Comparison of Clinical and Radiographic Outcomes Between Transforaminal Endoscopic Lumbar Discectomy and Microdiscectomy: A Follow-up Exceeding 5 Years
Article information
Abstract
Objective
To compare the long-term clinical and radiographic outcomes of transforaminal endoscopic lumbar discectomy (TELD) versus microdiscectomy (MD).
Methods
The data of 154 patients with lumbar disc herniation (LDH) who underwent TELD (n = 89) or MD (n = 65) were retrospectively analyzed. The patients’ clinical outcomes were evaluated using visual analogue scales for leg and low back pain, the Japanese Orthopaedic Association (JOA) score, and the Oswestry Disability Index (ODI). The evolution of radiographic manifestations was observed during follow-up. Potential risk factors for a poor clinical outcome were investigated.
Results
During a mean follow-up of 5.5 years (range, 5–7 years), the recurrence rate was 4.49% in the TELD group and 1.54% in the MD group. All scores significantly improved from preoperatively to postoperatively in both groups (p < 0.01). The improvement in the ODI and JOA scores was significantly greater in the TELD than MD group (p < 0.05). Forty-seven patients (52.8%) in the TELD group and 32 (49.2%) in the MD group had Modic changes before surgery, most of which showed no changes at the last follow-up. The degeneration grades of 292 discs (71.0%) were unchanged at the last follow-up, while 86 (20.9%) showed improvement, mostly at the upper adjacent segment. No significant difference was observed in the intervertebral height index or paraspinal muscle-disc ratio.
Conclusion
Both TELD and MD provide generally satisfactory long-term clinical outcomes for patients with LDH. TELD can be used as a reliable alternative to MD with less surgical trauma. Modic type II changes, decreased preoperative intervertebral height, and a high body mass index are predictors of a poor prognosis.
INTRODUCTION
Low back pain, sciatica, and numbness caused by mechanical and chemical irritation from lumbar disc herniation (LDH) can significantly impact patients’ quality of life [1]. Timely surgical intervention may be necessary for patients who do not respond well to conservative treatments.
Since open surgery was first applied to LDH treatment in 1934, multiple surgical techniques ranging from microdiscectomy (MD) to microendoscopic discectomy have been developed. The emergence of visual endoscopic technology has led to the development of transforaminal endoscopic lumbar discectomy (TELD), which is now gaining popularity as a treatment option [2,3]. Several studies have confirmed the excellent short-term clinical efficacy of TELD, and it is considered a treatment of choice for LDH because of its unique advantages of smaller incisions, decreased damage to soft tissues, and faster postoperative recovery [4-8]. However, the limited endoscopic field of view can increase the difficulty of the operation and the risk of incomplete resection of the nucleus pulposus [9,10]. This can lead to residual herniated tissue, which may affect the patient’s longterm prognosis and even result in recurrence during the late postoperative period [11]. Therefore, long-term follow-up is necessary to better evaluate the clinical outcomes of TELD.
In addition to the surgical procedure itself, pathologic radiographic manifestations such as Modic changes, intervertebral disc degeneration, paraspinal muscle atrophy, and decreases in intervertebral height have been associated with poor clinical outcomes following spine surgery [12-18]. Although the underlying mechanism remains unclear, researchers generally agree that spinal instability plays a significant role [19]. The intervertebral disc and paraspinal muscles play vital roles in maintaining the internal stability of the spine. Injury and degeneration of these structures can adversely affect their physiological function, leading to a poor prognosis after surgery [20]. To better evaluate the efficacy of TELD, it is essential to investigate its impact on the evolution of these radiographic manifestations, which have not been extensively studied.
In this study, we compared the long-term clinical outcomes of TELD versus MD over an average 5.5-year follow-up period. We also observed the evolution of pathologic radiographic manifestations during this time and identified any associations between clinical and radiographic outcomes.
MATERIALS AND METHODS
1. Patients and Materials
This study was approved by the Research Ethics Committee at Qilu Hospital of Shandong University (KYLL-2021(KS)-055). Prior to participation, informed consent was obtained from every subject. A total of 233 patients diagnosed with symptomatic LDH underwent spinal surgery (TELD in 96, MD in 137) to address back and/or leg pain along with typical sciatica symptoms. All procedures were performed by the same surgeon group (XL, SY, and YT) from March 2013 to June 2018.
The inclusion criteria were (1) ipsilateral, single-level LDH confirmed by magnetic resonance imaging (MRI) and computed tomography, (2) persistent radiculopathy consistent with radiographic findings, and (3) failure of at least 3 months of conservative treatment [21]. The exclusion criteria were (1) severe migration of an intervertebral disc (zones 1 and 4 according to the classification established by Lee et al. [22]) (Supplementary Fig. 1); (2) a high iliac crest (above the mid-L5 pedicle on lateral radiography) [23]; (3) severe calcification, bilateral symptoms, or other conditions that could be treated by MD but were unsuitable for TELD according to the surgeon’s experience [24]; (4) evidence of severe central lumbar spinal stenosis, segmental instability, infection, fractures, or tumors; (5) diseases involving other systems, such as cerebrovascular disease, that could potentially impact the clinical evaluation; and (6) a history of lumbar surgery [20,25]. A detailed flowchart is presented in Supplementary Fig. 2.
After application of the inclusion and exclusion criteria, 166 patients (TELD, n = 96; MD, n = 70) were enrolled in the study. Twelve patients were subsequently lost to follow-up, leaving 154 (TELD, n = 89; MD, n = 65) in the final analysis. Their average follow-up duration was 5.5 years (range, 5–7 years).
2. Surgical Procedure
The TELD and MD procedures were performed according to our previous study [21].
For TELD, after administration of a local anesthetic (0.5% lidocaine), a spinal needle was inserted into the target disc under fluoroscopic guidance. The position of the needle tip was confirmed to lay at the posterior vertebral bodyline and the medial pedicular line, and the nucleus pulposus was stained blue. A guide wire was inserted through the spinal needle, which was then removed. A 7-mm incision was made to facilitate insertion of a tapered cannulated obturator, and a bevel-ended, oval-shaped cannula was then placed along the guide wire. If cannula insertion was difficult, a trephine was applicated for foraminoplasty using a targeted foraminoplasty technique [26]. Finally, an endoscope was inserted, enabling removal of the herniated disc from outside to inside using endoscopic forceps.
For MD, the patients were placed in the prone position after induction of general anesthesia. A 2.5-cm posterior midline incision was made around the affected level, which was confirmed by C-arm fluoroscopy. Next, under direct microscopic visualization, the lamina and ligamentum flavum were removed using a lumbar Casper retractor and Kerrison rongeur. After exposure of the dura mater, nerve root, and protruded disc, discectomy was performed for complete decompression.
3. Clinical Outcome Evaluation
All enrolled patients underwent clinical assessments both before the operation and during follow-up. The evaluation items used to measure functional impairment were the visual analogue scale for leg pain (VAS-L), visual analogue scale for low back pain (VAS-B), Japanese Orthopaedic Association (JOA) score, and Oswestry Disability Index (ODI) score. Recurrence was defined as follows: (1) successful operation confirmed by a pain-free interval of at least 6 months; (2) recurrence of symptoms similar to those experienced preoperatively, significantly impacting the patient’s life and necessitating surgery, and (3) MRI confirmation of an ipsilateral, same-level herniation similar to the preoperative condition. Preoperative and postoperative data were then compared.
4. Radiographic Outcome Evaluation
Lumbar MRI was performed for all 154 patients before the operation and during the follow-up.
1) Modic change and disc degeneration
The Modic change was evaluated according to Modic et al. [27] and classified as types I, II, and III. The grading system established by Pfirrmann et al. [28] was used to evaluate lumbar disc degeneration at the operation segment (OS), upper adjacent segment (UAS), and lower adjacent segment (LAS).
2) Intervertebral height index
A modified distortion compensated Roentgen analysis [29] was used for intervertebral height measurement (Supplementary Fig. 3). The intervertebral height was defined as the average of the anterior, middle, and posterior distances from the upper and lower vertebral body to the midline. The Intervertebral height index (IHI) (the ratio of the intervertebral height to the anterior and posterior diameters of the upper vertebral body) was then calculated to eliminate individual differences.
3) Paraspinal muscle-disc ratio
To determine the actual paraspinal muscle content, we measured the functional muscle cross-sectional area (CSA), which is defined as the total CSA of the bilateral multifidus and erector spinae muscles minus the fat-infiltrated area. The ratio of the functional muscle CSA to the disc area at the same segment (paraspinal muscle-disc ratio [M/D]) was then calculated [30]. The threshold of distinguishing muscles from fat tissue was set at 120 (Image J, ver. 1.8.0; National Institutes of Health, Bethesda, MD, USA) (Fig. 1).
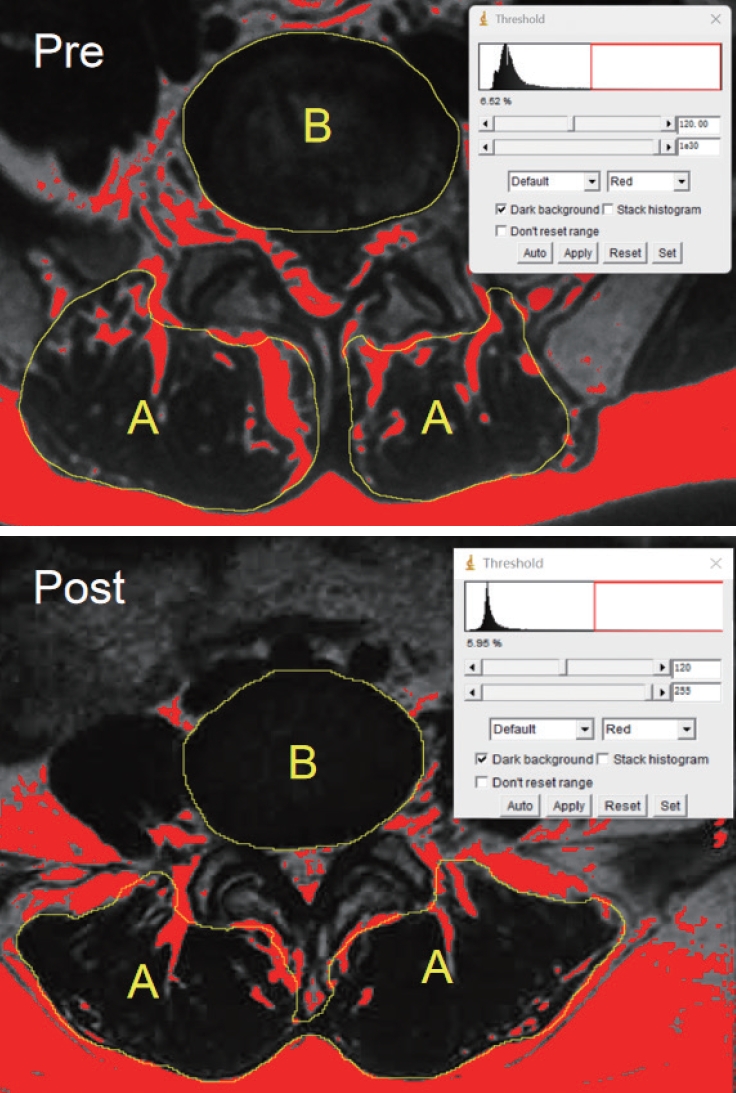
Pre- and postsurgery measurement of the functional muscle cross-sectional area (CSA)-disc ratio pre- and postsurgery. T1-weighted axial magnetic resonance imaging showing the (A) CSA of bilateral multifidus and erector spinae. (B) Disc area at the same segment. Fat tissue (Red) was distinguished from muscles with a threshold of 120.
5. Statistical Analysis
Statistical processing was performed using R Studio (Version 1.2.5033, Posit Software, BOSTON, MA, USA). Data distribution was assessed using the Shapiro-Wilk normality test. A paired t-test was used to analyze the changes in clinical scores and radiographic manifestations before and after surgery. Pearson analysis was performed to explore the associations between the 3 clinical scores and several radiographic manifestations. Linear regression analysis was performed to explore the associations between patients’ general information (sex, age, body mass index [BMI], surgical segment, Michigan State University classification [31], and disc migration classification [22]) and clinical outcomes. A p-value of < 0.05 was considered statistically significant.
RESULTS
1. Clinical Outcomes
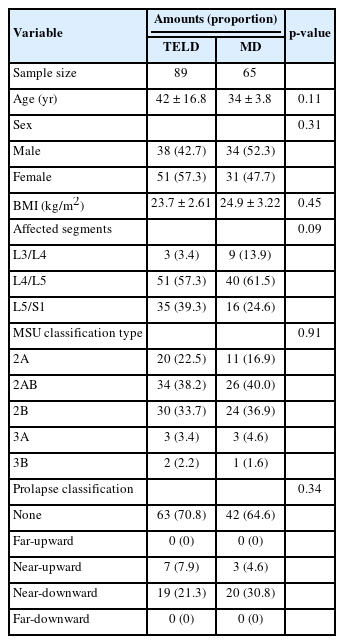
The demographic information of the final 154 recruited patients is summarized in Table 1. The entire dataset conforms to a normal distribution (Supplementary Table 1). During follow-up, the VAS-L, VAS-B, JOA, and ODI scores demonstrated significant improvements compared with the preoperative scores in both groups (p < 0.01) (Table 2). Additionally, the TELD group exhibited a significantly greater improvement (difference between preoperative scores and scores at the final follow-up) in the ODI and JOA scores (p < 0.05) compared with the MD group at the final follow-up.
The recurrence rate in the TELD group was 4.49%. Four patients developed recurrent LDH during follow-up, with 3 recurrences occurring in the fifth year after surgery and one in the third year after surgery. All patients were young (< 30 years old) with a BMI of > 25 kg/m2. Two recurrences were observed at L4/L5 (Michigan State University classification of intervertebral disc herniation [MSU type] 3A and 3B), and 2 were observed at L5/S1 (MSU type 3A and 2B). All patients underwent open revision surgery, resulting in successful recoveries. No cerebrospinal fluid leakage, infection, or other related complications were observed. The recurrence rate in the MD group was 1.54%. One 20-year-old patient with a BMI of 28 kg/m2 and an initial herniation at L5/S1 (MSU type 2B) developed a recurrent herniation in the fourth year postoperatively and underwent open revision surgery, resulting in successful recovery. No significant difference in the recurrence rates was observed between the 2 groups (p = 0.31).
2. Radiographic Outcomes
1) Modic change
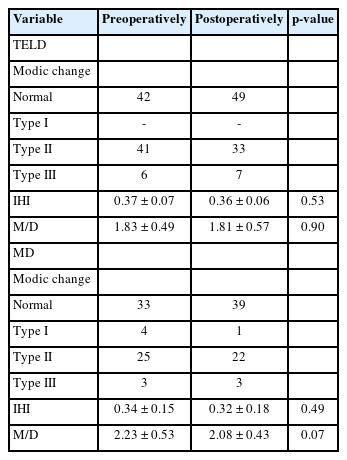
A total of 47 patients (52.8%) in the TELD group and 32 patients (49.2%) in the MD group exhibited Modic changes preoperatively. In the TELD group, 41 and 6 patients exhibited Modic type II and III changes, respectively. At the final follow-up, 8 patients who initially presented with Modic type II signals exhibited no signal. In addition, one patient who had no Modic changes preoperatively exhibited type III Modic changes at the posterior and upper end of the S1 vertebral body postoperatively. In the MD group, 4, 25, and 3 patients exhibited Modic type I, II, and III changes before the operation, respectively. During follow-up, all 4 patients with Modic type I changes showed conversion to type II. Seven patients who initially presented with Modic type II signals exhibited no signal at the final follow-up. One patient with no Modic changes before MD showed type I Modic changes at the OS postoperatively. The remaining patients showed no changes in the Modic type or extent (Table 3).
2) Disc degeneration
Disc degeneration was commonly observed at the OS, UAS, and LAS before and after the operation. In total, 411 intervertebral discs (TELD: 89 at OS, 89 at UAS, and 54 at LAS; MD: 65 at OS, 65 at UAS, and 49 at LAS) were analyzed in this study. The overall degeneration grades are shown in Fig. 2. In both groups, the degeneration grade of most of the discs showed no significant changes during follow-up, whereas 47 discs (20.2%) in the TELD group and 39 discs (20.0%) in the MD group showed improvement. Most improvements were observed in the UAS. Only a small number of discs (7.3% in TELD group and 8.2% in MD group) exhibited worsened degeneration. The aggravated intervertebral discs were primarily located in the OS, potentially resulting from the influence of surgical intervention.
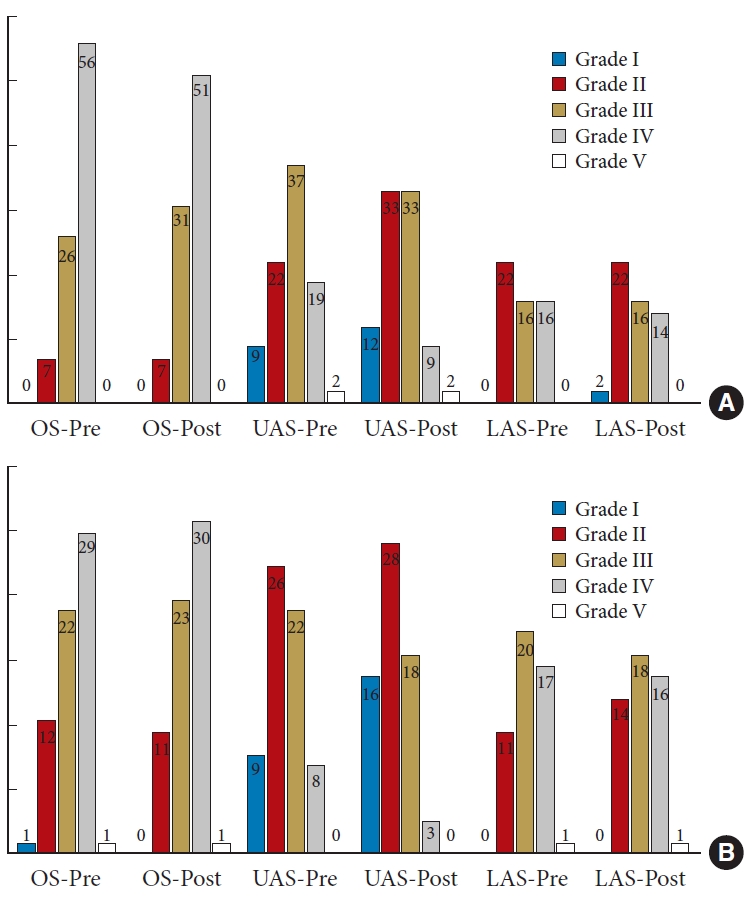
Evaluation of lumbar disc degeneration using Pfirrmann grading system. Bar graph showing the preoperative (PRE) and postoperative (POST) intervertebral disc degeneration grades of TELD (A) and MD (B) groups at the operation segment (OS), upper adjacent segment (UAS), and lower adjacent segment (LAS). TELD, transforaminal endoscopic lumbar discectomy; MD, microdiscectomy.
3) Intervertebral height
In the TELD group, the mean intervertebral height was 11.46 ±2.01 mm preoperatively and 11.16 ± 2.12 mm postoperatively (p = 0.59). The IHI decreased from 0.37 ± 0.07 to 0.36 ± 0.06 during follow-up, but the difference was not statistically significant (p = 0.53). In the MD group, the mean intervertebral height decreased from 10.55 ± 1.88 to 10.11 ± 2.19 mm (p = 0.22). The IHI was 0.34 ± 0.15 preoperatively and 0.32 ± 0.18 postoperatively (p = 0.49) (Table 3).
4) Paraspinal muscle-disc ratio
In the TELD group, the postoperative M/D was 1.81 ± 0.57, slightly lower than that before the operation (1.83 ±0.49, p = 0.90). In the MD group, the M/D decreased from 2.23 ± 0.53 to 2.08 ±0.57 (p = 0.07) (Table 3).
3. Association Analysis
In our analysis of the prognosis of all patients with different radiographic manifestations, we found that the preoperative VAS-L score in patients with Modic type II signals was significantly higher than that in patients without Modic change (6.8 vs. 5.6, respectively; p = 0.009). Patients with a higher preoperative IHI had significantly greater ODI improvement (difference between postoperative and preoperative scores) (p = 0.022, R=0.47). In addition, the preoperative IHI was positively correlated with VAS-B score improvement (p = 0.062, R = 0.39), although the difference was not statistically significant. No significant association was observed between disc degeneration and the M/D (Supplementary Table 2).
We also found that the preoperative VAS-B score was significantly higher in patients with a higher BMI (p < 0.01, R= 0.53). Additionally, there was a significant negative association between the BMI and the improvement in the VAS-B score (p = 0.012, R = -0.51). No significant association was found in the analysis of sex, age, surgical segment, MSU type (Supplementary Table 2). Three representative cases are shown in Figs. 3–5.
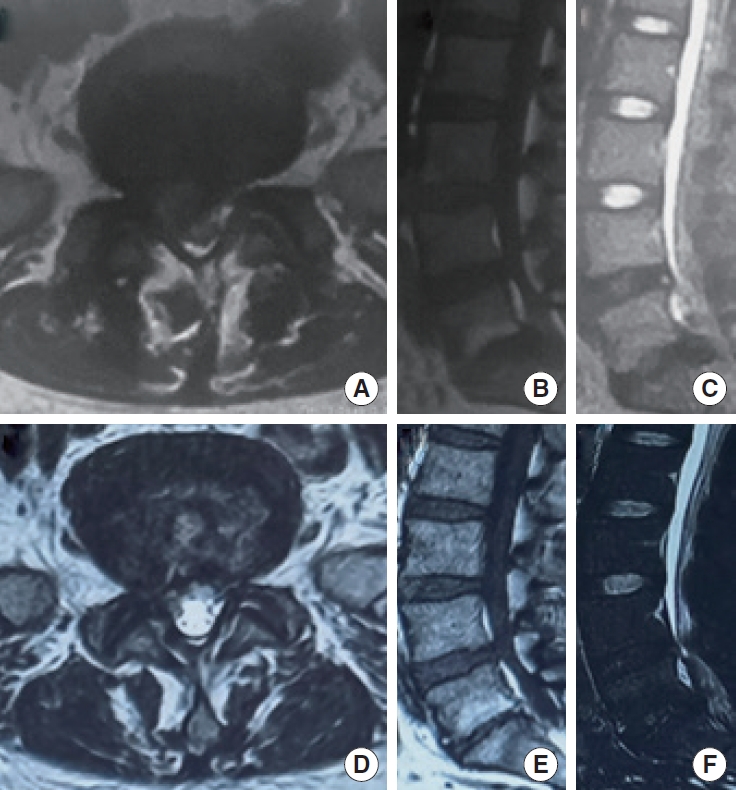
The radiographic evolution of a 37-year-old female patient underwent TELD during 7-year follow-up. (A-C) Preoperative magnetic resonance imaging (MRI) showing an MSU classification type 3B intervertebral disc herniation at L5/S1, with a Modic type II signal at L5/S1 upper and lower endplates. There was also a grade IV intervertebral disc degeneration at L5/S1 and a grade IV degeneration at upper segment (L4/L5). The prolapsed disc was in ZONE 4. (D-F) The MRI 7 years after TELD showing that the herniated tissue was basically removed, and the paraspinal muscles had no obvious atrophy compared with preoperative. The Modic type II change in the upper and lower endplates of L5/S1 was observed. The grades of intervertebral disc degeneration at L4/L5 and L5/S1 did not change, though the L5/S1 intervertebral space was significantly narrower than that before operation. TELD, transforaminal endoscopic lumbar discectomy; MSU classification, Michigan State University classification of intervertebral disc herniation.
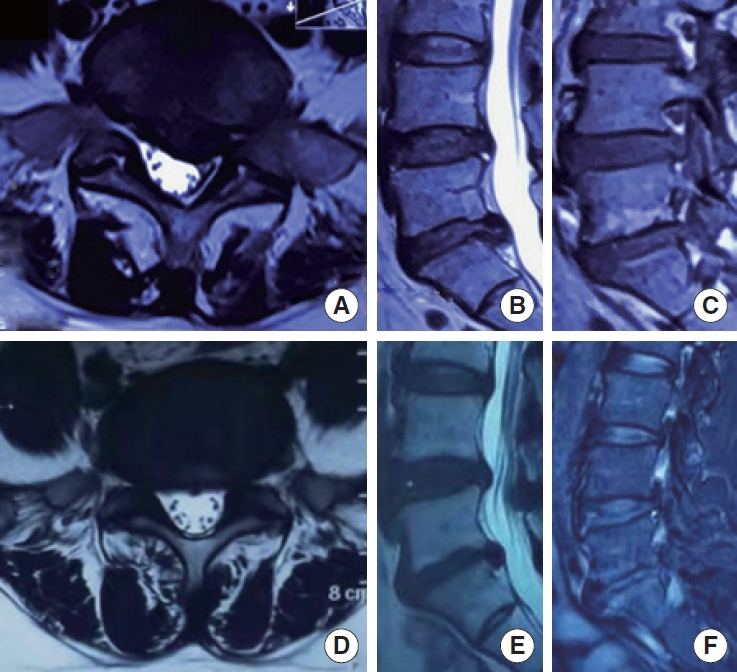
The clinical prognosis of a 46-year-old female patient underwent TELD with 6-year follow-up. At the last follow-up, this patient still suffered back and leg pain, with a VAS-L of 3, VAS-B of 2, a ODI of 10, and a JOA of 22, all of which did not reach the average level of our cohort. Intriguingly, this patient possessed Modic type II change and a high body mass index of 26.9 kg/m2, 2 predictors of poor prognosis in this study. (A-C) Preoperative magnetic resonance imaging (MRI). (D-F) The MRI 6 years after TELD. TELD, transforaminal endoscopic lumbar discectomy; VAS-L, visual analogue scale for leg pain; VAS-B, visual analogue scale for low back pain; ODI, Oswestry Disability Index; JOA, Japanese Orthopaedic Association.
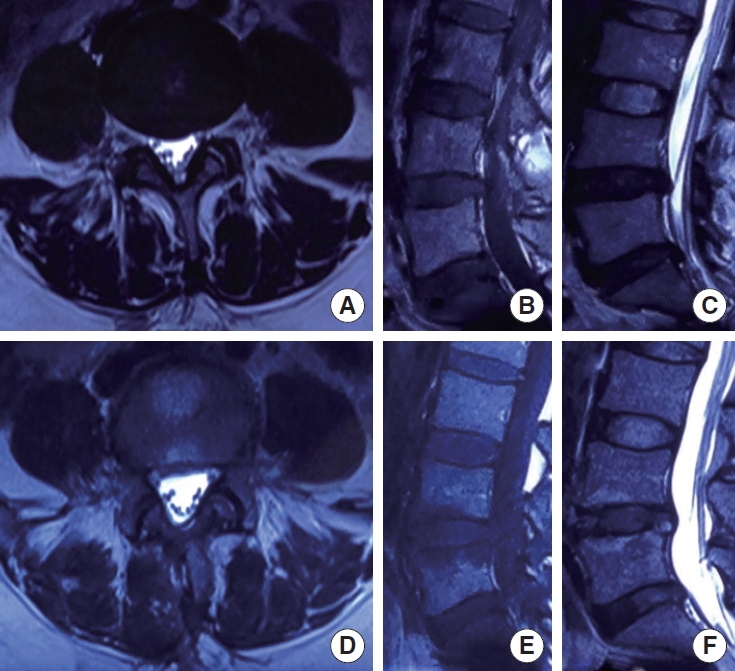
The radiographic evolution of a 42-year-old female patient underwent MD with 6-year follow-up. (A-C) Preoperative magnetic resonance imaging (MRI) showing the herniation at L4/L5 without Modic change. There were also a grade IV intervertebral disc degeneration at L4/L5 and L5/S1 and a grade II degeneration at upper segment (L3/L4). (D-F) The MRI 6 years after MD showing that the herniated tissue was basically removed, and the paraspinal muscles had a slight atrophy compared with preoperative. The Modic type I change in the upper and lower endplates of L4/L5 was observed postoperatively. The grades of intervertebral disc degeneration at L4/L5 and L5/S1 showed no significant change. MD, microdiscectomy.
DISCUSSION
Long-term follow-up is essential for a comprehensive evaluation of the outcomes associated with TELD, a relatively recent surgical technique. To accurately assess the application value of TELD, it is crucial to carefully select patients suitable for TELD and establish an appropriate control group [24,25]. We thoroughly assessed all patients in the present study, excluding those unsuitable for the transforaminal approach because of severe prolapse, a high iliac crest, and other conditions. Patients undergoing MD were then selected based on conditions aligning with those in the TELD group. Subsequently, a comparative analysis of the long-term outcomes between TELD and MD was conducted over an average follow-up period of 5.5 years. Our study also innovatively concentrated on the evolution of radiographic indicators during the extended follow-up. A thorough association analysis was carried out to explore potential risk factors associated with a poor prognosis. To our knowledge, this is the first comparative study to examine both clinical and radiographic outcomes of TELD and MD with a long-term follow-up.
Previous studies have revealed that the outcomes of TELD within 2 years are reliable and comparable to, or even better than, those achieved with microsurgical techniques [9,21,32]. In the present study, TELD also demonstrated stable clinical outcomes similar to those of MD over an average 5.5-year follow-up period. The improvements in both the VAS and ODI scores in both groups surpassed the minimum clinically important difference (MCID), which is 1.6 points for the VAS-L, 1.2 points for the VAS-B, and 12.8 points (25.6%) for the ODI [33]. In fact, the TELD group exhibited even greater ODI and JOA score improvements than the MD group. It is important to recognize that the ODI and JOA scores primarily reflect the extent of a patient’s daily life involvement and detailed neural symptoms, thus providing a more nuanced assessment than the VAS score. Because TELD offers advantages such as a smaller incision, less severe soft tissue trauma, and quicker postoperative recovery, these factors may have a lesser impact on patients’ daily lives after the procedure [32]. This might explain the observed differences in the ODI and JOA scores, although the difference between the 2 groups did not reach the MCID.
Multiple risk factors have been found to be associated with the recurrence of LDH, including intraoperative annulus preservation, early postoperative ambulation, the approach used (inside-out or outside-in technique), a high BMI, and a decreased CSA [18,34]. The reported recurrence rate of TELD varies widely among previous studies, ranging from 4.3% to 9.6% [30,35-37]. In our study, the recurrence rate was 4.49%, which is lower than the rates reported in most literature. This difference may be attributed to our use of the outside-in technique and our emphasis on preserving the annulus fibrosus. Notably, all recurrences were observed in young patients with a high BMI, and most were identified during the early stages of our TELD adoption, indicating a learning curve effect [38,39]. Three recurrences occurred in the fifth year after surgery and one in the third year after surgery. This finding suggests a relatively low short-term risk of recurrence after TELD and highlights the importance of long-term follow-up in the management of recurrence. The MD group exhibited a relatively lower recurrence rate in our study (1.54% vs. 4.49%, p > 0.05). In China, patients who undergo MD (which is associated with larger incisions and potentially more severe soft tissue damage) tend to mobilize later and engage in less immediate postoperative activity than patients who undergo TELD (who are encouraged to engage in early postoperative ambulation). This might explain the lower recurrence rate in the MD group. Abundant evidence also indicates an association between pathologic radiographic manifestations and LDH recurrence [13,18,30,40]. Interestingly, 2 of our patients who underwent TELD and developed recurrence showed Modic type II signals in the OS and a decreased postoperative intervertebral height, which is consistent with previous research.
We also observed the evolution of pathologic radiographic manifestations before and after surgery. Consistent with previous reports, our cohort showed that type II Modic signals accounted for up to 84% of all Modic signals [27]. Additionally, we observed 15 cases in which the endplates transitioned from Modic type II to a normal state. We speculate that surgical treatment somehow stimulates the regeneration of degenerated endplates. Intervertebral disc degeneration is a slow pathological process driven by multiple factors [41-43]. Although discectomy relieves nerve compression by herniated tissue, it may have little effect on the improvement of degeneration because of the complex pathological mechanism. This may explain why the degeneration of most discs in this study did not significantly change. Long-term loading can cause an irreversible decrease in intervertebral height [44]. A reduction of intervertebral height after discectomy has been consistently reported [45,46]. In our study, both the disc height and IHI decreased, but the differences were not significant. These results indicated relatively good postoperative intervertebral height maintenance and biomechanical improvement after TELD and MD. Surgery is one of the main causes of paraspinal muscle atrophy and fatty infiltration. Stretching of the paraspinal muscles during surgery can easily lead to muscle ischemia, degeneration, and necrosis [47,48]. In TELD group, the M/D was lower after than before the operation, while the difference was not statistically significant, indicating limited damage during TELD. In MD group, there was a relatively more pronounced muscle atrophy, though the difference was also nonsignificant (p = 0.07).
We also investigated the association between clinical outcomes and radiographic manifestations before and after surgery. Consistent with previous studies, we found that patients with type II Modic changes had more severe preoperative symptoms [17,49,50]. Our results also showed that the improvement in the ODI score was positively associated with the IHI, which is also consistent with previous studies [51-53]. No significant association was observed between the degree of intervertebral disc degeneration and the severity of pain, which is consistent with the results reported by Lurie et al. [12]. This may be explained by the fact that disc degeneration does not cause secondary problems such as decreased disc volume followed by intersegmental instability within a short period. One study revealed a positive association between paraspinal muscle atrophy and low back pain [54]. However, no such association was observed in our study.
Finally, we investigated other potential prognostic predictors. Excessive body weight increases the axial load on the spine, which may induce disc degeneration and decreased intervertebral height. Lidar et al. [55] found that patients who underwent bariatric surgery had a significantly increased postoperative intervertebral height and reduced low back pain. In our study, the preoperative VAS-B score was significantly higher in patients with a higher BMI (p < 0.01), and there was a significant negative association between the BMI and the improvement in the VAS-B score (p = 0.013). All these findings suggest that patients with a high BMI should control their weight before spine surgery.
One of the primary limitations of our study is that the data analysis was retrospective in nature. Moreover, our patients were required to undergo multiple MRI examinations during the follow-up period, and maintaining patient follow-up proved challenging. Thus, some patients were lost to follow-up, potentially introducing bias into the results. In the future, a prospective study with a larger cohort may provide greater clinical value.
CONCLUSION
Both TELD and MD provide generally satisfactory long-term clinical outcomes for patients with LDH. TELD can be used as a reliable alternative to MD with less surgical trauma. Modic type II changes, decreased preoperative intervertebral height, and a high BMI are predictors of a poor prognosis.
Supplementary Material
Supplementary Tables 1-2 and Figs. 1-3 can be found via https://doi.org/10.14245/ns.2347026.513.
Assessment of data distribution using the Shapiro-Wilk normality test
Summary of Results from Association Analysis.
The classification of herniated disc migration. Based on the direction and distance of disc migration, the migrated disc was classified into 5 types: zone 0 (no migration), zone 1 (far-upward migration), zone 2 (near-upward migration), zone 3 (near-downward migration), and zone 4 (far-downward migration). The migrated discs located in zone 1 and zone 4 were definite as severe disc migration.
Flow diagram showing the process of patient inclusion/exclusion. LDH, lumbar disc herniation; TELD, transforaminal endoscopic lumbar discectomy; MD, microdiscectomy.
Intervertebral height index measurement using the modified distortion compensated Roentgen analysis method. The intervertebral height was defined as the average of anterior, middle, and posterior distance from upper and lower vertebral body to the midline. Then the intervertebral height index, the ratio of intervertebral height to the anterior and posterior diameters of the upper vertebral body, was calculated to eliminate individual differences.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was supported in part by the National Nature Science Foundation (81874022 and 82172483 to Xinyu Liu; 82102522 to Lianlei Wang), Key R&D Project of Shandong Province (2022CXGC010503 to Xinyu Liu), Shandong Natural Science Foundation (ZR202102210113 to Lianlei Wang), Shandong Province Taishan Scholar Project (tsqn202211317 to Lianlei Wang) and National High Level Hospital Clinical Research Funding (2022-PUMCH-D-004).
Author Contribution
Conceptualization: XY, SZ, JS, XL; Data curation: XY; Formal analysis: XY, SZ, JS, SG; Funding acquisition: LW, XL; Methodology: XY, SZ, JS, SG, KZ, YT, LW, SY, XL; Project administration: XY, SZ, KZ, YT, LW, SY, XL; Visualization: XY, SG, YI, KZ; Writing - original draft: XY, JS, LW, XL; Writing - review & editing: XY, YI, XL.