The Endoscopic Lumbar Interbody Fusion: A Narrative Review, and Future Perspective
Article information
Abstract
Lumbar interbody fusion stands as a preferred surgical solution for degenerative lumbar spine diseases. The procedure primarily aims to establish lumbar segment stability, directly addressing patient symptoms associated with spinal complications. Traditional open surgery, though effective, is linked with notable morbidities and extended recovery time. To mitigate these concerns, minimally invasive surgery (MIS) has garnered significant popularity, presenting an appealing alternative with numerous benefits such as reduced soft tissue trauma, decreased blood loss, and expedited recovery. Among MIS procedures, full endoscopic spinal surgery, characterized by its minimal invasiveness, holds the potential to further minimize morbidities while enhancing surgical outcomes. Endoscopic lumbar interbody fusion, a novel procedure within this paradigm, has gained attention for offering advantages comparable to those of minimally invasive transforaminal lumbar interbody fusion. However, the safety, efficacy, and associated surgical techniques and instrument design of this method continue to be subjects of ongoing debate. This paper critically reviews current evidence on the safety, efficacy, and advantages of endoscopic lumbar spinal interbody fusion, examining whether it could indeed supersede existing mainstream techniques.
INTRODUCTION
Spinal surgery is increasingly shifting towards minimally invasive spine surgery (MISS) techniques, thanks to their significant advantages in reducing surgical morbidities such as minimized soft tissue dissection, decreased bleeding, and faster recovery [1-9]. Minimally invasive discectomy surgery represents the initial step in MISS, which further extends to minimally invasive decompression in spinal stenosis. The progression towards MISS fusion is evident as more surgeons continue to harness the benefits of this valuable approach.
The full endoscopic spinal surgery was first introduced in 2005 by Ruetten et al. for lumbar disc herniation, leading to the development of instruments that resolved technical issues from the initial period [10,11]. As a result, full endoscopic spinal surgery has become widely accepted for lumbar disc herniation surgery due to its proven efficacy and safety. Recent years have seen an expansion in its use for lumbar spinal stenosis without instability, yielding good clinical results [5,11-14]. Studies have demonstrated improvements in postoperative canal surface, AP diameter, interfacet distance, canal surface area, lateral recess height, and lateral recess angle following this approach [15].
In cases of spinal instability, spinal fusion becomes inevitable. There are various approaches and techniques to achieve spinal fusion. Open laminectomy and instrumented fusion with or without an interbody cage are fundamental techniques for lumbar fusion. To minimize invasiveness, minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) with microscopic assistance has become increasingly popular due to its advantages over the open technique [16-18]. However, compared to the endoscopic technique, MIS with a tubular retractor requires a larger incision, induces more soft tissue injury, results in more blood loss, and necessitates a longer recovery time [19-23].
Driven by the continuous evolution of endoscopic spinal surgery and concerns about patient morbidity, endoscopic lumbar spinal fusion has seen significant development over the past decade. While spinal decompression and fusion remain the primary goals of all surgical techniques, many spine surgeons are seeking the least minimally invasive procedures to minimize the risks of morbidity associated with open techniques, especially in elderly patients. The emerging goal appears to be maximizing outcomes while minimizing morbidity [24]. The purpose of this review is to summarize the advantages and disadvantages of endoscopic lumbar interbody fusion (endo-LIF), drawing on current evidence and projecting future trends.
WHAT ABOUT “THE CURRENT AVAILABLE ENDOSCOPIC LUMBAR INTERBODY FUSION”?
Endoscopic transforaminal lumbar interbody fusion (endo-TLIF) has recently emerged as a prominent minimally invasive spine technique. Its rise to prominence is attributed to reduced postoperative morbidities, safety, effectiveness, and favorable outcomes, including comparable postoperative complication rates and fusion rates to other techniques [24-26]. There are three endoscopic trajectories commonly used to achieve interbody fusion: full endoscopic trans-Kambin lumbar interbody fusion, endo-TLIF (posterolateral approach) or biportal endoscopic lumbar interbody fusion, and endoscopic-assisted oblique lumbar interbody fusion. When comparing these endoscopic LIF trajectories to MIS-TLIF, there are theoretical variations in the working area, size of the surgical corridor, incision size, involvement of facet joints, and extent of soft tissue dissection, as depicted in Figs. 1–3. These factors contribute to the differences in advantages and disadvantages, which are detailed in Table 1, along with indications for selection in each patient’s operation (Table 2). The endoscopic system was initially used for discectomy procedures. However, as new techniques and advanced instruments continued to be developed, the surgical indications for endoscopy have expanded [10,14].
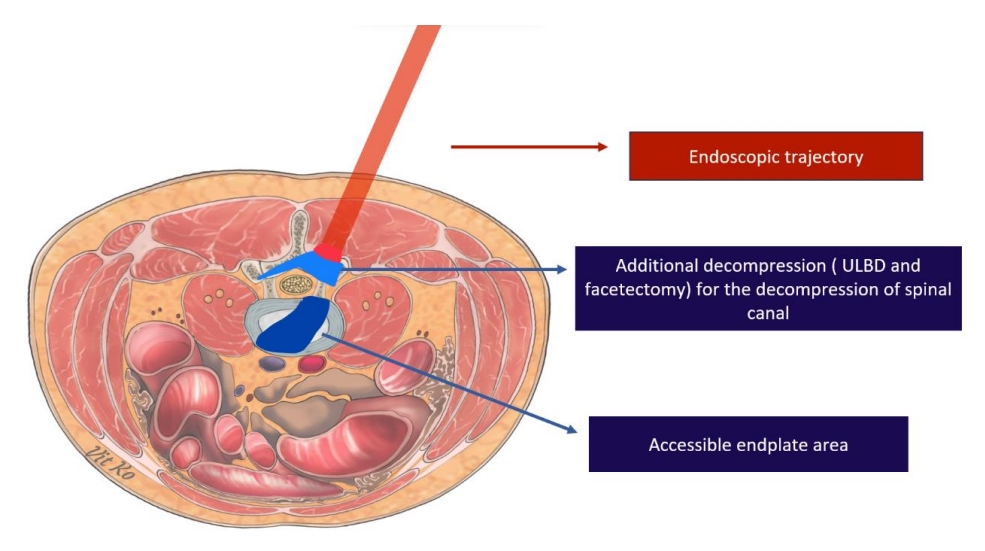
Trajectory of endoscopic lumbar interbody fusion approach by biportal endoscopic technique. ULBD, unilateral laminotomy for bilateral decompression.
The advent of endoscopic decompression has played a significant role in increasing its use in spinal decompression. More recently, the use of the endoscopic system has been extended to spinal fusion. Jacquot and Gastambide [27] first described the interbody fusion technique with a titanium cage via a transforaminal approach (Kambin triangle) in 2013. Yet, the postoperative complication rate was unfavorably high, up to 36%, including complications like paresthesia, radicular pain, cage migration, and screw migration. Owing to these complications, the author did not recommend proceeding without decisive technical improvement. Subsequently, improved techniques were continually published to avoid these complications and improve surgical safety [20,28-30]. There are investigators who described an innovative technique in cadaver models, which mitigated complications using a newly oriented superior articular process resection device, a parallel expandable cage, and an improved working channel [31]. One study introduced a new design for the endo-TLIF system, including a 2-part expandable tube (rigid C-shaped and flexible baffle), to prevent the risk of exiting nerve root injury, along with an expandable cage selection [32]. Another author developed a novel full endoscopic lumbar interbody fusion via an interlaminar technique with a new implantation cannula [33].
They reported better immediate postoperative clinical outcomes in the endoscopic group, with no significant difference in complication rates. Wang et al. [20] conducted a study to validate the feasibility of the endo-TLIF technique without the need for general anesthesia. Instead, conscious sedation was employed, enabling rapid recovery while ensuring live neurological monitoring by the patient. Moreover, they observed a reduction in postoperative pain scores, decreased side effects associated with general anesthesia, and clinical outcomes surpassing the minimum clinically important difference (MCID). The case series comprised ten patients who were promptly discharged from the hospital, with an average hospital stay of only 1.4 ± 1.3 nights and no reported complications. Another study also reported favorable results with awake spinal fusion using the endoscopic technique, which resulted in improved postoperative pain scores, reduced opioid requirements, shorter hospital stays, and accelerated rehabilitation [34]. Using conscious sedation reduced the side effects of general anesthesia and allowed for live neurological monitoring by patients. Importantly, all patient-reported outcomes exceeded the MCID. The authors concluded the expert’s classification of endoscopic lumbar interbody fusion based on the evidence presented in Table 3.
REDUCTION OF BLOOD LOSS
One significant advantage of endoscopic lumbar interbody fusion (endo-TLIF) over MIS-TLIF is the reduction in blood loss, which facilitates faster postoperative recovery. According to a study, the endoscopic group (transkambin approach) experienced significantly less blood loss compared to the MIS-TLIF group (45.1 ± 12.4 mL in endo-TLIF vs. 146.2 ± 41 mL in MISTLIF, p = 0.01) [31]. This finding was concordant with the results of a prospective cohort study by Ao et al. [19], which reported significantly reduced intraoperative blood loss in endo-LIF (transkambin approach) compared to MIS-TLIF (84.29 ± 44.34 in endo-TLIF vs. 171.79 ± 112.27 in MIS-TLIF, p < 0.001). Similarly, Ge et al. [35] found that endo-TLIF (posterolateral approach) led to a significant reduction of visible blood loss compared to MISTLIF (69.5 ± 30.3 in endo-TLIF vs. 144.8 ± 37.2 in MIS-TLIF, p < 0.001). Furthermore, they observed that the ratio of hidden blood loss to total blood loss was statistically greater in endoTLIF (91%) than in MIS-TLIF (87%).
Consistently, several other studies have reported significantly lower blood loss in the endo-TLIF group compared to the MIS-TLIF or open groups, eliminating the need for blood transfusions [19,36-39].
DECREASE BACK MUSCLE INJURY
Owing to its minimal incision and muscle dissection, endoscopic lumbar interbody fusion (endo-TLIF, transkambin approach) reduces muscle injury or collateral tissue damage, which corresponds to less blood loss [19]. There is a study that reported a significant difference in total incision length between endo-TLIF and MIS-TLIF (5.3 ± 0.8 cm in endo-TLIF vs. 7.8 ± 2.3 cm in MIS-TLIF, p = 0.000) [26]. Likewise, Ge et al. [35] found that endo-TLIF (posterolateral approach) significantly reduced total blood loss, visible blood loss, and hidden blood loss. These indirect signs suggest that endo-TLIF may provide better soft tissue protection than MIS-TLIF.
A prospective cohort study by Ao et al. [19] compared the serological markers of surgical trauma and muscle damage—C-reactive protein (CRP) and creatine kinase (CK)—between endo-TLIF (transkambin approach) and MIS-TLIF groups. They found significant differences in these markers at each postoperative peak time. For CRP level, which peaked three days postoperatively, the endo-TLIF group showed lower levels than the MIS-TLIF group (71.42 ± 39.89 vs. 106.62 ± 51.46, respectively; p = 0.002). Similarly, CK levels peaked on the first postoperative day and were significantly lower in the endo-TLIF group compared to the MIS-TLIF group (370.45 ± 145.7 vs. 469.81 ± 178.04, respectively; p = 0.011). The endo-TLIF group also consumed significantly less analgesics than the MIS-TLIF group. A nonrandomized clinical trial assessed muscle injury in endo-TLIF (transkanbin approach) and posterior lumbar interbody fusion (PLIF) groups by evaluating serum CK levels preoperatively, postoperatively, and at regular follow-up intervals. They used contrast-enhanced ultrasonography to measure the maximal cross-sectional area of the multifidus muscle (max-CSA) and blood perfusion around the surgical site. Their results showed that the mean CK level in the endo-TLIF group was significantly lower than the PLIF group at 1 day and 1 week postoperatively (p < 0.001).
Furthermore, the max-CSA in the endo-TLIF group was lower than the PLIF group at 1 week postoperatively but was significantly higher at 3 and 6 months. These results suggest that the PLIF technique may lead to more muscle injury and swelling immediately postoperatively and increased back muscle atrophy, which could be linked to a higher postoperative visual analog scale (back) score and a potential for future failed back surgery syndrome due to paraspinal muscle atrophy causing postoperative low back pain [40-43]. Nonetheless, more comparative studies between endo-TLIF and MIS-TLIF are needed to further confirm this hypothesis.
CLINICAL OUTCOMES
Several studies have presented remarkable conclusions regarding the postoperative clinical outcomes of endoscopic lumbar interbody fusion (endo-TLIF). These reports generally suggest that endo-TLIF yields favorable clinical results, exceeding the MCID, with no significant difference in the incidence of complications [44,45]. Compared to MIS-TLIF, several authors have noted that endo-TLIF improved short-term clinical outcomes, including postoperative low back pain, functional scores, and shorter recovery times. However, long-term clinical outcomes and fusion rates were comparable to MIS-TLIF, albeit with less surgical trauma [19,25,26,33,35,37,46-48].
A prospective randomized pilot study comparing endo-TLIF and MIS-TLIF showed lower visual analogue scale (VAS) back pain scores in the endo-TLIF group one day and three months postsurgery (p = 0.001). The length of postoperative hospitalization was also shorter for the endo-TLIF group (3.6 ± 1.6 days vs. 7.2 ± 2.7 days for MIS-TLIF, p = 0.01) [36]. In a similar, a prospective cohort study reported less postoperative low back pain and less surgical trauma from lower serum CK levels, suggesting faster recovery with endo-TLIF. Nonetheless, medium-short term surgical outcomes were not significantly different between endo-TLIF and MIS-TLIF [19].
A prospective randomized study comparing endo-TLIF to MIS-TLIF for single-segment lumbar spondylolisthesis, found significantly lower postoperative low back pain in the endo-TLIF group, though the fusion rates were comparable (95.85% in endo-TLIF vs. 90.7% in MIS-TLIF) [36]. Another studycompared three minimally invasive spinal surgery techniques for lumbar stenosis: biportal endoscopy, uniportal endoscopy, and microsurgery. They reported improvements in clinical outcomes for all techniques, with both endoscopies reducing immediate postoperative pain [49]. A recent systematic review and meta-analysis, concluded that endo-TLIF had better immediate outcomes regarding blood loss and immediate VAS back pain scores, while mid-term clinical outcomes and fusion rates were not different. Most studies suggest that endo-TLIF shows better results than MIS-TLIF, particularly regarding VAS back pain scores in the immediate postoperative and short-term follow-up periods, likely due to smaller incisions and less soft tissue trauma. Long-term clinical outcomes, however, were comparable between both techniques [22].
In a comparison between unilateral biportal endoscopic lumbar interbody fusion (ULIF) and conventional open PLIF, ULIF was associated with less immediate postoperative pain and no need for blood transfusions. VAS scores for back and leg pain, as well as Oswestry Disability Index, improved at the final follow-up, with comparable fusion rates in both groups. Given that ULIF is a less invasive technique which preserves the paravertebral muscles, it can achieve improved clinical outcomes and comparable fusion rates to the conventional technique [50]. Comparisons of ULIF with MIS-TLIF showed equivalent fusion rates, better short-term outcome improvements, and lower postoperative back pain than MIS-TLIF, likely due to less muscle injury and better visualization of the lateral recess and foraminal area without excessive tissue dissection as seen in conventional MIS-TLIF [51].
In a study by Xie et al. [52], ULIF and uniportal endoscopic lumbar interbody fusion (endo-TLIF) were compared. The authors concluded that ULIF offers several advantages over endo-TLIF, including a wider surgical field, greater maneuverability, larger working space for interbody fusion procedures, and better visibility during cage implantation. However, both ULIF and endoTLIF were deemed safe and effective, with good visibility during the procedure. Numerous studies have demonstrated that endo-TLIF provides excellent short-term clinical outcomes, particularly regarding reduced postoperative pain and faster recovery times, when compared to MIS-TLIF. Long-term clinical outcomes and fusion rates are generally similar between both techniques. ULIF, with its advantages of a less invasive approach, preservation of paravertebral muscles, and improved visibility, also presents a promising option for lumbar interbody fusion procedures. However, more research is needed to further compare these techniques and their long-term outcomes to establish best practices in the field.
RADOGRAPHIC OUTCOMES-SEGMENTAL LORDOSIS
One potential drawback of endoscopic lumbar interbody fusion (endo-LIF) appears to be related to the small cage size and the possibility of under-correcting sagittal parameters. These factors could result in a mismatch between pelvic incidence and lumbar lordosis (PI-LL mismatch), potentially leading to unsatisfactory clinical outcomes. One potential drawback of endoscopic lumbar interbody fusion (endo-LIF) is the use of small cages, which may lead to the under-correction of sagittal parameters. This can result in a mismatch between pelvic incidence and lumbar lordosis (PI-LL mismatch), potentially leading to less satisfactory clinical outcomes. While the TLIF procedure may not achieve superior results in local radiographic parameters such as anterior disc height, fused segmental lordosis, and disc angle compared to oblique lateral interbody fusion (OLIF) or anterior lumbar interbody fusion, there were no significant differences observed in postoperative global sagittal alignments, including sagittal vertical axis (SVA) and PI-LL mismatch [53].
As shown in a previous study, a significant correlation exists between PI-LL mismatch after short-segment fusion and worsening of back pain as measured by the VAS [54]. To prevent postoperative PI-LL mismatch, surgeons need to maintain segmental lordosis during fusion. The selection of cage size is often key to increasing disc height and achieving this lordosis. However, due to the small incision required for endo-LIF (trans-Kambin), the cage size is typically smaller than that used in other transforaminal lumbar interbody fusion (TLIF) techniques [29]. To address this issue, many surgeons opt to use expandable cages that can promote optimal segmental lordotic alignment [33]. Some studiy found that expandable cages could lead to longer-lasting restoration of disc height, foraminal height, and segmental lordosis compared to static cages. Yet, in contrast, a systematic review and meta-analysis found no significant differences in clinical and radiographic parameters between expandable and non-expandable cages in patients undergoing MIS-TLIF [55].
An alternative approach has been suggested in another study, which preferred to use trocar-matched narrow-surface cages to circumvent the issue of cage size. Their approach was associated with acceptable clinical outcomes, fusion rates, and a low risk of nerve damage [29]. In addition to potential issues related to disc height differentiation due to cage size, smaller cages might be associated with lower fusion rates and an increased risk of subsidence [56]. However, a multitude of previous studies have reported no significant differences in fusion rates between endoLIF and MIS-TLIF, despite the smaller cage size used in endo-TLIF [25,29,45,57]. In terms of the overall results (excluding endo/non endo considerations), it has been found that the use of expandable cages is linked to enhanced functional outcomes and the restoration of postoperative disc and foraminal heights in patients who undergo TLIF procedures. Furthermore, no statistically significant differences were noted in segmental lordosis, lumbar lordosis, pelvic parameters, cage subsidence, or fusion rate [58]. The superior visualization provided by endoscopic techniques enables surgeons to meticulously prepare the vertebral end plate without damaging the subchondral bone, thereby reducing the likelihood of cage subsidence and subsequent adverse outcomes. By ensuring thorough bone grafting and meticulous end plate preparation, surgeons can achieve satisfactory fusion rates with a low risk of cage subsidence or pseudarthrosis [56,59-61]. This is echoed by several studies that report no significant differences in fusion rates between endo-LIF and MIS-TLIF, despite the use of smaller cages in endo-LIF [33,45,47,50,56]. To enhance fusion rates, the insertion of double cages in biportal endoTLIF, which enlarges the cage footprint. This technique resulted in significantly improved clinical outcomes and excellent fusion rates, with a low incidence of cage subsidence [62]. In a prospective randomized clinical trial, the clinical and radiologic outcomes were compared between banana-shaped cages and straight cages in single-level MIS-TLIF [63]. The results showed a significant increase in disc height and restoration of the segmental lordotic angle in the banana-shaped cage group. However, a higher rate of cage subsidence was observed, and clinical outcomes decreased significantly throughout the follow-up period in both groups. Interestingly, the limitations related to cage size, end plate preparation, and correction of lumbar sagittal profile can be overcome by the OLIF procedure. OLIF offers the advantages of providing larger cages, minimal tissue destruction, and preservation of posterior structures. A retrospective study demonstrated that posterior OLIF-cage positioning provided a good indirect decompression effect, while anterior OLIF cage positioning resulted in a good segmental lordosis [64]. The clear visualization provided by the endoscopic view enables surgeons to achieve direct decompression of neural elements and improved end plate preparation, leading to better fusion. Thus, a combination of OLIF and endoscopic procedures theoretically offers the best surgical results. There is a study which reported successful decompression of neural elements compressed by herniated discs through endoscopic anterior-to-psoas lumbar interbody fusion [65]. Satisfactory radiographic improvement in disc height index, sacral slope, and SVA, along with improved clinical outcomes at the 24-month follow-up, resulted in an overall success rate of 77%. However, endo-OLIF carries the risk of major vascular injury or sympathetic injury and should be carefully selected for each patient based on their specific condition.
Endo-TLIF has also been successfully applied in the treatment of degenerative scoliosis. Previous study reported that endo-TLIF is a safe and effective procedure for treating mild to moderate degenerative scoliosis. The procedure provided good early clinical results and improvements in coronal Cobb angles [66].
FACET JOINT VIOLATION
The biomechanics of the lumbar spine highlight the crucial role that posterior elements play in maintaining stability under flexion and rotational forces. The stability can potentially be compromised following facetectomy combined with posterior fixation [58]. Therefore, preserving these structures can help reduce the risk of postoperative spinal instability and prevent the degeneration of adjacent disc and ligamentous structures [67].
Minimally invasive surgery (MIS) techniques are designed with the aim of conserving these posterior structures, thereby minimizing destabilization while simultaneously achieving adequate decompression [68]. These procedures provide excellent visualization and optimal viewing angles, enhancing the ability to undercut the facet joint [69]. In this regard, endo-TLIF trans-Kambin technique has demonstrated an advantage over MIS-TLIF in preserving the facet joint [66]. The superiority of the endo-TLIF (trans-Kambin) technique lies in its ability to directly access the disc without the need for excision of posterior structures, such as the lamina, posterior ligamentous complex and ligamentum flavum. Additionally, the endo-TLIF (trans-Kambin) technique requires minimal bone removal, with only ventral facetectomy necessary in some cases. This stands in stark contrast to traditional MIS-TLIF and biportal endoscopic LIF trajectory, which have the potential to cause postoperative instability. Therefore, the endoscopic trans-Kambin TLIF technique effectively mitigates the risk of iatrogenic postoperative instability [19,70]. In other words, the trans-Kambin technique offers comparable surgical access to the disc space as the lateral LIF approach, while also providing an advantage in terms of avoiding major vessels and vital structures at risk [70]. Similarly, the endoscopic-assisted OLIF procedure enables the preservation of bilateral facet joints while facilitating effective end plate preparation and the insertion of large cages.
THE TECHNICAL ADVANCEMENT OF EXPANDABLE TECHNOLOGY AND FUTURE TREND
Expandable cages have emerged as a revolutionary solution that effectively addresses several limitations associated with MIS. Notably, the compact size of traditional cages presents challenges such as an increased susceptibility to cage subsidence and limited access corridors for height and lordotic adjustments. To overcome these issues, 2 primary types of expandable cages have been developed: the medial-lateral expansion type and the caudal-cranial expansion type. These innovative cage designs hold great potential in rectifying existing problems encountered during MIS procedures [71].
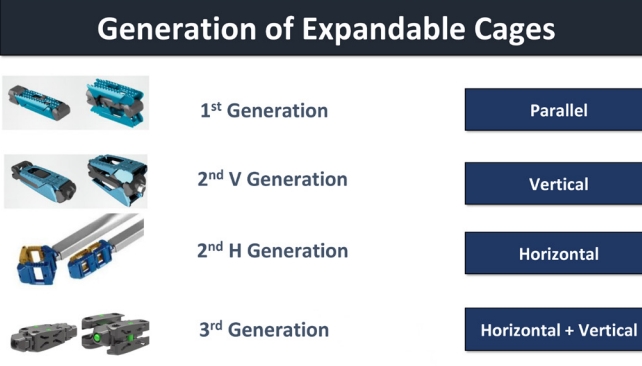
Considering the biomechanical impact of an expandable cage on the intervertebral disc, a vertical expandable cage is designed to improve radiological and clinical results, while a horizontal expandable cage is designed to have a large foot-print enhancing the fusion rate and reduce complications such as subsidence. The medial-lateral expansion cage offers a larger footprint, thereby mitigating the issues of cage subsidence and fusion complications. Biomechanical studies have substantiated this claim, revealing enhanced segmental stability when compared to cages with smaller footprints [72]. Conversely, caudal and cranial expansion cages aim to improve disc height and facilitate lordotic correction, thereby further expanding the scope of their benefits. Therefore, expandable cages can theoretically achieve a good indirect decompression effect through the restoration of disc height and correction of the lumbar segmental angle. However, spine practiceners should keep eyes on the timeline of technical advancement and the current availability of expandable cages (Table 4). The most common type of the first generation of expandable cages only support the height expansion with fixed minimum angle (Fig. 4). Then the second generation of expandable cages support not only the disc height but also lordosis with adjustable lordosis up to 20° or 22°.
1. 1st Generation
The initial expandable cages allow vertical expansion for controlled enlargement within the intervertebral disc space. They offer the option of fixed lordotic angle for alignment but lack horizontal expansion or adjustable lordosis.
2. 2nd – V. Generation
In the second vertical-oriented generation, cages retain vertical expansion, featuring adjustable sizing post-placement. Additionally, this introduces adjustable lordosis, aligning with the spine’s curvature. However, like the 1st generation, it lacks horizontal expansion or adjustable lordosis (Fig. 4).
3. 2nd – H. Generation
The second horizontal-oriented generation lacks vertical expansion and adjustable lordosis. It focuses on controlled horizontal expansion within the intervertebral space, excluding horizontal expansion with lordosis adjustment (Fig. 4).
4. 3rd Generation
The third generation maintains vertical expansion and fixed lordosis. It also adds controlled horizontal expansion, alongside a fixed lordotic angle (Fig. 4).
5. 4th Generation
The fourth generation retains vertical expansion and introduces adjustable lordosis. It advances further ith horizontal expansion and adjustable lordosis, enabling size and alignment customization. Notably, the 4th Generation signifies an upcoming advancement but is not yet available in the market.
The systematic review and meta-analysis, which compared surgical outcomes between expandable cages and static cages, reported that the expandable cage group exhibited significantly greater anterior disc height and segmental lordosis. However, no significant differences were observed in terms of restoring lumbar lordosis, cage subsidence rate, and clinical outcomes [73]. Despite the potential advantages, doubts have been raised regarding the utility of expandable cages. Other research suggests that segmental lordosis, disc height, and sagittal alignment restoration achieved with expandable cages are comparable to those attained with non-expandable cages. Nonetheless, the study indicated a higher incidence of intraoperative cage subsidence in the expandable cage group, raising concerns about the cost-effectiveness of caudal-cranial expandable cages [74]. As a result, uncertainties have emerged regarding the clinical benefits of expandable cages when compared to static cages [73]. However, regarding the timeline of the development of expandable cages the early series are mostly vertical expandable cages with small footprints. From the perspective of an expert, one of the reasons for the higher rate of subsidence in the papers might be due to technological immaturity related to the expandable technology. A meta-analysis concluded the positive effect of expandable cage to postoperative disc height and foraminal height together with functional outcomes in TLIF but no significant in lumbar sagittal profiles (SS, LL, PT, PI-LL) and fusion rate [58]. Consequently, multidirectional expandable cages have emerged as the preferred choice for MIS procedures. Notably, a retrospective review conducted comparing multidirectional expandable cages to static cages demonstrated superior restoration of disc height, foraminal height, and reduction of spondylolisthesis [75]. As such, multidirectional expandable cages represent a safe and rational alternative to conventional MIS cages.
The dual-direction expandable cage offers several advantages, including decreased point loading, a wider footprint, increased bony contact, and disc height restoration. Another study reported successful biportal lumbar interbody fusion using a dual-direction expandable titanium cage, which resulted in a significant increase in disc height, segmental lordotic angle, and lumbar lordotic angle at 6 months postoperative [76]. Furthermore, no complications related to nerve root injury were observed, and there was an improvement in clinical outcomes. Nevertheless, it is imperative to conduct further investigation through comprehensive long-term clinical and radiographic studies to assess the true value and efficacy of multidirectional cages in practice. These studies will offer valuable insights into the potential advantages and long-term outcomes associated with the use of multidirectional expandable cages in MIS procedures. As outlined in our classification of expandable cage generations (Table 5), our current focus lies on third-generation expandable cages. Looking ahead, fourth-generation expandable cages may prove instrumental in enhancing the effectiveness of endoscopic lumbar interbody fusion procedures. To explore this potential further, it is advisable to seek input from experts at NASS (North American Spine Society). This underscores the reason for emphasizing the role of expandable cages in the context of endoscopic lumbar interbody fusion, as previously referenced [77].
In most endo-LIF procedures, fluoroscopic-guided imaging is the primary choice for spine surgeons due to its availability. However, issues can arise from unclear images caused by patient size (obesity and thick fat). Multiple shots and high dose fluoroscope increase radiation exposure to the surgeon and the operating team. While using pulse mode with reduced kV can lessen radiation exposure, there are still annual exposure limits [78]. To enhance accuracy, ensure adequacy of decompression, and reduce radiation exposure, numerous innovative intraoperative spinal imaging technologies have been developed.
O-arm-based navigation is gaining popularity in clinical practice due to its advantages: reducing radiation exposure, providing accurate surgical site identification, visualizing trajectory, and enabling real-time intraoperative checking of decompression adequacy [79]. However, optic-based navigation systems have limitations including increased operation time due to re-registration, issues with tracking light blockage, and limitations in pedicular screw placement assistance.
The electromagnetic-based navigation system, on the other hand, resolves many of these issues and requires a shorter learning curve. Additionally, it is compatible with relevant instruments, allowing surgeons to receive real-time assistance throughout the entire surgical process [80]. Previos investigation reported endo-TLIF assisted by o-arm-based navigation could reduced the radiation exposure and surgical time compared to fluoroscopy-based procedure, with comparable clinical outcomes [81].
Robotic-assisted endoscopic surgery is a newer innovation aimed at enhancing surgical safety. It enables optimal surgical point determination during preoperative planning and increases accuracy in pedicle screw insertion compared to fluoroscopic guidance [82]. A prospective cohort study on endoscopic robot-assisted transforaminal lumbar interbody fusion, noting significant improvement in screw placement accuracy, reduction in surgical trauma and radiation exposure, and facilitation of rapid postoperative recovery with good clinical outcomes. However, a steep learning curve was required [83].
Augmented reality (AR) assisted endoscopic surgery is a novel assistive tool in spinal surgery. The use of smart glasses that provide navigation data while allowing visualization in a surgical field eliminates the need for eye shuffling between multiple monitors, reducing disorientation often experienced with other navigation systems [79]. Moreover, AR can visualize nonbony anatomy such as discs and nerve roots, enhancing safety to neural structures and offering potential advantages for revision cases [84]. However, a change in the reference frame position during operation can offset some benefits of AR.
The advent of 3-dimensional (3D) printing technology aims to transition from mass-produced to patient-specific implants. In spinal surgery, 3D printing can produce implants that perfectly fit a patient’s anatomy, effectively distributing stress and shearing forces while promoting osteointegration with a low complication rate [85].
The previous studies proposed that the outcomes after MIS-TLIF procedure with a 3D-printed titanium cage were comparable to the polyetheretherketone (PEEK) group in terms of the incidence of cage subsidence [86]. However, the 3D-printed cage showed significantly better fusion grade than the PEEK group at the 1-year follow-up. 3D printed spinal implants may be an upcoming technology to develop ideal, noteworthy implants that improve surgical outcomes in both single- and multilevel fusions [85].
Due to the small cage size and limited space for bone grafting, the success of fusion also depends on biological factors. In addition to the iliac crest autograft, allograft options including demineralized bone matrix (DBM) and recombinant bone morphogenetic protein-2 (rhBMP-2) have been proven successful in achieving spinal fusion. A new synthetic allograft called “anorganic bone matrix/15-amino acid peptide fragment (ABM/P-15)” was investigated for its efficacy in lumbar interbody fusion [87]. The study revealed a satisfying fusion rate (97.9%), particularly with the shortest average time to union compared to the rhBMP-2 and DBM groups, along with favorable clinical results and a low complication profile. Therefore, ABM/P-15 could potentially serve as an alternative to reduce fusion failure. Nonetheless, advancements in assistive technologies offer alternatives to improve safety, accuracy, and reduce unnecessary radiation exposure, there is a risk of over-reliance on technology. Surgeons must be aware of their limitations and precautions.
In general, the success of the endoscopic lumbar interbody fusion procedure relies on 3 key steps. Firstly, it is crucial to perform meticulous decompression of the spinal canal and nerve roots while minimizing damage to soft tissues, utilizing a minimally-invasive surgery concept. Secondly, careful attention should be given to end plate preparation in order to prevent any damage, as this can significantly improve the fusion rate. Lastly, the selection of the cage design plays a vital role. Collaboration with the industrial sector can help generate the most suitable cage design, which in turn can increase the fusion area and enhance the quality of disc height restoration, ultimately improving alignment and reducing the risk of fusion failure. Nevertheless, achieving satisfactory outcomes for patients undergoing the endo-LIF procedure hinges on several factors, with the most critical being the proper selection of suitable cases. This careful case selection stands as the foremost key to success.
CONCLUSION
This approach offers benefits like smaller incisions, reduced blood loss, faster recovery, and shorter hospital stays. By minimizing muscle injury, it reduces back pain scores and soft tissue scarring. The endoscopic fusion technique shows superior short-term outcomes for postoperative back pain reduction. Advancements in cage design and instrument technology have the potential to improve safety and lumbar lordosis restoration. Long-term outcomes and fusion rates necessitate additional investigation through extensive trials.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: JSK, PP, VK; Formal analysis: PP, JSK; Investigation: PP, JSK; Methodology: JSK, PP, VK; Project administration: PP, JSK; Writing – original draft: PP, JSK, YL; Writing – review & editing: PP, JSK, YL, VK.