Predictors of Cerebrospinal Fluid Leak Following Dural Repair in Spinal Intradural Surgery
Article information
Abstract
Objective
We aim to compare the effectiveness of dural closure techniques in preventing cerebrospinal fluid (CSF) leaks following surgery for intradural lesions and seek to identify additional factors associated with CSF leaks. Surgical management of spinal intradural lesions involves durotomy which requires a robust repair to prevent postoperative CSF leakage. The ideal method of dural closure and the efficacy of sealants has not been established in literature.
Methods
We performed a retrospective analysis of all intradural spinal cases performed at a tertiary spine centre from 1 April 2015 to 29 January 2020 and collected data on patient bio-profile, dural repair technique, and CSF leak rates. Multivariate analysis was performed to identify predictors for postoperative CSF leak.
Results
A total of 169 cases were reported during the study period. There were 15 cases in which postoperative CSF leak was reported (8.87%). Multivariate analysis demonstrated that patient age (odds ratio [OR], 0.942; 95% confidence interval [CI], 0.891–0.996), surgical indication listed in the “others” category (OR, 44.608; 95% CI, 1.706–166.290) and dural closure with suture, sealant and patch (OR, 22.235; 95% CI, 2.578–191.798) were factors associated with CSF leak. Postoperative CSF leak was associated with the risk of surgical site infection with a likelihood ratio of 8.704 (χ² (1) =14.633, p < 0.001).
Conclusion
Identifying predictors for CSF leaks can assist in the counselling of patients with regard to surgical risk and expected postoperative recovery.
INTRODUCTION
The surgical management of spinal intradural lesions involves opening of the dura [1] which requires a robust repair to prevent postoperative cerebrospinal fluid (CSF) leakage. Despite best efforts, reported rates of CSF leakage range from 6.6% [2] to 10% [3]. Failure to achieve adequate dural closure could lead to potential complications of CSF fistulas [4], wound infection [5], nerve root entrapment [6] and meningitis [3]. To avoid these debilitating complications, watertight closure is advocated and is typically achieved with a meticulous dural suture. Biological materials have also been developed in recent decades to supplement dural closure primarily with the use of sealants such as fibrin which provides an adhesive effect from the formation of fibrin cross-linking plymers [7], allowing improved watertight closure in animal models [8]. The clinical evidence for sealants is still debated, with a recent review [9] recognizing the paucity of randomized control trials (RCTs) examining sealants in dural closure. Specific to intradural pathologies, retrospective studies have not demonstrated the superiority of fibrin sealants [10,11]. Although RCTs have demonstrated the efficacy of autologous fibrin tissue [12] and hydrogel sealants [13], a systematic review by Choi et al. [14] suggested that sealants as an adjunct to primary closure did not reduce the rate of CSF leak.
Dural management is especially challenging in intradural lesions which vary in circumferential position and behavior. Resection of meningiomas, especially in recurrent cases, may lead to large dural defects [15] which cannot be reliably closed primarily. To overcome the risk of CSF leak, dural patch techniques have evolved from epidural fat patches [16] to biomaterials such as polyglactin acid sheets [17], collagen scaffolds [18] and hemostatic pads [19]. There is currently a lack of high-quality evidence demonstrating the efficacy of dural augmentation products. Of note, a retrospective study has shown higher rates of CSF leaks in cases treated with dural augmentation and no reliable comparison between the materials could be made [20].
Besides dural closure techniques, other factors could increase the risk of CSF leak in the management of intradural lesions. Sin et al. [21] described that patients’ age and level of surgeon’s training were factors that predicted dural tear in patients undergoing lumbar surgery. In terms of surgeries requiring intended durotomies, however, predictive factors have yet to be identified in current literature [2]. Filling this knowledge gap will help guide surgeons in the counselling of patients with regards to postoperative course and complications, in addition to aiding the preoperative preparation of dural augments and sealants.
Given this deficiency in literature, we aim to compare the effectiveness of dural closure techniques in preventing CSF leak following surgery for intradural lesions. Additionally, we seek to identify additional factors associated with CSF leak and evaluate their predictive ability, including patient bio-profile, the presence of a dural defect and the nature of the intradural lesion.
MATERIALS AND METHODS
We retrospectively analysed all intradural spinal cases performed at a tertiary spine centre from 1st of April 2015 to 29th January 2020 by a team of 3 fellowship-trained neurosurgeons. Operative notes, clinic notes, inpatient records, and imaging studies recorded in our electronic patient records were recorded in an anonymized dataset that did not include patient identifiers. The following selection criteria was applied: (1) surgical procedure must have included surgical opening of the spinal dura mater, (2) clear documentation of the dural closure technique applied and the material used.
The dural closure techniques were categorized into the following groups: (1) running stitch closure, (2) stitch closure plus application of dural sealant, (3) stich closure plus application of dural patch on top and dural sealant, (4) other closure techniques (e.g., application of dural clips, duraplasty techniques).
A dural defect was defined as a durotomy that could not be opposed primarily in a tension-free environment as assessed by the primary surgeon, usually due to dural adhesion to the tumor such as in meningiomas. These dural defects tend to result in a broader durotomy compared to a linear durotomy which could be primarily repaired. In these cases, the dural edges were sutured to the dural patch graft to maintain a tension-free closure. In cases without a dural defect, dural closure was performed using a 6-0 prolene running, nonlocking suture. The use of dural sealant, however, was not standardized and is based on surgeon choice. The dural sealant preferred in our unit during the duration of the study was Tisseel (Baxter Healthcare Corp., Deerfield, IL, USA). The dural patches used were either TissuePatchDural (Tissuemed Surgical Technologies, Leeds, UK), Duraform (Depuy Synthes, Raynham, MA, USA) or Neuro-Patch (B Braun, Tuttlingen, Germany). In instances where dural patch grafting was required or performed, DuraGuard (Baxter Healthcare Corp., Deerfield, IL, USA) was used. In all instances, after dural closure, a Valsalva manoeuvre was performed by the anaesthetic team to assess for absence of CSF egress, indicating the completeness of CSF closure.
A postoperative CSF leak was defined in this study as a CSF discharge from the operative wound or CSF collection which required prolonged bed rest, intervention during the same index admission or a subsequent readmission. In the cases where the nature of the fluid discharge was unclear, CSF was differentiated from serous exudate by the use of B-2 transferrin assay. Management of postoperative CSF leak was dependent on the surgeon’s preference but included at least 24 hours of bedrest. Time of onset of CSF leak was recorded as either: (1) within 48-hour postoperation, (2) after the initial 48-hour postoperation and until hospital discharge, (3) post hospital discharge. Postoperative instructions such as flat bed rest were analysed to see if they have any impact on the rate of CSF leak. Age, sex, and body mass index (BMI) data were also collected together with recorded comorbidities. The presence of surgical site infection was also noted, including both superficial and deep infections as defined by The National Institute for Health and Care Excellence (NICE) guidelines [22].
Data analysis was performed using IBM SPSS Statistics ver. 27.0 (IBM Co., Armonk, NY, USA). Chi-squared analyses were performed to evaluate the correlation between presence of postoperative CSF leak and categorical factors of sex, BMI category, type of intradural lesion and suture technique employed. For multivariate analysis, we included the following variables: age, sex, BMI, type of intradural tumor, dural closure technique, the presence of a dural defect, whether a wound drain was placed and whether the patient was placed on bed rest following surgery. A logistic regression model was constructed to analyze the multivariate association between postoperative CSF leak and the abovementioned variables. Odds ratios and 95% confidence intervals were generated from logistic regression models. The Hosmer-Lemshow Goodness-of-Fit test was used to evaluate the lack of fit in the constructed model. Wald values of examined variables were evaluated and p-values of < 0.05 were considered statistically significant.
As the data used for the study was retrieved from an anonymized audit registry, informed consent was deemed exempt from requirement. Institutional Review Board approval was not required as the data was based on an anonymized audit registry.
RESULTS
A total of 169 cases were reported during the study period in a tertiary multisurgeon neurosurgical centre. No patients were excluded for unclear documentation of dural repair technique. All durotomies were created in the dorsal midline or dorsolateral overlying the intradural lesion. There were 15 cases in which postoperative CSF leak was reported (8.87%) in the following periods: 1 case (6.67%) of early (≤48 hours) CSF discharge which required suturing on the ward, 6 cases (40%) of CSF leakage during the same index admission ( > 48 hours till discharge) and 8 cases (53.3%) of late presentation (after hospital discharge) with pseudo-meningoceles. The patients are dichotomized into groups which did not experience CSF leak (no leak) and those with CSF leak (leak) with their profile described in Table 1.
In terms of patient demographics comorbidity profile, sex was not associated postoperative CSF leak, but univariate analysis revealed that patients with CSF leak tend to be younger (53.7 ± 19.4 years vs. 41.7± 14.5 years, p=0.020). Patients were categorized by their BMI with overweight defined as BMI of 25 kg/m2 and above. The majority of patients with CSF leak (66.7%) did not have significant comorbidities nor were they smokers (86.7%). Neither smoking (p =0.280) nor diabetes (p =0.898) was associated with postoperative CSF leak.
The main pathologies treated were spinal cord schwannomas/neurofibromas (n=53, 31.36%) followed by intradural meningiomas (n =39, 23.08%) and intramedullary tumors (n =27, 15.98%) as shown in Fig. 1. Rare intradural cases such as spinal cord teratomas, epidermoid or dermoid tumors, spinal cord herniation, open spinal cord biopsy were labelled as “others” (Fig. 1, Table 1). The proportion of patients in each indication who experienced CSF leak is also described in Table 1.
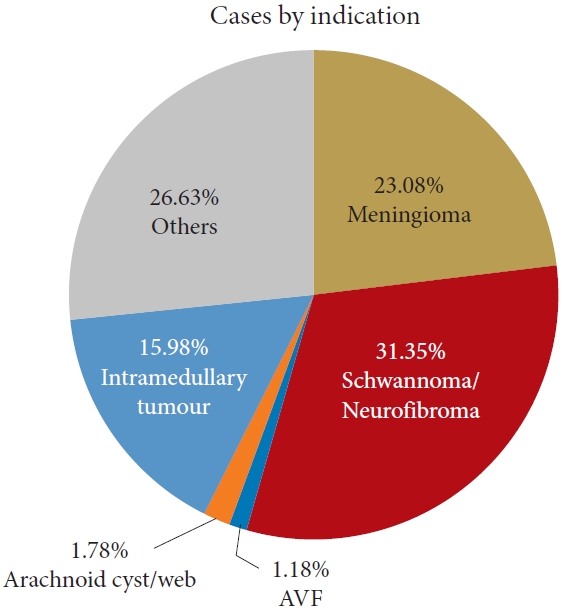
Pie-chart of indications for intradural surgeries with listed percentages. AVF, arteriovenous fistula.
Dural closure technique is categorized into suture only, suture with sealant, suture with sealant and patch or others. Dural defects were reported in 56.4% of cases. The CSF leak rate based on technique is described in Table 1. Chi-square test of independence revealed that dural closure technique was associated with postoperative CSF leak, χ2 (3, N=160)=8.4, p=0.004. Dural closure technique was also associated with the reported presence of a dural defect, χ2 (3, N=160)=16.9, p< 0.001.
Peri- and postoperative factors such as the effect of the presence of dural defects, prolonged bed rest and wound drains were evaluated and chi-square test of independence did not show a significant association between these factors and postoperative CSF leak. Forty-six point seven percent of patients with CSF leak and 51.8% of patients without CSF leak were allowed unrestricted ambulation and this proportion was not significantly different between the 2 groups (p=0.704).
Multivariate analysis was performed using both patient profile factors and peri/postoperative factors as described above via logistic regression. The studied predictors and their respective odds ratios are summarized in Table 2. In summary, a younger patient age (OR, 0.942; 95% CI, 0.891–0.996), surgical indication listed in the “others” category (OR, 44.608; 95% CI, 1.706–166.290) and dural closure with suture, sealant and patch (OR, 22.235; 95% CI, 2.578–191.798) were associated with postoperative CSF leak.
Surgical site infection was present in 4 of 15 (26.7%) of cases with CSF leak and 5 of 152 (3.3%) of cases without CSF leak. Postoperative CSF leak was associated with the risk of surgical site infection with chi-square test of independence demonstrating significance χ2 (1, N=167)=14.633, p< 0.001 and a likelihood ratio of 8.704. The management of the postoperative CSF leak cases consisted of surgical re-exploration and repair (n=4), wound stitch (n=1) and conservative management via pressure dressing (n=10). The re-exploration strategies consisted of ventriculo-peritoneal shunts (n=2), thoraco-peritoneal shunt (n=1) in patients with risk factors for increased intracranial pressure and lumbar drain insertion (n=1).
DISCUSSION
Our analysis revealed a CSF leak rate of 8.87%, comparable to rates reported in previous studies [3,23] and in a recent review [2]. Postoperative CSF leak adversely affects patient recovery, with an increased likelihood of infection (likelihood ratio 8.704) and one-third of patients requiring operative reintervention. It is therefore useful to be able to identify risk factors that predispose to CSF leak and evaluate the efficacy of various dural closure techniques.
Patients with postoperative CSF leak were significantly younger that those without. We postulate that this could be related to the age predilection as well as behavior of the tumor. Spinal meningiomas typically have a benign course with low rates of morbidity [24,25], although they are less common in younger patients and more likely associated with aggressive histological subtypes [26] in these age groups. Additionally, they vary in location with a higher prevalence of cervical tumors in younger patients, complicating their management [27]. These factors contribute to a more aggressive clinical course in younger patients [28] and the histological aggressiveness with dural invasion [29] may explain the possible increase in dural complications. This differential behavior is similarly observed in intramedullary lesions which follow a more insidious course in younger populations and possibly presenting at a later, more advanced stage [30,31].
Intramedullary lesions were associated with a higher rate of CSF leak. Surgical resection of these lesions can be challenging due to the anatomical disruption of the spinal cord orientation and infiltrative lesions obscuring clear margins. Proper exposure with an extensive durotomy is necessary to plan the myelotomy. We hypothesize that increased CSF protein content from tumor and blood breakdown products, combined with the dissection required in these cases, can also disrupt CSF drainage and perivascular Virchow-Robin spaces [32], leading to local accumulation of CSF which adversely affects dural healing. This is further compounded by the disruption to the arachnoid plane, which may be preserved in some cases of extramedullary lesions such as certain meningiomas or schwannomas, leading to potential arachnoid adhesions. The surgery can be further complicated by the presence of hydrocephalus in some patients which increase the risk of CSF leak. Some of these difficulties have been recognized by Samartzis et al. [1] and proper perioperative planning and identification is essential to the prevention of CSF leak in intramedullary lesions. Interestingly, the reported presence of a dural defect was not associated with CSF leak in our analysis which may be attributed to duroplasty techniques that decrease the local CSF pressure effects exerted on the dural closure.
Given the importance of a watertight seal following durotomy, we evaluated the efficacy of the various closure techniques. Although the use of sealants was associated with lower CSF leak rates, this association was not evident after multivariate analysis. Also, while patches were used more often in dural defect cases, they were not identified as interacting factors in multivariate analysis. The analysis revealed that cases in which a patch was used had higher odds of having a dural leak postoperatively. Despite the use of multivariate analysis, we acknowledge that this finding may be circumstantial in view of the retrospective nature of the study. A possible explanation could be that patches were used in cases where difficulties in securing a watertight dural closure were anticipated pre or perioperatively due to factors not captured in multivariate analysis. This result was also reflected in a series which found that the use of multiple dural substitutes led to increased CSF leak rates [20]. An alternative explanation could be that patches may disrupt proper dural healing, as a study has demonstrated that collagen patches incite an initial inflammatory response that may disrupt anastomotic healing [33]. Basic science studies investigating the healing of the dura after application of dural substitutes are lacking in this regard. We acknowledge that various methods of dural repair, such as inlay, onlay, or direct suture techniques, may affect the quality of dural repair, although the numbers in each subgroup did not permit multivariate analysis. In our centre, a combined inlay with onlay technique is commonly used with the inlay patch functioning to prevent CSF pressure effects on the dural closure by establishing a 1-way valve effect, and the onlay providing a buttress to the repair. In addition to dural closure techniques, intraoperative lumbar subarachnoid drainage has been investigated as an adjunct to decrease subcutaneous CSF accumulation postoperatively [34,35].
We acknowledge important limitations to the study. As there was a lack of consistent photographic data, we were unable to capture accurate information on the location, size and shape of durotomies which could influence the rate of CSF leak [36,37]. Inherent to the uncommon nature of intradural tumors, especially postoperative CSF leaks, the relatively small numbers may decrease the robustness of a prediction model. Additionally, no prediction model can capture every significant variable and other factors such as the use of steroids, radiotherapy and patient fragility scores. Despite this, our analysed factors accounted for the majority of data variance and achieved a respectable classification rate for postoperative CSF leak. It is also important to note that the detection of postoperative CSF leak was dependent on early surgeon recognition and patient report, thus asymptomatic, occult cases of postoperative CSF leak are not captured in this analysis. Lastly, as the study did not prescribe a standardized protocol for the management of durotomies, the results are not controlled and may not be easily generalized to other practices managing a different profile of patients with durotomies.
CONCLUSION
Postoperative CSF leak following intradural tumor resection is associated with morbidity and can lead to re-operation and infection. Identifying predictors for CSF leak can assist in the counselling of patients with regards to surgical risk and expected postoperative recovery. Additionally, it may aid the surgeon in anticipating dural closure difficulties and preparation of augments.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: AB, MSK, AK, RT, JF; Data curation: AB, MSK; Formal analysis: LJ, AB, MSK, EG; Methodology: MSK, JF; Project administration: JF; Writing - original draft: LJ; Writing - review & editing: LJ, EG, AK, RT, JF.


