Risk Factors of Restenosis After Full Endoscopic Foraminotomy for Lumbar Foraminal Stenosis: Case-Control Study
Article information
Abstract
Objective
To investigate risk factors associated with postoperative restenosis after full endoscopic lumbar foraminotomy (FELF) in patients with lumbar foraminal stenosis (LFS).
Methods
A single-center, retrospective case-control study was conducted on patients diagnosed with foraminal stenosis who underwent FELF between August 2019 and April 2022. The study included 56 patients, comprising 18 cases and 38 controls. Clinical data, radiologic assessments, and surgical types were compared between the groups. The cutoff values of radiologic parameters that differentiate the 2 groups were investigated.
Results
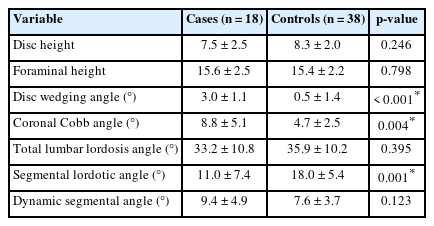
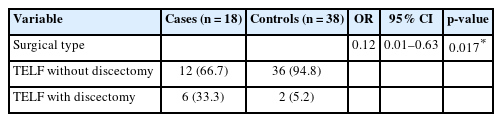
No significant difference in age, sex distribution, or presence of adjacent segment disease or grade I spondylolisthesis was observed between the groups. Cases had a higher degree of disc wedging angle (DWA) (3.0°±1.1° vs. 0.5°±1.4°, p < 0.001), larger coronal Cobb angle (CCA) (8.8°±5.1° vs. 4.7°±2.5°, p = 0.004), and smaller segmental lumbar lordosis (SLL) than controls (11.0±7.4 vs. 18.0±5.4, p = 0.001). Optimal cutoff values for DWA, CCA, and SLL were estimated as 1.8°, 7.9°, and 17.1°, respectively. A significant difference in surgical types was observed between cases and controls (p = 0.004), with the case group having a higher distribution of patients undergoing discectomy in addition to TELF.
Conclusion
The study identified potential risk factors for restenosis after FELF in patients with LFS, including higher DWA, larger CCA, smaller SLL angle. We believe that discectomy should be perform with caution during FELF, as it can lead to subsequent restenosis.
INTRODUCTION
Lumbar foraminal stenosis (LFS) is responsible for approximately 8% to 11% of degenerative lumbar spine diseases [1]. As the clinical characteristics of this condition have become more widely recognized, the importance of surgical intervention has grown. Surgical treatments for LFS can generally be categorized into neural decompression and fusion procedures. Fusion is often necessary due to the frequent association of intervertebral foraminal stenosis with various degenerative changes. However, elderly patients or those with chronic diseases face a higher risk of postoperative complications related to general anesthesia and blood loss, leading to an increased preference for minimally invasive treatment methods in recent years.
Consequently, endoscopic surgery, a form of minimally invasive spine surgery, has undergone significant advancements over the past 2 decades, providing a beneficial treatment option for elderly patients at high risk for complications related to general anesthesia [2,3]. Historically, the use of endoscopic spine surgery was limited to the lumbar spine, but through the efforts of various researchers, its applicability has expanded to include the cervical and thoracic spine as well as a broader range of pathological conditions, such as spinal stenosis, intervertebral foraminal stenosis, and complex lesions [4-6]. Recent studies have even reported successful endoscopic surgical outcomes for tumor lesions, comparable to traditional surgery [7]. One of the most significant achievements of endoscopic surgery is its ability to largely replace fusion procedures in the treatment of intervertebral foraminal stenosis.
Nonetheless, it is uncertain whether the duration and durability of symptom relief and the risk of stenosis recurrence with endoscopic decompression alone of the intervertebral foramen can be compared to the fusion technique, which offers both neural decompression and structural stabilization [8]. This represents a key challenge that the endoscopic approach needs to address. Therefore, this study aims to examine the risk factors associated with postoperative restenosis, a primary reason for reoperation following full endoscopic lumbar foraminotomy (FELF) in patients with LFS. To the best of our knowledge, this is the first study to compare restenosis cases after FELF to a control group.
MATERIALS AND METHODS
1. Study Populations
Prior to the start of this study, the Institutional Review Board of Chosun University Hospital approved for the research design (CHOSUN 2023-04-029). This retrospective study was conducted in a single center on patients diagnosed with foraminal stenosis who underwent full FELF for foraminal stenosis from August 2019 to April 2022. Participants were selected based on the following inclusion criteria: (1) 18 years of age and older with a diagnosis of symptomatic moderate or severe intervertebral foraminal stenosis [9], (2) clear evidence of foraminal stenosis observed on lumbar magnetic resonance imaging (MRI) with corresponding lower extremity radicular pain, (3) symptom not improving after at least 3 months of nonsurgical treatment.
Exclusion criteria were as follows: (1) inconsistency between MRI findings and symptoms, (2) patients where low back pain is the main symptom rather than lower extremity radicular pain, (3) presence of spondylolytic spondylolisthesis of grade 2 or higher, (4) presence of segmental instability, (5) coexistence of severe grades 3–4 central spinal canal stenosis [10], (6) coexistence of other pathological conditions such as infection, trauma, or tumors, (7) cases of lumbar disc extrusion or sequestration.
2. Selection of Cases and Control Group
The definition of restenosis after FELF in this study, which served as the criteria for selecting the patient group, was as follows: (1) improvement in lower extremity radicular pain for at least one month after FELF surgery, (2) recurrence of lower extremity radicular pain in the same location within 1 year after surgery, (3) radiologic confirmation of foraminal stenosis recurrence at the same site as the initial surgery. The control group criteria were set as not having any clinical symptom recurrence and no radiological evidence of restenosis for at least one year after FELF surgery. To increase the statistical power of the independent variables, a control group twice the size of the case group was collected.
3. Clinical Data Collection
Basic clinical indicators of all selected patients, including age, sex, surgical level, and follow-up period, were collected. Clinical outcomes were assessed using the visual analogue scale (VAS), preoperatively, postoperatively and at 6-month follow-up. In terms of surgical technique, whether the basic TELF procedure was performed and whether additional discectomy was performed were investigated.
4. Radiologic Assessment
To evaluate the relationship between imaging findings and restenosis, a range of baseline radiologic parameters were obtained from preoperative static and dynamic simple radiograph of all study participants in both the case and control groups. The differences between the groups were then analyzed using statistical methods. Parameters that showed significant differences between groups were analyzed to calculate receiver operating characteristic curve, area under curve (AUC), optimal cutoff value, sensitivity, and specificity using statistical software. Their respective measurement methods are as follows (Fig. 1): (1) Total lumbar lordotic angle: angle measured between the upper endplate of L1 and the upper endplate of S1, (2) Segmental lordotic angle (SLA): angle measured between the upper endplate of upper vertebra and the lower endplate of lower vertebra, (3) Coronal Cobb angle (CCA): angle measured between the most tilted top vertebrae and the most tilted bottom vertebrae, (4) Disc height (DH): half the sum of the anterior and posterior heights of disc, (5) Foraminal height (FH): distance between the pedicles, (6) Disc wedging angle (DWA): angle between the inferior endplate of the upper vertebra and superior endplate of the lower vertebra. A positive value indicates disc wedging towards the side of the lesion, while a negative value indicates disc wedging on the side opposite the lesion. In case of L5/S1 level, angle between the upper endplate of L5 and the line connecting the top of bilateral sacral ala [11], (7) Dynamic SLA: difference of SLA between flexion and extension posture.
5. Statistical Analysis
Statistical evaluations were conducted using R ver. 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). All variables underwent descriptive statistical analysis, and appropriate statistical methods were used for comparisons between groups when necessary. For continuous variables, the normality of values was assessed for each variable, and either Welch T-test or Wilcoxon rank-sum test was used for analysis, depending on the appropriate method. Categorical variables were analyzed using either Pearson chi-square test or Fisher exact test, after checking the expected frequencies in the contingency table cells. A statistical significance level of 0.05 was set for evaluating significance.
6. Surgical Procedure
Intramuscular midazolam (0.05 mg/kg) and intravenous fentanyl (0.8 μg/kg) were administered as preoperative treatments, and the patient was conscious and underwent surgery with local anesthesia and a transforaminal epidural block. All patient was placed in the prone position on a radiolucent operating table, with the knees and hips slightly flexed to reduce the lumbar lordotic curve. All procedures were performed via a full endoscopic transforaminal approach, and an out-in technique was used to minimize exiting nerve root (ENR) irritation of the narrowed intervertebral foramen and facilitate resolution of the circumferential stenosis [12]. For sufficient decompression of the ENR, the extent of bone work was planned through preoperative MRI, computed tomography, and x-ray evaluation, and the need for bone work in the superior articular process as well as the isthmus, lower pedicle of upper body, and upper endplate of lower body was determined preoperatively [13]. If preoperative examination revealed a vertical or circumferential foraminal stenosis, removal of the protruding disc and osteophyte was planned [1]. After removing the protruding disc and osteophytes, if the possibility of disc reprotrusion was expected, interbody discectomy was performed (Fig. 2). So, surgical procedures were classified into 2 types as follows: (a) TELF without discectomy, (b) TELF with discectomy.
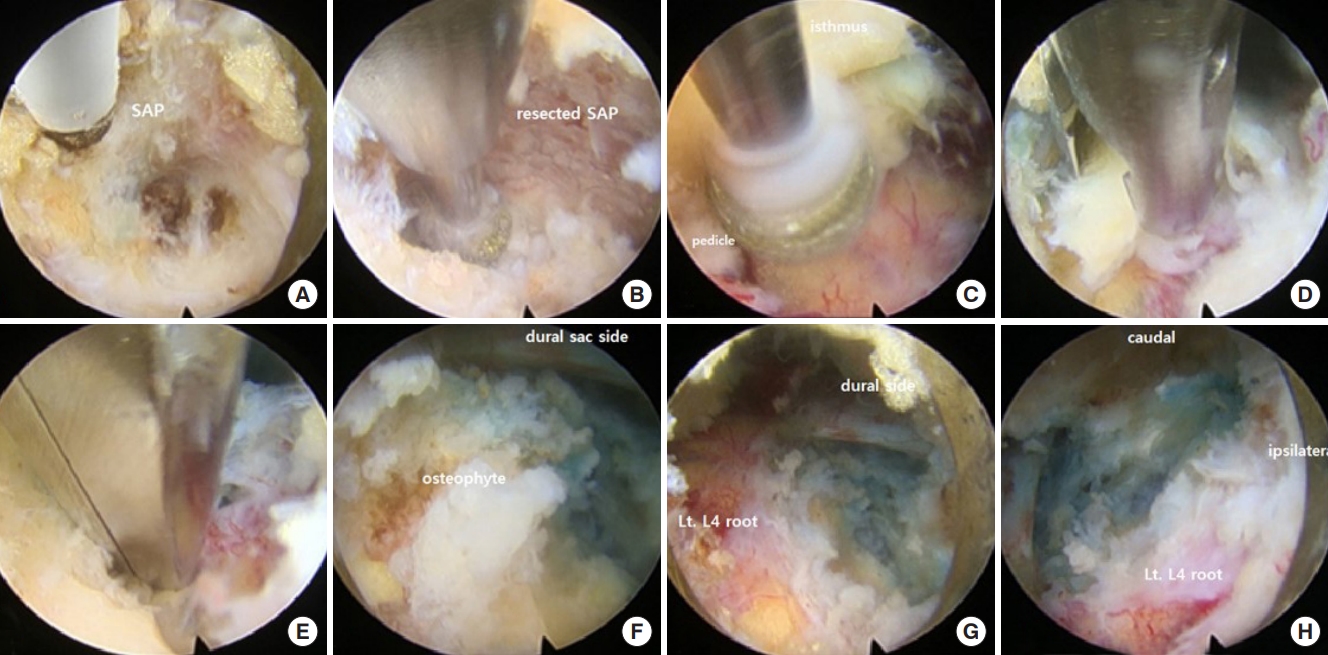
Endoscopic view of surgical procedure (TELF with fragmentectomy for left foraminal stenosis at the L4/L5 level). (A) Placement of a bevel-ended working sheath on the left L5 base of the superior articular process (SAP). (B) Bone work on the SAP using an endoscopic burr. (C) Bone work on the isthmus, lower part of the upper pedicle, and selectively on the lower body upper endplate. (D, E) Removal of the ligamentum flavum and foraminal ligament to expose the exiting nerve root (ENR). (F) Selective removal of the protruding disc and osteophyte after retracting the ENR. (G, H) Exposure of the axillary side of the dural sac and decompression of the ENR from medial to lateral. SAP, Superior articular process; Lt., left.
(a) TELF without discectomy: Exposure of the ENR has been confirmed by sufficient bone resection and soft tissue removal such as ligamentum flavum, foraminal ligament, and fatty tissue. And then, the ENR was retracted to remove the protruding disc and osteophyte, if deemed necessary by the preoperative imaging evaluation. (b) TELF with discectomy: After the (a) procedure, interbody disc removal was performed.
7. Illustrative Case
A 62-year-old male patient presented with left leg radiating pain from the lateral thigh down to the anterolateral leg, with no history of previous back surgery. Preoperative MRI revealed narrowing of the left L3/4 intervertebral foramen (Fig. 3A), preoperative x-ray showed increased CCA and decreased total and SLA, and disc wedging to the lesion side (positive DWA) with overall DH reduction at L3/4 (Fig. 3D, E). Postoperative MRI showed dilatation of the L3/4 foramen (Fig. 3B), but approximately 6 months later, due to recurrent left lower extremity radicular pain, an MRI scan was performed and the L3/4 foramen was found to be narrowed again (Fig. 3C).

An illustrative case of 62-year-old male patient. (A) Preoperative magnetic resonance imaging (MRI) showing foraminal stenosis at the L3/L4 level (arrow). (B) Postoperative MRI illustrating foraminal decompression following resection of the superior articular process, lower endplate, removal of ligamentum flavum, protruding disc, bony spur, and interbody disc (arrow). (C) Postoperative 6-month MRI revealing recurred foraminal restenosis at the L3/L4 level (arrow). (D, E) Preoperative simple radiograph demonstrating increased coronal Cobb angle, decreased lumbar lordotic angle (total lumbar lordotic angle, segmental lordotic angle), and increased disc wedging angle on the lesion side. (F, G) Intraoperative endoscopic view presenting the decompressed exiting nerve root.
RESULTS
1. Patient Characteristics
A total of 56 patients were included in this case-control study, comprising 18 cases and 38 controls. The average age was 68.9 years for cases and 65.6 years for controls, with no significant difference in age (p=0.3384) or sex distribution (p=1.000) between the 2 groups. In the case group, the most common surgical level was L4–5 and L5–S1 and followed by L3–4. While in the control group, the most common surgical level was L5–S1, followed by L4–5 and L3–4. There was no significant difference in the presence of adjacent segment disease or grade I spondylolisthesis between the groups. Preoperative and postoperative VAS scores was comparable between the 2 groups. However, VAS scores at 6 months follow-up were significantly higher in cases compared to controls (5.8±1.1 vs. 2.2±0.9, p<0.001). The follow-up period was longer for cases than controls (22.3±6.8 months vs. 17.2±3.5 months, p=0.007). The mean time to recurrence for cases was 7.4±2.4 months. Complications were rare, with only 1 case each of dysesthesia and motor weakness. In the control group, dysesthesia occurred in 2 patients, and no motor weakness was reported. Other than that, there were no other major complications. These findings are summarized in Table 1.
2. Radiologic Parameters
Baseline radiologic parameters were compared between case and control groups (Table 2). There were no significant differences between the groups in DH, FH, total lumbar lordosis angle and dynamic segmental angle. However, cases had a higher degree of DWA (3.0°±1.1° vs. 0.5°±1.4°, p<0.001) and larger CCA (8.8°±5.1° vs. 4.7°±2.5°, p=0.004) than controls. SLA was significantly smaller in cases compared to controls (11.0°±7.4° vs. 18.0°±5.4°, p=0.001) (Fig. 4). The cutoff values for the radiologic parameters significantly associated with restenosis, which yielded the best sensitivity and specificity, were identified using R statistical software, and are presented in Table 3. For the DWA, a cutoff of 1.8° resulted in sensitivity and specificity of 94.4% and 92.1% respectively, with an AUC of 0.969. The CCA had a cutoff value of 7.9°, yielding a sensitivity of 55.6% and specificity of 92.1%, with an AUC of 0.757. For the SLA, a cutoff value of 17.1° provided a sensitivity of 83.3% and a specificity of 60.5%, with an AUC of 0.775.
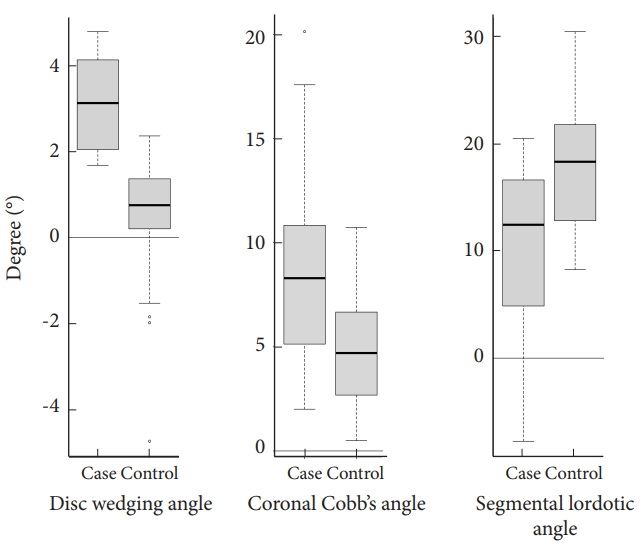
Boxplot illustrating the distribution of disc wedging angle, coronal Cobb angle, and segmental lordotic angle by groups.
3. Surgical Types
Table 4 shows the comparison of surgical types between case and control groups in detail. In the case group, the distribution of surgical types was as follows: TELF without discectomy (12 patients, 66.7%) and TELF with discectomy (6 patients, 33.3%). In the control group, the distribution was: TELF without discectomy (36 patients, 94.8%), TELF with discectomy (2 patients, 5.2%).
The case group had a higher rate of patients undergoing discectomy in addition to TELF rather than the control group. In the control group, most patients underwent TELF without discectomy. With an odds ratio of 0.12, not performing discectomy was found to be more favorable for the development of restenosis. This difference was statistically significant (p=0.017), indicating an association between surgical types and the risk of restenosis after endoscopic foraminotomy for LFS.
DISCUSSION
Apart from foraminal lesions, simple central stenosis, disc herniation, and other lesions without accompanying instability can typically be treated initially using nonfusion techniques. Unstable LFS with segmental instability, however, is commonly addressed with fusion techniques as the gold standard. In contrast, stable LFS without segmental instability has traditionally been treated with fusion techniques, which are the primary target subset in minimally invasive spine surgery to be replaced by nonfusion surgery. Consequently, numerous authors have sought to perform foraminotomy without fusion using both microscopic and endoscopic methods, achieving significant results. Longestablished microscopic approaches have been introduced by Ozeki et al. [14] and Park et al. [15]. Now, with the development of equipment such as endoscopic drills, lasers, and radiofrequency probes, the applicability and therapeutic effects of full endoscopic foraminotomy have been demonstrated by various authors [12,16-21].
Foraminotomy for LFS offers the benefits of motion preservation and reduced concern about adjacent segment degeneration, but it also appears to have disadvantages compared to fusion. According to the review of Ju and Lee [22]. for complications following foraminotomy, the primary complications include recurrent stenosis with incidental durotomy, motor weakness, and dysesthesia. Technical issues, such as the surgeon’s level of experience, may cause complications other than recurrent stenosis, but recurrent stenosis can occur even with complete nerve decompression achieved during surgery. This complication should be treated as a problem inherent in the surgical method called FELF, relating to its reliability and durability. It has been a significant concern for endoscopic surgeons due to limitations like the inability to entirely replace fusion surgery in cases of restenosis, resulting in the need for additional revision fusion surgery. Thus, identifying the risk factors for restenosis is a crucial first step in refining FELF into a more reliable and robust surgical technique.
In our study, preoperative baseline radiologic parameters indicated that DH was lower and FH was higher in the case group compared to the control group, DWA and CCA at the surgical level were angled towards the lesion, total lumbar lordosis angle and SLA were reduced, and dynamic segmental angle was high. Among these parameters, DWA, CCA, and SLA were found to be statistically significant risk factors, with DWA being the strongest. Additionally, SLA showed a more significant difference between the groups than total lumbar lordosis angle, suggesting that local alignment deserves more attention than global alignment. Data published by Haimoto et al. [23] studying risk factors for restenosis after microscopic foraminotomy reported similar findings, with DWA being the most statistically significant risk factor. Some differences in statistical significance between the 2 studies may be due to the difference in power related to the number of samples. The data of Yamada et al. [24] on recurrence after microscopic decompression for LFS identified degenerative lumbar scoliosis as a significant risk factor, suggesting that coronal alignment significantly affects restenosis. These findings are similar to those of this study. However, we found no other significant differences in risk factors attributable to technical differences between microscopic and endoscopic techniques for LFS.
When performing TELF, the rationale for conducting additional discectomy in the interbody space alongside TELF for protruded discs is to prevent the potential risk of postoperative disc material leakage and symptom recurrence. However, our data showed that the rate of TELF without discectomy in the control group was significantly higher, and the rate of TELF with discectomy in the case group was higher than the control group, with an odds ratio of 0.12, with a statistically significant difference. This result suggests that additional discectomy of the interbody space, which has been performed based on surgeon preference to prevent postoperative recurrence of foraminal disc herniation, is a risk factor for recurrence. While preemptively performing discectomy can remove the nucleus pulposus that may herniate later, it can also lower the DH on the ipsilateral side. Therefore, from the pathophysiological perspective of foraminal stenosis, it may develop the vertical or circumferential stenosis and cause symptoms to recur [1]. As a result, it is essential to achieve neural decompression through proper bone work, soft tissue removal, and careful resection of protruding disc and bony spur. However, it is recommended to avoid performing discectomy in the interbody space.
This study is a retrospective case-control investigation with a limited sample size, which implies that the evidence is relatively weak. To obtain stronger evidence, it would be essential to validate these findings in a larger prospective study.
CONCLUSION
The study findings indicate that greater DWA toward the lesion, larger CCA, smaller SLA as a baseline radiologic parameter, and the inclusion of discectomy as part of the surgical procedure may be associated with a higher likelihood of restenosis after FELF for LFS. As a result, it is recommended that surgeons exercise caution when planning FELF surgery, taking care to avoid unnecessary discectomy during the procedure. By adopting these measures, it is anticipated that the risk of restenosis can be minimized, thereby optimizing patient outcomes, and reducing the need for further interventions.
Notes
Conflict of Interest
The authors declare no conflicts of interest.
Funding/Support
This study was supported by research fund from Chosun University (2021).
Author Contribution
Conceptualization: JHS, CIJ, SWK, SML; Data curation: JHS; Formal analysis: PK; Funding acquisition: PK; Methodology: CIJ, SWK, SML; Project administration: SML; Visualization: CIJ, PK; Writing - original draft: JHS, PK; Writing - review & editing: JHS.