Comparative Efficacy of Clinical Interventions for Sacroiliac Joint Pain: Systematic Review and Network Meta-analysis With Preliminary Design of Treatment Algorithm
Article information
Abstract
Objective
We aimed to identify the most effective clinical treatment method for sacroiliac joint (SIJ)-related pain based on the systematic review and network meta-analysis (NMA) to evaluate the comparative efficacy of clinical interventions for sacroiliac joint pain by pooling the randomized controlled trials (RCTs).
Methods
Our team conducted a systematic review and NMA of RCTs to determine the most effective clinical treatment for SIJ-related pain. We searched the PubMed (MEDLINE), Web of Science, Cochrane Library, and Scopus databases for RCTs until February 2023. The PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines were followed. Pairwise and network meta-analyses were conducted using a random effects model.
Results
Based on the search strategy and inclusion criteria, our systematic review and NMA included 9 randomized studies with 652 participants. Research has mainly focused on various radiofrequency sources, but their number is still low. In the network analysis, according to the NMA and mean ranking probabilities for the improvement of pain intensity (PI) and quality of life (QoL), sacroiliac joint fusion and cooled radiofrequency were associated with high treatment rank for improving PI and QoL in patients with sacroiliac joint pain.
Conclusion
This NMA suggest that SIJ fusion and cooled radiofrequency could be potential options for improving the QoL and relieving pain in patients with SIJ-related pain. Comparison studies of outcomes between these 2 procedures with solid methodology and a low risk of bias would be very beneficial to identify the optimal treatment option for this challenging disease.
INTRODUCTION
Sacroiliac joint (SIJ) pain is a common etiology of chronic low back pain worldwide, with an approximate prevalence of 10%–33% [1-3]. The joint acts as a solid bridge connecting the lumbosacral spine and the ilium while transferring the load from the axial spine to both lower extremities. Due to its complex morphology, various innervation patterns, and overlapping clinical findings from various sources of possible pain generators around the lumbosacral spine and hip girdles, decisions regarding diagnosis and treatments are very challenging.
In general, the clinical manifestations of SIJ pain vary. Possible regions of aching pain are the lower back (up to 38%) [4,5], followed by the pelvis or buttock, hip or groin, thighs, or rarely the lower legs [6]. A history of sitting intolerance, the number of child deliveries, trauma, previous treatments, and back surgery are also important factors associated with SIJ pain. SIJ pain may be strongly correlated with SIJ pathologies, such as SIJ instability or adjacent segment disease [7,8]. Many free-ending nerve fibers and mechanoreceptors from the SIJ joint have been observed [9,10], and high levels of dermatome in the SIJ can also make the clinical presentation of pain uncertain. This could also cause SIJ pain to be underdiagnosed [11], thus leading to premature bias in treatment decision-making [4].
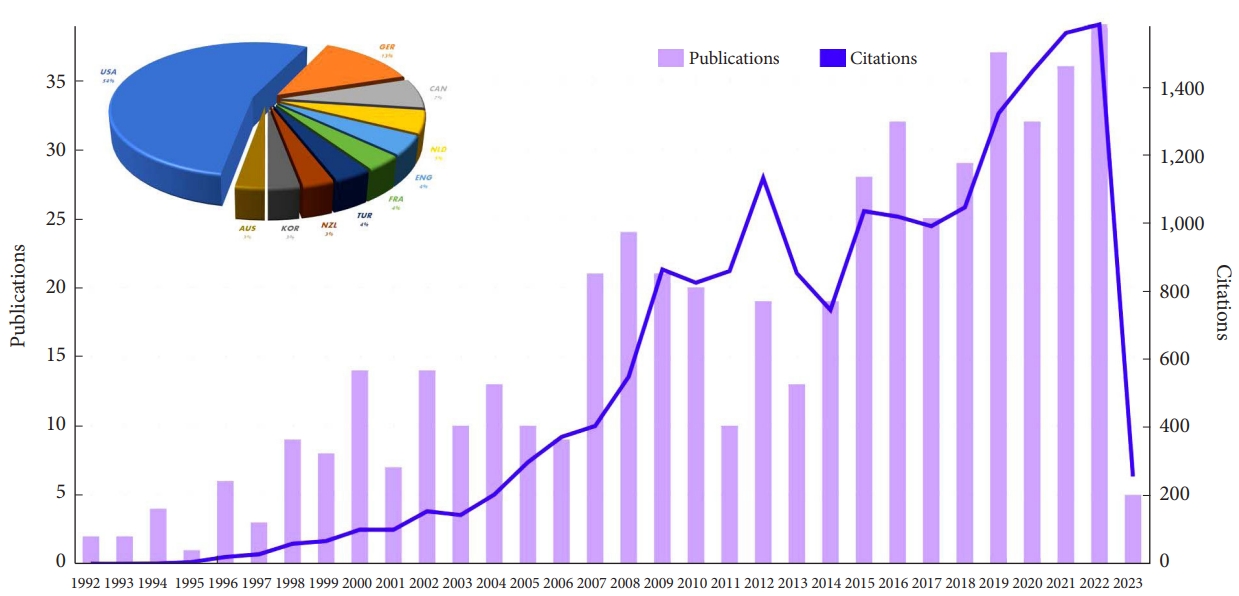
In recent years, there has been a growing interest in research related to SIJ interventions, driven by the increasing prevalence of SIJ pain and the continuous search for effective treatments. Advancements in diagnostic techniques and novel therapeutic approaches have contributed to a better understanding of the SIJ’s complex nature and its related pain. Consequently, a surge in scientific publications has been observed, reflecting the expanding body of knowledge on SIJ interventions and their potential benefits (Fig. 1). Against this background, this study aims to present a systematic review and network meta-analysis (NMA) to assess the comparative efficacy of various types of current clinical interventions for treating SIJ pain.
MATERIALS AND METHODS
1. Search Strategy and Data Extraction
This study was reported in accordance with the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines [12]. The study protocol for this systematic review and meta-analysis was registered on the PROSPERO (International Prospective Register of Systematic Reviews; No. CRD42 023401777). For nonhuman interventional research, ethical approval and informed consent are not needed. We searched the PubMed (MEDLINE), Cochrane Library, Scopus, and Web of Science electronic databases from inception to 2 February 2023. The search strategy for each database is shown in the Supplementary Material. We also manually searched for published, preprint RCTs. In accordance with the Cochrane Handbook for Systematic Reviews of Interventions, 2 independent investigators extracted the demographic (author, publication year, followup time) and intervention data (surgical technique, measurement metrics).
We included RCTs comparing various clinical interventions for treatment without restrictions regarding age, sex, or race. The primary diagnosis of SIJ pain should be performed by the standard clinical operationalized method. According to the predefined categories, the treatment methods were grouped into different homogeneous groups. Among them, various details of treatment methods were examined because the purpose of our research was to focus on technique differences. There were also no restrictions regarding the threshold ranges of the demographic baseline, the minimum number of participants, and the technology device used. YT and SS independently extracted data to Excel (Microsoft Corp., Redmond, WA, USA, 2018. Microsoft Excel) using a structured and standardized form. In addition to outcomes, information on a vast array of clinical and methodological trial characteristics were extracted, as described in the protocol. In cases of discrepancies among the evaluators concerning the extracted data, a third reviewer (JSK) was consulted to achieve a consensus. The following data were extracted from eligible studies: the author’s name, publication date, study design, age, sample size, follow-up duration, intervention measures, and outcome indicators.
2. Risk of Bias Assessment and Outcome Indicators
Two reviewers (YT and SS) independently assessed the risk of bias (RoB) of included studies using the revised tool to assess RoB 2 tool) in randomized trials. A visualization tool (Robvis) was used for visualizing risk of bias assessments in our systematic review and NMA [13]. The RoB 2 tool comprises 5 domains for assessing the RoB. We included 2 parameters in our NMA model: pain intensity (PI; visual analogue scale or Numerical Rating Scale) and quality of life (QoL; Oswestry Disability Index [ODI], EuroQoL-5 dimension, or 36-item Short Form health survey). For these indicators, a treatment hierarchy was estimated by the surface under the cumulative ranking curve (SUCRA) analysis, which ranks the order of superiority of each clinical intervention based on the probability values compared against others [14,15].
3. Data Synthesis and Statistical Analysis
First, we planned to perform pairwise meta-analyses using a fixed or random effects model for direct comparisons with at least 2 studies based on the heterogeneity test results. The test for heterogeneity was performed using a standard chi-square and I2 statistic. All results associated with the 95% confidence interval (CI) of the pairwise meta-analysis and heterogeneity estimates are presented in the Supplementary Material. According to the Cochrane Handbook, the range of I2 values indicating substantial heterogeneity higher than 75% [16].
Second, we performed the NMA in Stata with the “network” and “mvmeta” packages [17,18], the analytic process based on the random effects model frequentist framework, to synthesize the results reported by the RCTs [19]. This program assumes that all included treatment contrasts have the same heterogeneity variance. Then, we defined our research characteristics as indirect treatment comparison or mixed treatment comparison based on the loop results of network geometry [20]. If a study involves different arms with a minor difference in the clinical intervention, the similar arms will be merged with the single arm. For all missing data, we will contact the original author to support the data. Otherwise, the appropriate statistical methods will be applied to estimate the blank. In the NMA, a random effects model was considered to estimate and pool the heterogeneous outcomes. We strictly limited the methodology based on our inclusion criteria and exclusion criteria (Supplementary Material).
To ensure the validation of the overall effect size, a series of prior statistical assumptions were conducted to verify the consistency of the NMA model. The network geometry was illustrated by drawing a network plot to intuitively visualize the connection characteristics between the included studies [21]. Then, inconsistency was further statistically tested in each treatment with the node-splitting method. Then, we estimated the effect size and uncertainties in pairwise comparisons included in our NWA by using the confidence interval and predictive intervals considering the heterogeneity level to better assess the proportions of contribution to each study’s indirect and direct comparison. The consistency is statistically examined to ensure no significant statistical discrepancy of outcomes between the direct and indirect evidence from different treatment effects in NWA by using hypothesis logical inference that allows for inconsistency [22]. In addition, we formally assessed the publication bias in NMA by the asymmetry of the comparison-adjusted funnel plot to detect the potential small study size effect [23]. We constructed a contribution plot using weighted squares based on the effect sizes with variances to represent the different pieces of evidence within the NMA. Moreover, we evaluated the inconsistency and uncertainties separately in each closed loop of networks of interventions using the method of moments estimator [19]. Finally, the treatment hierarchy was drawn under the cumulative ranking (SUCRA) to illustrate the probability ranking of interventions. Statistical analysis and graph construction were performed using the network packages in Stata using Stata/MP (StataCorp., 2021. Stata Statistical Software: Release 17. College Station, TX, USA: StataCorp LLC).
RESULTS
1. Study Characteristics
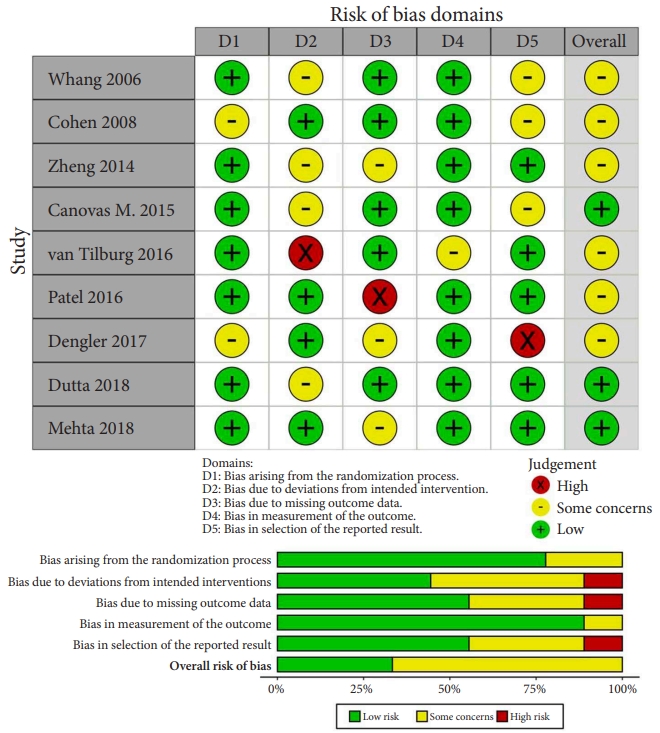
The process of evaluating studies according to the inclusion and exclusion criteria is illustrated in Fig. 2. After screening the abstracts and reviewing the complete texts, 9 RCTs with 652 patients were ultimately included in the NMA [24-32]. As shown in Table 1, a total of 6 different clinical interventions were reported: conservative therapy, cooled radiofrequency (CRF), thermal radiofrequency (TRF), pulsed radiofrequency (PRF), intra-articular injection (IJ), and SIJ fusion. The most common groups were the SIJ fusion (23.47%) and IJ group (23.62%), followed by TRF (23.31%), CRF (9.05%), and PRF (2.30%). The mean age of all participants was 49.88 years. Overall, 66.7% of the included studies were reported in the United States or Europe from 2006 to 2018. Six studies were considered to have a moderate level of bias. Three studies were considered to have a high risk of missing data due to the large proportion of patients who were lost to follow-up as well as bias in the selection of the reported results due to an oversized effect size, which was induced by support from the device manufacturer. The most common domain in the RoB tool was deviations from the intended interventions, which was due to concealed treatment allocation (Fig. 3). Of the 9 studies, only one provided data for one clinical outcome parameter. For the standard pairwise meta-analyses in NMA, the assessment results of heterogeneity and inconsistency are reported in the Supplementary Material.
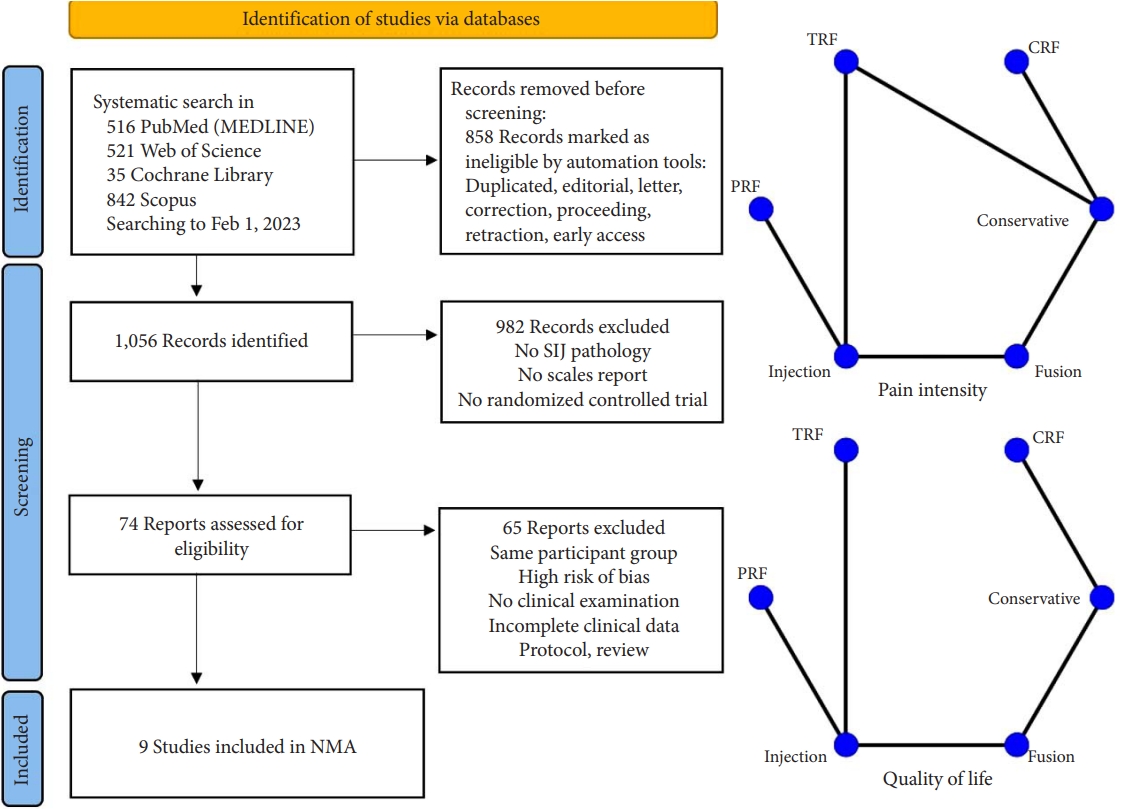
Flowchart diagram illustrating the articles included in the bibliometric analysis. Network geometry of the network meta-analysis (NMA). TRF, thermal radiofrequency; CRF, cooled radiofrequency; PRF, pulsed radiofrequency; SIJ, sacroiliac joint.

Overview of clinical trials on sacroiliac joint pain interventions: comparative study design, outcomes, and results
2. Pairwise Meta-analysis
In the pairwise meta-analysis of PI, a significant treatment difference, as measured by the VAS, was reported for CRF versus conservative (-2.95; 95% CI, 1.53–4.37; p=0.085; I2=59.4%) and for TRF versus IJ (-1.85; 95% CI, -2.88 to 0.83; p=0.116; I2=59.6%). The results indicated that CRF is superior to conservative treatment and that TRF is superior to IJ for SIJ pain relief. On the other hand, in the pairwise meta-analysis of QoL, a significant treatment difference, as measured by the QoL scores, was observed for conservative versus CRF (0.95; 95% CI, 1.20–0.70; p=0.133; I2=50.4%). The results showed that CRF is superior to conservative treatment for improving QoL. More data are needed to for additional pairwise comparisons. The contribution distribution from each direct pairwise study in the mixed and indirect models was estimated by calculating the effect size and variances in the contribution plot (Fig. 4).
3. NMA and Ranking Probabilities
The head-to-head comparison results of the PI and QoL for each intervention for SIJ pathology are shown in the net league (Fig. 5). In terms of PI (9 RCTs, 628 participants), the top clinical intervention for SIJ pain was SIJ fusion (-3.29; 95% CI, -4.83 to 1.74; p≤ 0.001), followed by CRF (-2.94; 95% CI, -4.26-1.63; p ≤ 0.001), PRF (-0.82; 95% CI, -3.36 to 1.73; p = 0.529), TRF (-0.24; 95% CI, -1.80 to 1.32; p=0.763), IJ (1.32; 95% CI, -0.40 to 3.03; p=0.132). The results of SIJ fusion and CRF showed a significant decrease in PI scores compared to conservative therapy. There was no other statistical evidence among any other 3 clinical interventions. In terms of QoL (8 RCTs, 568 participants), the top clinical intervention was SIJ fusion (5.54; 95% CI, 3.13–7.96; p≤ 0.001), followed by CRF (2.36; 95% CI, 0.93–3.79; p=0.001), TRF (4.26; 95% CI, 0.08–8.43; p=0.046), PRF (1.34; 95% CI, -2.93 to 5.60; p = 0.539), IJ (-1.92; 95% CI, 3.13–7.96; p = 0.272). Additionally, we depicted the comparative efficacy of different clinical interventions from each individual study for treating SIJ pain (Fig. 6). The results suggested that SIJ fusion, CRF, and TRF led to significant statistical improvement in QoL compared to conservative therapy. There was no statistical evidence among any other 2 clinical interventions compared to conservative treatment.
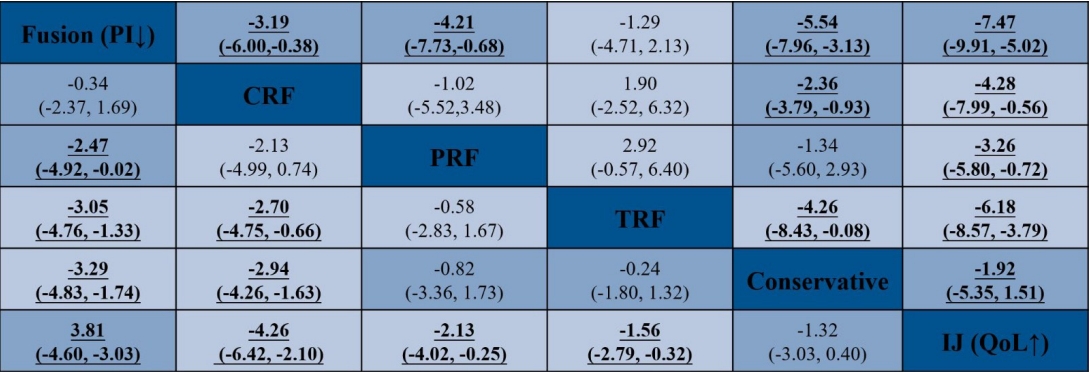
Network meta-analysis net league for pain intensity (PI) and quality of life (QoL). Numbers underlined represent statistically significant results. CRF, cooled radiofrequency; PRF, pulsed radiofrequency; TRF, thermal radiofrequency; IJ, injection.
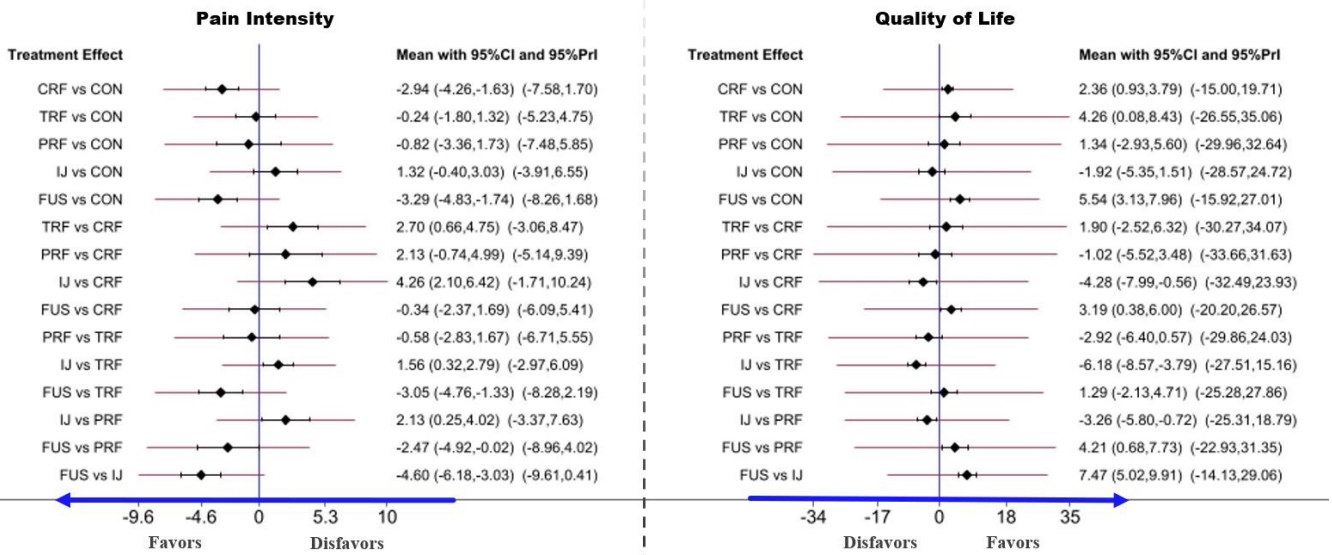
Forest plot of comparisons in pain intensity (PI) and quality of life (QoL) to estimate the effect size and uncertainty. The red horizontal lines represent the predictive confidence interval (CI). CRF, cooled radiofrequency; TRF, thermal radiofrequency; PRF, pulsed radiofrequency; IJ, injection; FUS, fusion; 95% Prl, 95% predictive interval.
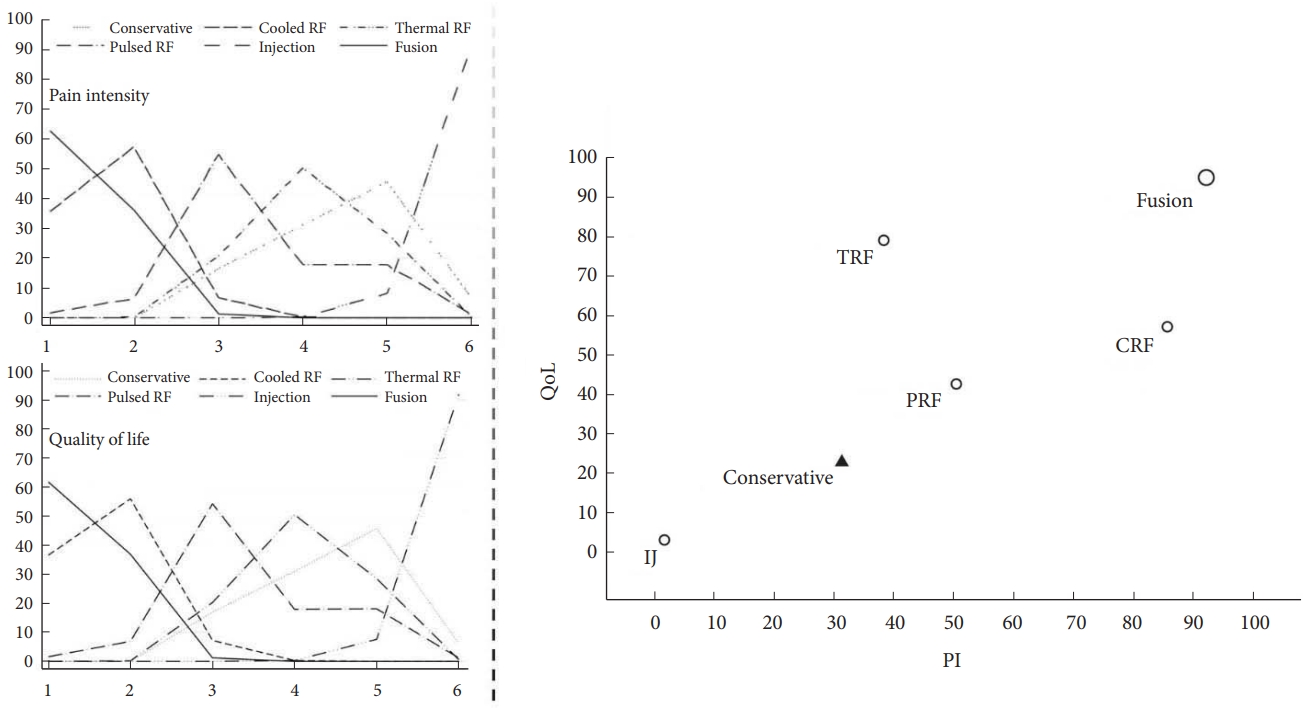
According to the ranking probabilities and SUCRA for improving PI and QoL, the probabilities efficacious of treatment rank are illustrated in Fig. 7. Based on our analysis, SIJ fusion had the highest probability (SUCRA, PI: 92.2%, QoL: 95%) of being the most efficacious procedure for relieving SIJ pain and improving QoL, followed by CRF (SUCRA, PI: 85.7%, QoL: 57.2%), TRF (SUCRA, PI: 38.4%, QoL: 79.1%), and PRF (SUCRA, PI: 50.5%, QoL: 42.7%). In contrast, IJ (SUCRA, PI: 1.8%, QoL: 3.2%) was the least efficacious compared to conservative therapy; this difference was not significant.
4. Consistency Test and Heterogeneity Analysis
According to our statistical analysis, the design-by-treatment interaction inconsistency model is fit with no evidence for inconsistency in PI and QoL using the restricted maximum likelihood. In the pairwise meta-analysis of PI and QoL, the pooled effect size showed no heterogeneity and no evidence of intraloop inconsistency in PI (I2=1.30, p = 0.254; loop, IF = 1.954, p=0.014, τ2=0) (Supplementary Material). However, the ODIs do not have a reticulated loop structure that cannot perform loop-specific tests. Therefore, heterogeneity arises in the test of inconsistency. To determine whether heterogeneity was due to external or internal models, node-splitting was performed to determine whether heterogeneity still existed between the included studies. The results of node-splitting showed no statistical inconsistency in estimating the effect size between the direct and indirect intervention analyses. Furthermore, there was no evidence of any potential small study effect bias by inspecting the comparison-adjusted symmetrical funnel plots of PI and QoL (Supplementary Material).
DISCUSSION
Our research advances a structured, stepped treatment algorithm for SIJ pain management, recommending CRF as the primary intervention owing to its minimally invasive character. This comprehensive approach provides an effective roadmap for handling SIJ pain, thereby improving patient outcomes. It underscores CRF’s advantage in minimizing surgical trauma linked to more invasive procedures like open SIJ fusion, hence permitting clinicians to alleviate pain while mitigating patient risk and discomfort. Our study also highlights the effectiveness of SIJ fusion, CRF, and even the older technique of TRF in pain relief and enhancing QoL, implying their suitability as alternatives to conservative treatments. These findings could assist the development of health policy and guidelines. Furthermore, our results support the ongoing healthcare trend towards less invasive procedures and emphasize the need for personalized patient care, considering factors such as comorbidities, specific pain patterns, and patient preferences.
In our study, we implemented both pairwise meta-analyses and NMA. The pairwise meta-analyses were used to compare the effectiveness of 2 interventions at once, whereas NMAs provided an avenue for us to compare multiple interventions concurrently. This is especially advantageous when direct evidence from RCTs is not sufficiently robust. We adopted the surface under the cumulative ranking curve as a measure to rank the efficacy of each intervention. Further, we assessed the consistency and heterogeneity among the studies integrated into the meta-analysis. The term consistency in our context relates to the agreement level between direct and indirect sources of evidence within the network. Heterogeneity, on the other hand, pertains to the variability observed in the outcomes of the studies. The results we found show that SIJ fusion, CRF, and TRF are the preferred operations to improve the patient’s postintervention parameters in the PI or QoL evaluation scales. Among them, SIJ fusion seemed to be the best choice compared to other interventions. Generally, the treatment of SIJ pain can be divided into nonsurgical and surgical management. Nonsurgical management (NSM), such as medication and physical therapy, is usually considered the first line of treatment [33-35]. However, if those conservative measures fail, nonsurgical interventions will come into play [36]. SIJ injection is the standard procedure to enhance the accuracy of the diagnosis of SIJ pain. Several physician societies have recommended the positive threshold to be 50% to 75% [37-39]. Local anesthetic is used as a diagnostic agent, but the appropriate amount of volume is still under debate [40]. Corticosteroids can also be added to facilitate the combined therapeutic effect [41]. Although several studies have demonstrated promising results in pain control after corticosteroid injection of the SIJ [42-44], others revealed its short-term benefit with poor longevity compared to other interventions [45,46].
Radiofrequency ablation (RFA) is usually the next step of NSM if the patients have already confirmed the diagnosis of SIJ pain and the effects of the initial SIJ injection have weaned out with relapsing pain. It is a minimally invasive procedure that aims to provide more longevity of pain control by destroying the nerve endings using an insulated needle [31,47,48]. PRF originates from the concept that voltage fluctuation will cause a strong magnetic field and could relieve pain more than direct tissue destruction by heat [49]. Therefore, the surrounding temperature could be controlled to avoid permanent nerve tissue damage (< 45°C) [50]. TRF uses the ‘palisade’ technique to place bipolar electrodes and facilitate long continuing thermal lesions along the course of several lateral branch nerves [27]. The recently developed CRF enhances larger lesions to ablate the nerve to be created. This is because the specialized probe has an internal cooling system that prevents the surrounding tissue from charring, allowing the lesion radius to increase by up to 3 times compared to TRF [51]. Many studies have reported comparable or better CRF outcomes than other NSM techniques [52-54]. Mild complications, such as soreness or numbness at the intervention site, were reported, which could be resolved entirely within 2 weeks [55,56]. Comparing the 3 RFA interventions, one systematic review and meta-analysis showed that CRF was more efficacious than TRF and PRF; however, the differences were not significant [48].
Recently, RFA has also been proven to preserve the surrounding muscles well. Oswald et al. [57] retrospectively reviewed symptomatic facet joint pain patients treated with RFA. They used magnetic resonance imaging analysis to compare the lumbar paraspinal musculature before and at least 6 months after the surgery. No fatty degeneration occurred on the operated side. Therefore, less iatrogenic damage to surrounding tissue after RFA could be expected. The findings from this study could be aligned well with the aim of minimally invasive procedures, as they could preserve the patient’s function while achieving better outcomes.
Fusion is the main objective of surgical management in patients with chronic back pain from the SIJ. It should be the last resort of options in cases of failed NSM or pain relapse. Those patients also needed to prove the cause of chronic low back pain to be from the SIJ in origin (e.g., relieving pain more than 50%–75% from previous SIJ injection) [33]. To summarize, we have proposed a comprehensive algorithm for diagnostic and treatment pathways for chronic low back pain, specifically for SIJ pathology (Fig. 8). Building on previous discussions pertaining to the established positive threshold for pain reduction following SIJ interventions as advocated by various medical associations [37-39], we have subsequently defined the efficacy of our algorithm to be 50%. Minimally invasive SIJ fusion was introduced using the iFuse Implant System (SI-BONE Inc., Santa Clara, CA, USA), followed by many afterward. Many studies have reported good results regarding using iFuse compared with other NSMs [58-60]. Polly et al. [58] reported 2-year outcomes from a multicenter RCT study comparing SIJ fusion using iFuse and NSM. The SIJ fusion group yielded more significant improvements in clinical parameters than the NSM group. Another RCT compared conservative management and SIJ fusion outcomes and reported similar results. They concluded that the iFuse implant system was safe and more effective [59]. However, complications, such as increased pain around the injection site, hematoma, deep wound infection, or nerve root impingement, are all possible up to 16.4% [60,61]. Shamrock et al. [62] also reported an overall complication rate of 11.1%, of which the most common are wound infection and nerve root impingement, with occurrence rates of 2% and 1.6%, respectively.
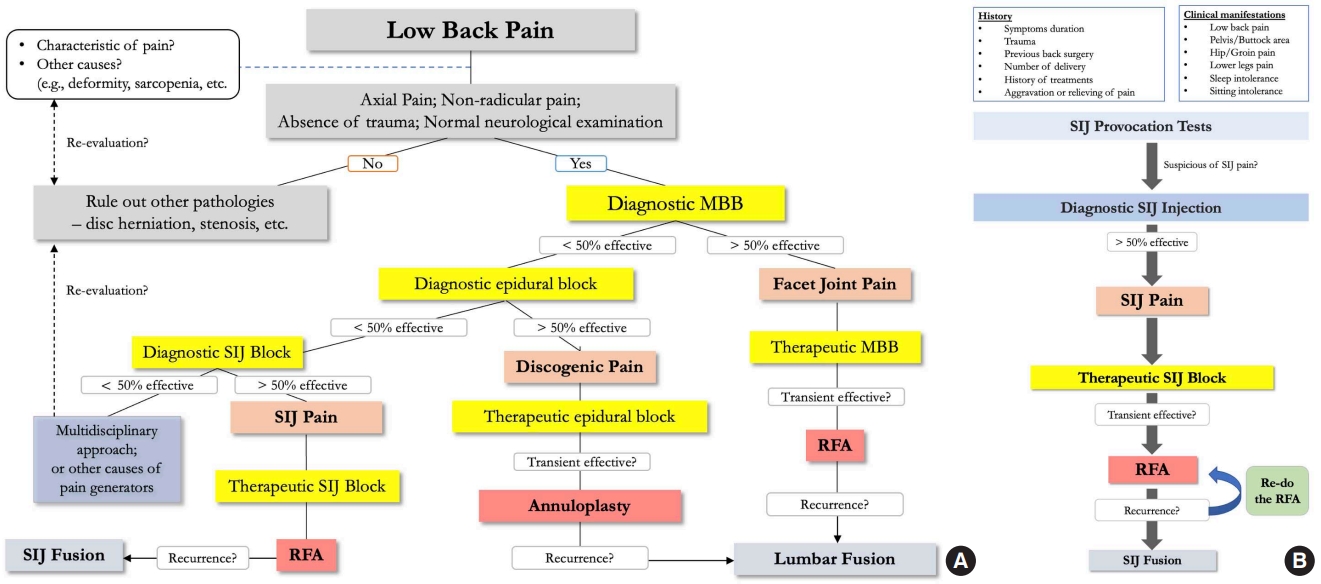
Algorithm of diagnosis and treatment for low back pain (A) and sacroiliac joint pain (B). MBB, medial branch block; SIJ, sacroiliac joint; RFA, radiofrequency ablation.
Previous systematic reviews and meta-analyses also revealed similar outcome results. Dengler et al. [63] conducted a pooled analysis of 2 multicenter RCTs and 1 single-arm prospective trial. They concluded that SIJ fusion leads to better treatment outcomes than NSM. Another systematic review and meta-analysis by Abbas et al. [64] showed that the standardized mean difference regarding SIJ fusion was better than that of conservative treatment at the 6-month follow-up. They concluded that SIJ fusion is a potential option in surgical management regarding SIJ pathology. However, the confidence level in the evidence presented in their review was mentioned as ‘very low.’ Moreover, 5 out of 6 studies included in their research were industry-funded. Therefore, there was a high risk of publication bias. This finding is also consistent with the quality assessment of the included papers performed in our study.
More concerns regarding SIJ fusion studies exist. Although most of them are RCTs, all of those studies conducted a comparison study between iFuse and NSM [58,59]. NSM consists of various kinds of treatment, ranging from medication or physical therapy to interventions such as SIJ injection or many types of RFA. They also have different levels of therapeutic effects [27,31,46-48,56]. Therefore, this may lead to information bias. Surprisingly, there is no direct comparison of the efficacy between these 2 procedures and their cost-effectiveness in previous literature. Future studies directed at comparisons between SIJ fusion and a specific kind of NSM, such as RFA (with or without endoscope assistance), as well as its cost-effectiveness, will fill this enormous knowledge gap.
Recently, endoscopic spine surgery (ESS) has evolved rapidly in the last decade and has successfully proven its use in many spinal procedures, such as discectomy, spinal canal decompression, or interbody fusion [65-68]. The use in more complicated spine diseases, for example, spinal infection or malignancy [69,70], was also reported. ESS has also proven good results in assisting neuroablation surgery in patients diagnosed with facet joint pain [71-73]. However, its use in patients with SIJ pain was initially reported by Choi et al. [74]. It yielded good results with improved PI and QoL scales immediately and could be maintained at least 6 months after the surgery. The proposed advantages of the endoscope are better visualization of bony landmarks, as it could better identify the lateral branches of S1 to S3. Furthermore, it could locate the area that has already been ablated. This could prevent excessive damage to the surrounding soft tissue and lessen postoperative pain and dysesthesia risk [74]. Subsequent studies regarding endoscopic-assisted RFA, with or without the use of navigation guidance, also yielded good results with decent longevity after the procedure when treating SIJ pain in chronic low back pain patients [75-78].
For future research, it is recommended that more high-quality RCTs be conducted to further compare the effects of different clinical interventions on SIJ pain, especially the less common ones like PRF. Future studies could also focus on the long-term effects of these interventions. Additionally, studies could also explore the reasons behind the effectiveness of SIJ fusion and CRF to better understand their mechanisms and potentially improve their efficacy even further. It’s worth mentioning that the findings should be interpreted with caution due to the moderate level of bias identified in 6 of the studies included in this NMA. As a result, future research should strive to minimize bias, such as concealed treatment allocation, and should carefully account for potential confounding factors. Researchers should ensure to report all relevant results to avoid selection bias.
There are several limitations to our study. First, half of the included studies reported concern about the RoB due to unclear or intact allocation concealment that impacts the reliability of the pooled effect size of clinical intervention on SIJ pathology. Second, the consolidation of treatment arms with minor differences could potentially oversimplify interventional disparities, possibly masking subtle but clinically relevant nuances. Although we aimed to objectively classify ‘minor differences,’ the inherent subjectivity of this process could introduce bias. To counteract this, we ensured transparency by providing a detailed table outlining each study’s specific characteristics. Third, a notable limitation of our NMA is the absence of closed loops within the network map in the assessment of QoL. This gap potentially constrains the depth of our comparative findings, limiting our ability to robustly cross-validate the relative effectiveness of these interventions. Fourth, we only considered the efficacy of interventions for SIJ pathology based on clinical statistical scales. We could not extract the data from the current data with satisfaction as the binary outcome to estimate the acceptability of participants facing interventions. Fifth, our strategy for managing missing data had to resort to statistical imputation methods. We acknowledge that statistical imputation can facilitate more comprehensive analyses, it cannot completely substitute for actual data. The specific extent of this bias is often challenging to quantify due to the underlying assumptions made during the imputation process, which may or may not hold true. When data are missing completely at random, the potential for bias is reduced, but our study becomes less precise, leading to a larger uncertainty interval. Conversely, if missing data are not random but are linked to factors within our study, this can introduce bias. Moreover, the potential impact of missing data is more complicated due to the complex network structure and how it affects both direct and indirect comparisons. Therefore, we hope readers consider the potential impact of these data limitations when interpreting our findings. Additionally, the application of SIJ interventions may vary, leading to differences in surgical techniques and potentially increasing the heterogeneity of the results.
CONCLUSION
The systematic review and NMA suggested that for the consideration of clinical intervention methods, SIJ fusion and CRF could be the first 2 clinical options to relieve PI and improve QoL in patients who complained of SIJ pain. However, due to RoB, the current evidence cannot lead to a definite conclusion. Additionally, more RCTs, especially those comparing SIJ fusion and CRF, are recommended to identify the best treatment option for this long-standing challenging disease.
Supplementary Material
Supplementary Material can be found via https://doi.org/10.14245/ns.2346586.293.
Notes
Conflict of Interest
The corresponding author is a consultant of Richard Wolf, GmbH, and Elliquence, LLC. The other authors have no conflicts of interest to declare.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: JSK; Data curation: YL, SS; Formal analysis: YL, SS; Funding acquisition: JSK; Methodology: YL, SS; Writing - original draft: YL, SS; Writing - review & editing: YL, SS, JSK.