Delay of Surgery for Spinal Metastasis due to the COVID-19 Outbreak Affected Patient Outcomes
Article information
Abstract
Objective
The present study is to analyze the effects of the coronavirus disease 2019 (COVID 2019) outbreak and the subsequent lockdown on the outcomes of spinal metastasis patients.
Methods
The study was a retrospective analysis of data from a prospective cohort study. All patients underwent surgical intervention for spinal metastases between January 2019 and December 2021 and had at least 3 months of postoperative follow-up. The primary outcome was overall mortality during the 4 different stages (pre-COVID-19 era, COVID-19 pandemic except in Taiwan, national lockdown, lifting of the lockdown). The secondary outcomes were the oncological severity scores, medical/surgical accessibility, and patient functional outcome during the 4 periods as well as survival/mortality.
Results
A total of 233 patients were included. The overall mortality rate was 41.20%. During the Taiwan lockdown, more patients received palliative surgery than other surgical methods, and no total en bloc spondylectomy was performed. The time from surgeon visit to operation was approximately doubled after the COVID-19 outbreak in Taiwan (75.97, 86.63, 168.79, and 166.91 hours in the 4 periods, respectively). The estimated survival probability was highest after the national lockdown was lifted and lowest during the lockdown. In the multivariate analysis, increased risk of mortality was observed with delay of surgery, with emergency surgery having a higher risk with delays above 33 hours, urgent surgery (below 59 and above 111 hours), and elective surgery (above 332 hours).
Conclusion
The COVID-19 pandemic and related policies have altered daily clinical practice and negatively impacted the survival of patients with spinal metastases.
INTRODUCTION
The coronavirus disease 2019 (COVID 2019) pandemic has had tremendous impacts on the global economy and daily life. Additionally, it has altered medical practice, and has led to a lack of medical resources, insufficient medical treatment capacity, and delays of nonemergency interventions.
Compared to countries worldwide, the outbreak of COVID-19 was delayed until 2021 in Taiwan, resulting in a national lockdown lasting approximately 2 months after mid-May 2021. After 2 months of indefatigable struggle, Taiwan became the only country to return to normal daily life in late July 2021 (Fig. 1).

Cumulative coronavirus disease 2019 (COVID-19) cases for global cases, domestic cases, and imported cases.
Spinal cancer patients, especially those with metastatic lesions, may suffer from neurological symptoms as the disease gradually progresses. This study analyzed the effect of the COVID-19 outbreak and the subsequent lockdown on spinal metastasis patients admitted to a tertiary referral cancer center compared to those in the previous 2 years.
MATERIALS AND METHODS
All patient data were retrieved from a prospective spinal cancer registry. The collection of databases was conducted at the National Taiwan University Hospital in accordance with the applicable local regulations and the Declaration of Helsinki and was approved by the Institutional Review Board of the same institution (IRB No. 201810043RIND). Written informed consent was obtained. Data from all patients who received surgical intervention for spinal metastatic tumors from January 2019 to December 2021 at our institution were retrospectively analyzed. Data collection for postoperative follow-up stopped on March 31, 2022, ensuring that all patients had at least 3 months of postoperative follow-up. The origins of metastatic tumors were identified and recorded, and synchronous metastases were defined as a diagnosis of a spinal metastasis within three-months of the primary cancer diagnosis.
Data were collected on the following variables: age, sex, body mass index (BMI), time from symptom onset to surgeon visit (the sum of time from symptoms onset to hospital presentation and time from hospital presentation to specialist visit), time from surgeon visit to operation, urgency of operation (emergency, urgent, or elective), type of surgical intervention (palliative, debulking, or total en bloc spondylectomy [TES]), epidural spinal cord compression (ESCC) scale score, spinal instability neoplastic score (SINS), Tomita score, modified Tokuhashi score, Frankel grade (preoperative, and postoperative 1 month and 3 months), time from surgery to postoperative radiotherapy/chemotherapy, postoperative outpatient follow-up, and survival time.
Different levels of the urgency of operation were established according to patient neurological status. In patients with an insidious course of disease, elective surgery was feasible. A radiologist was consulted to evaluate the necessity of preoperative transarterial embolization for all patients with hypervascular metastasis [1], and preoperative transarterial embolization was performed before surgery when necessary. Urgent surgery was defined as recommending that patients undergo surgery within 24 hours, and was considered for patients with minor neurological deficits who could receive more delicate preoperative studies and management, such as preoperative transarterial embolization, to decrease the surgical risk. In patients with acute neurological deterioration, emergency surgery was arranged to save neurological function. Emergency surgery was defined as recommending that patients should undergo surgery within 4 hours.
Palliative surgery was defined as simple posterior decompression of the thecal sac and/or posterior fixation without aggressive excision of the tumor within the vertebral body, which is performed only for neurological purposes [2]. In patients with advanced disease, such as multiple spinal metastases, multiple visceral metastases, or poor performance, palliative surgery was performed. Debulking surgery was defined as aggressive intralesional or gross total excision of the tumor of all aspects in which the margin is not tumor-free. TES was defined as a wide excision of the tumor with a tumor-free margin. In patients with solitary and oligometastatic spinal metastases with favorable histological findings, TES is the treatment of choice [3].
The patients were stratified by the following 4 different periods based on the date of surgery: The pre-COVID era (January 1, 2019 to January 21, 2020), global pandemic except in Taiwan (January 22, 2020 to May 18, 2021), national lockdown in Taiwan (May 19, 2021 to July 26, 2021), and after lifting the national lockdown in Taiwan (July 27, 2021 to December 31, 2021). Comparing these 4 periods allowed determination of the possible impact of the COVID-19 pandemic on spinal cancer patients.
The primary outcome was overall mortality during the 4 periods. The secondary outcomes were the oncological severity scores, medical accessibility, surgical accessibility, and functional outcome of patients during the 4 periods. In addition, we reviewed all patient data to identify possible groups vulnerable to mortality. We compared available data among these 4 periods to determine the actual impact of the COVID-19 outbreak on surgical accessibility and clinical outcomes in spinal metastasis patients.
Descriptive statistics of continuous variables are expressed as the mean ± standard deviation, those of categorical variables are presented as the frequency and percentage, and Kaplan-Meier survival curves were constructed to estimate the survival rates of patients.
In the univariate analysis, differences in the distributions of continuous variables, categorical variables, and survival outcomes were examined among the 4 pandemic periods using the Kruskal-Wallis test, chi-square test, Fisher exact test, and log-rank test as appropriate for the data type. Univariate analysis was also used to compare differences in the distributions of continuous variables and categorical variables between patients surviving at the end of the study and those that did not survive (hereafter, the surviving and nonsurviving groups, respectively).
Multivariate analysis was conducted by fitting Cox proportional hazards models with time-dependent covariates (called the “Cox’s model” for simplicity) to estimate the adjusted effects of risk factors, prognostic factors, and predictors on death. Specifically, we had defined several time-varying covariates such as “lifting of lockdown (i.e., 4 periods),” “back to outpatient clinic less than once per month (at event time),” “Frankel scale A, B, or C vs. D and E (at event time),” and “Frankel scale A, B, or C vs. D and E× overall survival time in weeks (at event time)” (see Table 1) and considered them in fitting Cox models. Technically, we reconstructed the original wide-form data (n = 233) into a long-form structure (m=13,873) using the so-called counting process style of input for our survival outcome, time to death. Next, at each ordered event time (i.e., time of death), we computed the values of all time-dependent covariates for each of the patients at risk in the transformed long-form data set. Finally, we fitted Cox models to these long-form survival data with all relevant time-fixed and time-varying covariates. Hence, although the 4 time periods had different durations, the follow-up period varied between the patients, the statistical methodology of Cox model properly handled covariates with values varying over time, so we could evaluate the effects of time-fixed and time-dependent covariates on the log-hazard rate of death in our patients during the 4 time periods.
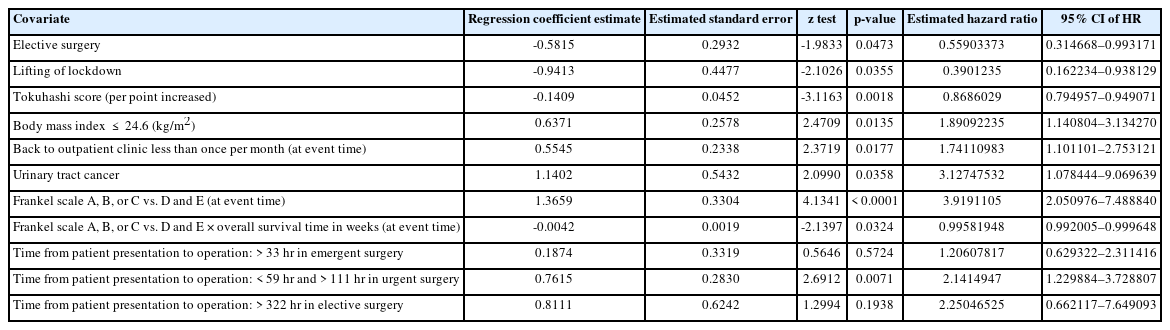
Multivariate analyses of the predictors for time to death by fitting Cox models with the modern stepwise variable selection method
All statistical analyses were performed using R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). A 2-sided p-value of ≤ 0.05 was considered statistically significant.
RESULTS
During the study period, 257 patients underwent surgery to manage spinal cancers at our institution. Twenty-four patients were excluded due to pathological diagnosis of primary spinal tumors; therefore, a total of 233 patients with spinal metastases were included in the final analysis. None of the patients in this study were infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Patient characteristics are summarized in Table 2. Of the 233 patients, 85 (36.48%) were admitted in the pre-COVID era, 100 patients (42.92%) were admitted during the global pandemic except in Taiwan, 14 patients (6.01%) were admitted during the national lockdown in Taiwan, and 34 patients (14.59%) were admitted after the national lockdown was lifted. Lung cancer, hepatocellular carcinoma, and breast cancer were the top 3 primary malignancies. The overall mortality rate was 41.20% (n = 96).
The rate of palliative surgery exceeded that of other surgical methods during the national lockdown (57.14%). In this period, no TES was performed. The percentage of patients with poor Frankel grades (A and B) was higher during the national lockdown than in other periods. The time from surgeon visit to operation was also prolonged during the national lockdown and persisted after the lockdown was lifted (168.79 and 166.91 hours, respectively). These delays were approximately twice those of the pre-COVID era and global pandemic period. Additionally, after the national lockdown, a lower proportion of patients received postoperative radiotherapy and chemotherapy within 1 month. The proportion of patients who received preoperative radiotherapy increased during the national lockdown and after it was lifted. All of the abovementioned differences were statistically significant.
The mortality rate of patients was slightly increased during the global pandemic period. The Kaplan-Meier survival curves indicated that the estimated survival probability was highest after the national lockdown was lifted, followed (in descending order) by the pre-COVID era, global pandemic period, and national lockdown period (Fig. 2). Table 3 presents a comparison between the groups of patients who did not survive and those who did.
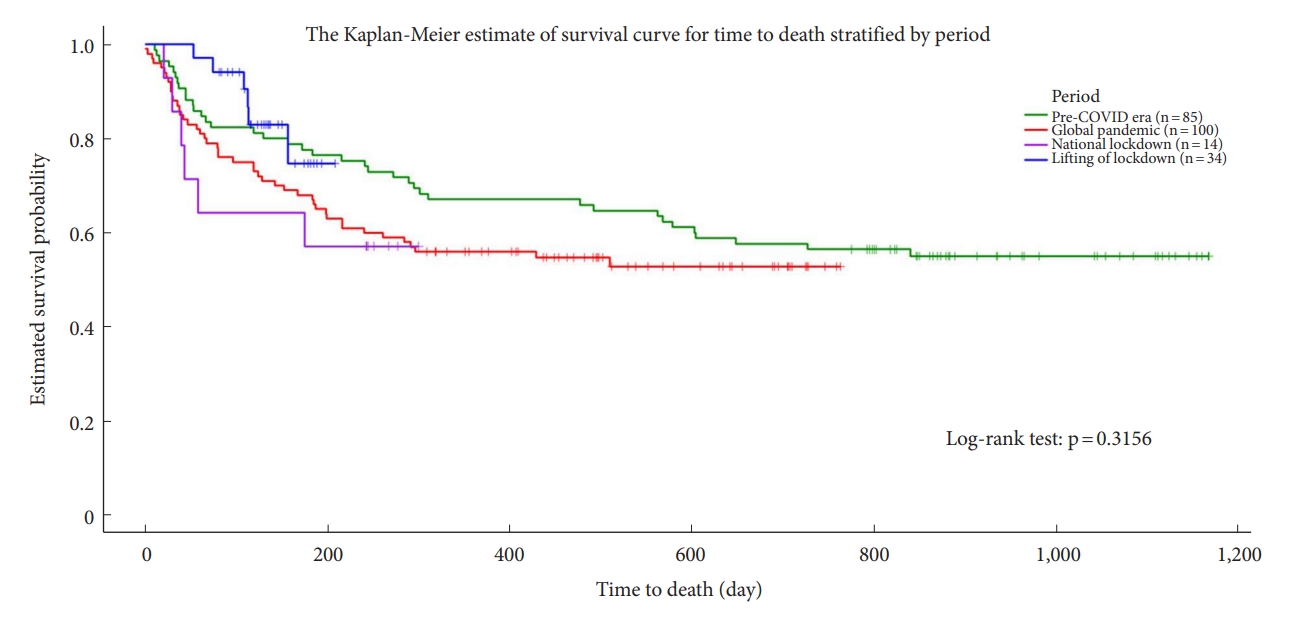
Kaplan-Meier survival curves estimating the survival time of patients, stratified by pandemic period. COVID, coronavirus disease.

Comparisons of demographic and clinical characteristics between the alive and dead spine metastasis patients
In the multivariate analysis, patients who underwent elective surgery and those who underwent surgery after the national lockdown was lifted had a lower risk of mortality. A higher modified Tokuhashi score was also a protective factor; specifically, the mortality risk decreased 0.870-fold per 1-unit increase in modified Tokuhashi score. In contrast, patients with BMI ≤ 24 kg/m2, those who returned to the outpatient clinic less than once per month, and those with urinary tract cancer had a higher risk of mortality. Compared to patients with Frankel D–E, patients with Frankel A–C had a higher mortality risk at any time point. The Cox model indicated that the beneficial effects of “Frankel A–C” on the hazard rate of overall survival decreased with increasing time after the operation. All of the abovementioned results were statistically significant. Regarding the emergency level of operation and the time from surgeon visit to operation, emergency surgery resulted in a higher risk of mortality with delays > 33 hours, urgent surgery resulted in a higher risk of mortality with delays < 59 hours and > 111 hours, and elective surgery resulted in a higher risk of mortality with delays > 332 hours (Table 1).
DISCUSSION
The COVID-19 outbreak started in Wuhan, China in December 2019; by March 2020, the World Health Organization had officially declared it a pandemic [4,5]. This pandemic has increased demands and pressure on health systems worldwide, and health care priorities and treatment paradigms for most diseases have undergone rapid alterations in this unforeseen context [6-8]. Most national and international healthcare societies proposed halting all elective procedures and maintaining only emergency and urgent procedures; thus, most individuals with surgical subspecialties experienced changes in their work regardless of whether they were primarily involved in treating COVID-19 [8,9].
In contrast to most of the world, Taiwan did not experience an outbreak until May 2021. With the national outbreak of COVID-19, Taiwan implemented a national lockdown for approximately 2 months, and the government introduced harsh zero-COVID policies. Many emergency measures for medical practices were implemented to cope with the outbreak. Elective surgeries, procedures, and examinations were postponed; regular operating theater capabilities essentially ceased, providing only the minimum available resources for emergency surgeries. Prior to all procedures and surgeries, patients were required to undergo nasopharyngeal swabs to detect SARS-CoV-2 in polymerase chain reaction (PCR) tests. Therefore, even for emergency situations, the interval between patient arrival and management was significantly prolonged. The prolonged arrival-to-management time remained problematic as the policy of SARS-CoV-2 PCR tests persisted with equal strictness even after the declaration of the end of lockdown in July 2021.
Due to the prolonged arrival-to-management time, clinical practices have been delayed to various degrees. In the present study, the time from the surgeon visit to operation almost doubled after the national lockdown. This finding is important. In general, medical practices cannot be delayed for any reason because of patient safety. However, during the COVID-19 pandemic, surgery was postponed due to disease control policies, and the prevention of COVID-19 became the top priority in daily practices. Thus, the COVID-19 pandemic provided an opportunity for us to evaluate the consequences of delays in elective surgery, which cannot be investigated in normal medical practice without violating ethical principles. Riley et al. [10] reported a similar finding, in that the mean time from admission to operating theater was 3.7 days (2.1 days for noninstrumental cases and 5.4 days for instrumental cases). The present study reported much longer delays, which might have resulted from the stringent zero-COVID policies. First, preprocedural SARS-CoV-2 PCR results should be obtained for both patients and their families. In emergency situations in which waiting for the results to become available was not feasible, all the staff and environment had to be equipped with adequate protections. These protective measures may have also prolonged the surgery. Second, for all procedures, including examinations, a lengthy duration must be spent cleaning the environment between patients. The capacity for examinations and procedures was thus further decreased. Which may cause delayed diagnosis and delayed treatment. In multivariate analysis, the association between the urgency of operation and the time from surgeon visit to operation significantly impacted patient prognosis. In the patients who underwent urgent surgeries, a significantly higher risk was found when operations were performed less than 59 hours and more than 112 hours after the surgeon visit. This finding might indicate that some of these patients needed emergency or earlier intervention. In patients who underwent emergency surgeries and elective surgeries, a higher mortality rate was found when operations were performed more than 33 hours and 322 hours after the surgeon visit, respectively. Although the differences were not statistically significant, they might indicate the benefits of earlier management.
A comparison of patient prognosis among the pandemic periods in the univariate analysis revealed that the length of survival after operation decreased and that this decrease was significant. This might be confounded by the follow-up duration. In univariate analysis, the mortality rate was lower during national lockdown and was the lowest after the national lockdown was lifted. However, when other variables were controlled, Kaplan-Meier survival curves indicated that the estimated survival probability was worst during national lockdown, and highest after the national lockdown was lifted. In multivariate analysis, the mortality risk was significantly lower (0.39-fold) in “lifting of lockdown.” A more favorable prognosis was observed solely in the univariate analysis for the “during the lockdown” period, whereas it remained consistent across all statistical methods for the “lifting of lockdown” period. This could be elucidated by the following factors. Firstly, the outcome of the univariate analysis may have been influenced by differences in follow-up duration, given that patients who underwent surgeries before the COVID era had longer follow-up periods. Secondly, the univariate analysis results could have been affected by limited case numbers during the national lockdown. This may have contributed to a slightly higher survival rate “during the lockdown” period compared to “before the lockdown.” Thirdly, the superior prognosis observed during the “lifting of lockdown” might be attributed to the fact that a majority of nonemergency cases (with better prognoses) postponed their treatment during the national lockdown, subsequently receiving treatment after the lockdown was lifted. The majority of surgeries performed after the “lifting of lockdown” were either urgent or elective, which lends support to this finding.
In the multivariate analysis, the mortality risk was higher in patients with urinary tract cancer, with a hazard ratio of 3.12. This finding is consistent with that of a previous report, with 5-year survival rates of 6.8% in metastatic urothelial carcinoma [11]. We also found a higher mortality risk in patients with Frankel A–C, with the mortality risk approximately 3.92-fold higher than that of patients with Frankel D–E. In the Cox model, the influence of “Frankel A–C” slightly decreased the overall survival after operation. Krause et al. reported an increased mortality risk in patients with Frankel A−C compared with Frankel D–E [12]. In a meta-analysis, the mortality rate was higher in those with Frankel A neurological injury [13].
In a previous study of the impact of the COVID-19 pandemic on outcomes from cancer surgery, the COVID-19 pandemic has indeed drastically reduced oncologic operations and survival outcomes [14,15]. A worldwide web-based survey even indicated that neurosurgical activity was significantly reduced in most centers (79%), and surgical planning was changed in most institutions (92%) [8]. Alternative treatment recommendations were released by numerous neurosurgical societies to serve as guidance for neurosurgeons worldwide [16]. Many adaptive strategies for spinal surgery have been reported, including delayed surgery with prompt referral to radiotherapy or observation could be adopted during the pandemic [4,6,10,17]. However, pathologies that compress the spinal cord or the cauda equina have a time-critical component for treatment [18]. Timing and indications of surgery were the most important factors to reduce mortality risk while maintaining the expected outcomes. For spine surgeries that cannot be postponed, alternative surgical plans and less invasive options may be considered. Immediate surgical intervention is indicated in spinal metastasis patients with a risk of instability (SINS > 12) and neurological involvement (Bilsky grade or ESCC score 2–3). Among patients with intermediate SINS (8–12) those with mid-thoracic vertebrae fractures are recommended to undergo immediate cement augmentation. During the national lockdown, no patient underwent TES. In many studies, TES significantly decreases the rate of local recurrence and can provide long-term survival for selective patients. Patients with solitary and oligometastatic spinal metastases with favorable histological findings might be benefit from TES [3,19-21]. However, successful TES depends on the surgeon’s technique and patient’s condition. TES is a complex and life-threatening surgery that imposes considerable physiological stress, and strict patient selection is necessary [3,6]. There were no suitable cases during the national lockdown.
Both the rates of postoperative radiotherapy and postoperative chemotherapy within one month were significantly lower after the national lockdown. These changes might have resulted from reduced capacity for treatment, and the avoidance of chemotherapy regimens that may cause immunosuppression during the COVID-19 pandemic [9,22]. The proportion of patients receiving preoperative radiotherapy was also higher during the national lockdown and after it lifted. This difference might have resulted from the referral of nonemergency patients to radiotherapy during the national lockdown.
This study has some limitations. First, the present study was a single-center study with a small number of cases, although our institution is the largest university hospital with the most cancer patients in Taiwan. As the patients were unwilling to travel over a long distance during domestic spread of COVID-19, the actual impact of the pandemic might have been underestimated. Second, although all the patients in the present study died from cancer progression or related comorbidities, the detailed cause of death was not analyzed. However, none of the patients in this study were infected with SARS-CoV-2 or died from COVID-19. These results simplify and eliminate a potential mortality bias of SARS-CoV-2 infection.
This is the first survey conducted to assess the impact of the COVID-19 pandemic on spinal metastasis patients, covering the periods from the pre-COVID era to after the national lockdown in Taiwan was lifted. The COVID-19 pandemic also provided an opportunity to evaluate the impact of delayed surgery on patient survival, which cannot be explored in daily medical practice. We believe that data from this study can help global health organizations understand the actual impact of the COVID-19 pandemic on clinical practice.
CONCLUSION
The COVID-19 pandemic and related policies altered clinical practices and negatively impacted the survival of patients with spinal metastases. Even after the national lockdown was lifted, variation in delays during the medical management of cases persisted. For spinal metastasis patients, optimization of the timing and indications for surgery is needed to reduce risk and maintain favorable patient outcomes.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: CJH, FYT; Formal Analysis: CJH, FYT; Investigation: CJH, CYW, YHL, YCH, WCY, TWWC, WLM, WHL, FMH, FX, SHY, DML, CMC, SYC, FYT; Methodology: CJH, FYT, FX, SHY, DML, CMC; Project Administration: FYT; Writing – Original Draft: CJH; Writing – Review & Editing: FYT.