in younger patients depended on the effect of tumor removal or neuroplasticity or both.
3. IMSCT Surgical Indications in the Elderly
Our results indicated that history of cancer and heart disease are independent prognostic factors contributing to overall survival in patients with IMSCTs and should be considered when determining the indication for surgery. While not statistically significant, subgroup analysis suggested these conditions contributed to an increased risk rather in younger patients. This might indicate that a reciprocal association between length of morbidity history and better management of comorbidities in the older patients, but further research is needed in this regard. Subgroup analysis showed significantly more favorable outcomes in younger patients for cervical spine lesions and astrocytoma, without a significant difference in the interaction. There were significant outcome differences between younger and older patients, with the cervical lesion who had preoperative mMCs of III and with astrocytomas who had preoperative mMCs of I. In older patients, there were no favorable outcomes if their preoperative mMCs were V. In the astrocytoma group, no older patients had favorable outcomes if their preoperative mMCs were I and III or higher. The reason for the poor prognosis of cervical spine lesions in the elderly may partly be due to higher spinal cord lesions generally presenting with more extensive neurological symptoms and injuries at the cervical level allowing for considerably more spontaneous recovery than injuries at the thoracic level [30], and older patients have worse neuroplasticity than younger patients [31,32]. If the tumor type can be determined preoperatively, the indication for surgery can be considered according to the above in older patients. Considering the challenges in achieving a differential diagnosis of IMSCT through preoperative assessment, and acknowledging that intraoperative rapid pathology exhibits only 70% concordance with definitive pathology specimens [33], it appears that surgical intervention may not be advisable for older patients presenting with a preoperative McCormick scale of V and a high suspicion of astrocytoma. Subgroup analysis showed significantly better overall survival in younger patients for mMCs of I/II, without a significant difference in the interaction. This indicates that surgical intervention in older IMSCTs patients who are neurologically independent preoperatively requires more attention than in younger patients. The indications for IMSCT surgery in older patients suggested by this study are summarized in Supplementary Fig. 2.
4. Limitations
Because of the nature of IMSCTs, it will continue to be ethically difficult to determine the efficacy of surgery in the elderly through randomized trials. We believe that our study might provide insight into the optimal treatment strategies for IMSCTs in the older population.
This study has several limitations, including the retrospective design. Each center decided to perform surgery, and only surgically treated cases were included. Therefore, we were unable to evaluate the clinical course of conservatively treated patients with IMSCTs in Japan, and the complete coverage rate was insufficient. Second, a 22% attrition rate was observed at the 6-month time point. These findings suggest inevitable selection bias. It is important to note that our results show no difference in the effectiveness of surgery between older and younger patients with IMSCTs, although they do not indicate homology. To our knowledge, this is the largest study of IMSCTs, but we cannot rule out the possibility of differences as the sample size increases. A large prospective, multinational, longitudinal study on this issue needs to be conducted in the future.
CONCLUSION
We believe that a thorough evaluation of each individual’s conditions such as preoperative neurological status, comorbidities, disease spinal level, and expected pathological diagnosis rather than age itself is crucial for determining the surgical indications for elderly patients with IMSCTs. By striving to minimize operative time, blood loss, and surgical complications, and avoiding overly aggressive total resection, we can enhance the safety and efficacy of the procedure. It is essential to consider the differences in recovery between older and younger patients. This comprehensive approach will contribute to more precise and appropriate surgical decision-making, ultimately leading to improved patient outcomes.
Supplementary Material
Supplementary Tables 1, 2 and Figs. 1, 2 can be found via https://doi.org/10.14245/ns.2346390.195.
Study flowchart. Among the 1,080 patients initially enrolled in the registry, we excluded nonsurgical cases (n=20), cases of spinal lipoma (n=18) and myxopapillary ependymoma (n=11), cases that underwent external decompression only (n=1), and those that underwent surgery before 2008 (n=3). We classified pediatric patients (<18 years) (n=58), younger patients (aged 18–64 years) (n=739), and older patients (over 65 years) (n=230), respectively. In total, 81 younger and 47 older patients did not have modified McCormick scale (mMCs) results at 6 months and were excluded from this analysis. Finally, 841 adult patients with available mMCs data at 6 months were examined in this analysis. There were 658 younger (78.2%) and 183 (21.8%) older patients.
Indications for intramedullary spinal cord tumor (IMSCT) surgery in older patients. GTR, gross total removal; STR, subtotal removal. Appendix: The ultimate determination regarding surgical indications remains at the discretion of the treating physician, and this algorithm should be regarded as a general guide rather than an inflexible rule. Older age is not an independent poor prognostic factor, but older patients should be informed that it is difficult to achieve the same degree of neurological recovery as younger patients. Older patients of preoperative mMC III with cervical lesions should be informed that approximately 40% of patients will have postoperative neurological deterioration. Older patients of preoperative mMCs II/IV suspected astrocytoma should be informed postoperative independence difficulties. A history of cancer or heart disease independently contributed to overall survival in patients with IMSCT and should be factored into surgical decision-making.
Multivariate analysis incorporating comorbidities as adjustment factors
Sensitivity analyses
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study was financially supported by the Neurospinal Society of Japan.
Author Contribution
Conceptualization: HK, KT, TE, MM; Data curation: HK, TE, MM; Formal analysis: KT; Funding acquisition: TE; Methodology: HK, KT; Project administration: HK, KT, TE, KH, MM; Visualization: HK, KT, TE, MM; Writing - original draft: HK, KT; Writing - review & editing: HK, KT, SY, TE, KH, MM.
Acknowledgements
Investigators of Intramedullary Spinal Cord Tumors in the Neurospinal Society of Japan -- Masahito Hara and Masahiro Aoyama: Aichi Medical University. Taku Sugawara: Akita Cerebrospinal and Cardiovascular Center. Hiroaki Shimizu: Akita University. Kotaro Ogihara: Iwakuni Clinical Center. Atsushi Sugawara: Iwate Medical University. Phyo Kim and Kazushige Itoki: Utsunomiya Brain and Spinal Cord Center. Seiji Matsui and Seiji Shigekawa: Ehime University. Noritsugu Kunihiro: Osaka City General Hospital. Kentaro Naito: Osaka City University. Shinji Yamamoto: Ohnishi Neurological Center. Takao Yasuhara: Okayama University. Motoyuki Iwasaki: Otaru City Hospital. Yasuyuki Miyoshi: Kawasaki Medical School. Hideki Hayashi: Kitano Hospital. Nakayama Noriyuki and Toru Iwama: Gifu University. Daisuke Umebayashi: Kyoto Prefectural University of Medicine. Hiroshi Nakagawa and Manabu Sumiyoshi: Kushiro Kojinkai Memorial Hospital. Yasukazu Hijikata: Spine and Low Back Pain Center, Kitasuma Hospital. Hisaaki Uchikado: Kurume University. Hitoshi Fukuda: Kochi University. Tomoaki Nakai and Takashi Sasayama: Kobe University. Kazuhiko Mishima: Saitama Medical University, International Medical Center. Tomoo Inoue: Saitama Red Cross Hospital. Shunsuke Yano and Toru Sasamori: Sapporo Azabu Neurosurgical Hospital. Nobuhiro Mikuni and Yukinori Akiyama: Sapporo Medical University. Tsuyoshi Hara: Juntendo University. Gakuji Gondo: Shonan Kamakura General Hospital. Mitsuhiro Yoshida: Yokkaichi Municipal Hospital. Shigeo Ueda and Minoru Hoshimaru: Shin-Aikai Spine Center. Hideki Komatani and Yuichi Takahashi: Shin Komonji Hospital. Kiyoshi Ito: Shinshu University. Hisaharu Goto and Node Yasuhiro: Shin-Yurigaoka General Hospital. Mizuki Watanabe: Seirei Hamamatsu General Hospital. Yasunobu Ito: Tokyo General Hospital. Yoshitaka Hirano: Southern Tohoku Research Institute for Neuroscience. Teiji Tominaga: Tohoku University. Hirokazu Takami: Tokyo University. Jun Karakama: Tokyo Medical and Dental University. Hiroki Ohashi: The Jikei University School of Medicine. Naoyuki Harada: Toho University. Ryu Kurokawa: Tetsuro Shingo and Satoshi Kawajiri, Dokkyo Medical University. Tomohiro Yamauchi: Tomakomai City Hospital. Tetsuji Uno: Tottori University. So Fujimoto and Keisuke Takai: Tokyo Metropolitan Neurological Hospital. Yasufumi Otake: Nakamura Memorial Hospital. Yasuhiro Takeshima and Hiroyuki Nakase: Nara Medical University. Akihiko Saito: Niigata City Hospital. Daijiro Morimoto and Kyongsong Kim: Nippon Medical School. Tatsuya Ohtonari: Brain Attack Center, Ota Memorial Hospital. Takafumi Mitsuhara: Hiroshima University. Yosuke Kuromi: Fukushima Medical University. Toshiyuki Takahashi and Ryo Kanematsu: Fujieda Heisei Memorial Hospital. Tatsushi Inoue: Fujita Health University. Toshitaka Seki and Kazuyoshi Yamazaki: Hokkaido University. Izumi Koyanagi: Hokkaido Neurosurgical Memorial Hospital. Kazuhisa Yoshifuji: Hokkaido Medical Center for Child Health and Rehabilitation. Masashi Fujimoto: Mie University. Misao Nishikawa: Moriguchi-Ikuno Memorial Hospital. Takashi Yagi and Hiroyuki Kinouchi: University of Yamanashi. Hidetoshi Murata: Yokohama City University. Mari Kitayama: Wakayama Medical University.