Clinical Experiences of Uncommon Motor Neuron Disease: Hirayama Disease
Article information
Abstract
Hirayama disease, juvenile muscular atrophy of the distal upper limb, is a rare disease predominantly affecting the anterior horn cells of the cervical spinal cord in young men. This cervical myelopathy is associated with neck flexion. It should be suspected in young male patients with a chronic history of weakness and atrophy involving the upper extremities followed by clinical stability in few years. Herein, we report 2 cases of Hirayama disease on emphasis of diagnostic approach and describe the pathognomonic findings at flexion magnetic resonance imaging.
INTRODUCTION
Hirayama disease, juvenile muscular atrophy of the distal upper limb, is an uncommon cervical myelopathy associated with neck flexion movements24513). This disorder usually develops in the late teens and early twenties with a male preponderance. Although the underlying causative mechanism remains unclear, findings in recent studies reveal that the pathogenetic mechanism of this disease is anterior shifting of posterior dura of the lower cervical dural canal during neck flexion, often asymmetric, flattening of the lower cervical cord24578101314). This disorder, mostly nonfamilial, is typically exhibits an insidious onset, slow progression, and often a self-limiting course3451013). We report 2 cases of Hirayama disease and describe the pathognomonic findings at flexion magnetic resonance imaging (MRI).
CASE REPORTS
Case 1
An 18-year-old man visited Gyeongsang National University Hospital with progressive weakness and atrophy of right upper arm since 3 months ago. Initially, several months ago, his symptom appeared as twinge sensation on posterior thoracic to sacrum when he flexed his neck. More recently, he noted slowly progressive weakness and atrophy of upper arm, especially right shoulder. In visits, we found grossly atrophic changes in right shoulder triceps and biceps muscles. Neurologic examination revealed that the deep tendon reflexes were symmetrically normal and sensation to sharp pain, vibration and light touch was intact. No pathologic signs, such as Horner sign, Hoffman sign, and Babinski sign were detected. He had no previous history of any other disease or trauma. None of his family had the same symptoms. For further evaluation, we checked image work up. His dynamic views of cervical spine showed segmental instability in C4-5, C5-6 levels (Fig. 1), and atrophic spinal cord and increased signal intensity, C5-6 level in MRI (Fig. 2A). No significant pathologic lesions at neutral position MRI, but we found prominent posterior epidural space with engorged epidural venous plexus at flexion view (Fig. 2B, C). The patient was applied with a neck collar to prevent neck flexion, and after a year of follow-up, presents no signs of disease progression in physical examination.
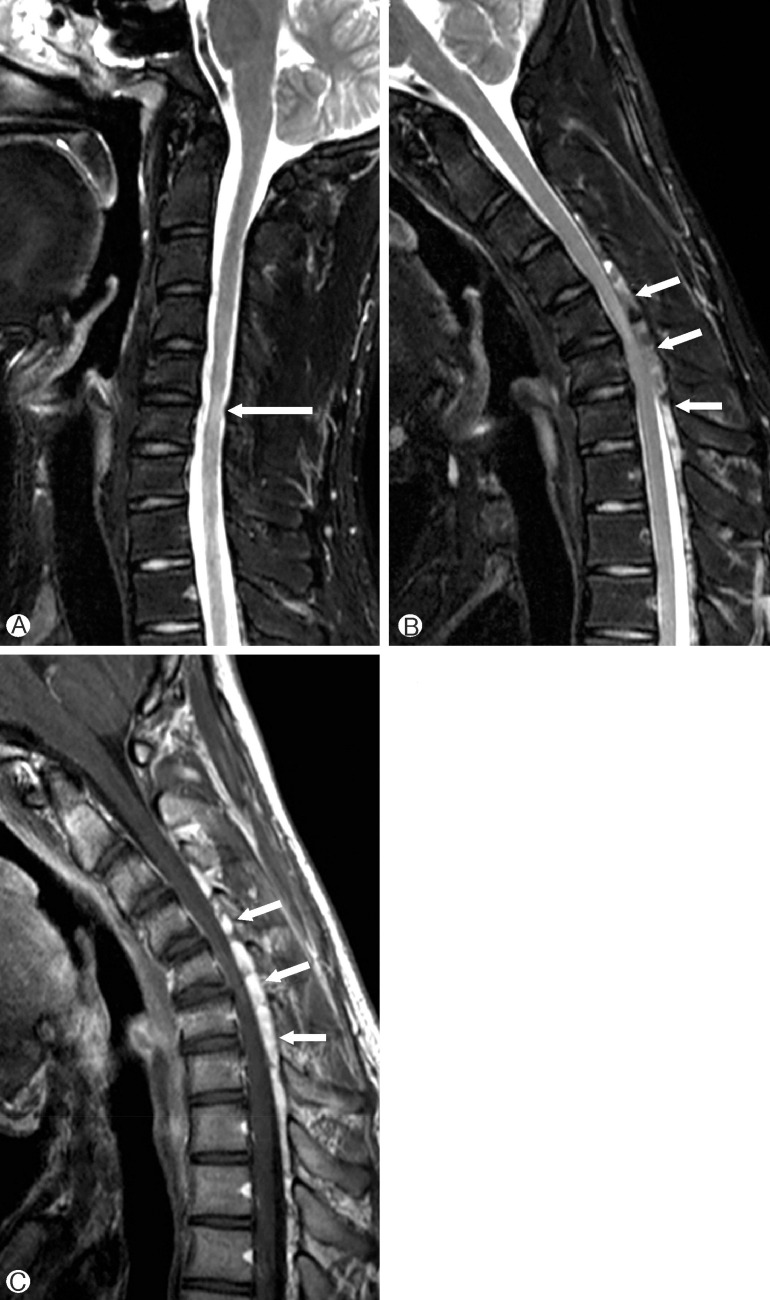
(A) T2-weighted sagittal image obtained at neutral position shows a high intensity lesion and segmental atrophy of the cervical spinal cord at C5-6 level (long arrow). (B) T2-weighted image of flexion neck position reveals a newly appeared heterogeneous intensity lesion in the posterior epidural space of the cervical and upper thoracic spinal canal (short arrows). The spinal cord is compressed by the lesion and anteriorly displaced dura. (C) On contrast-enhanced T1-weighted image, the epidural space lesion is strongly enhanced(short arrows).
Case 2
A 15-year-old man with no prior medical history presented to our institution with a 2-year history of progressive weakness and atrophy of right hand. He also felt numbness at his arm and weakness at 4th, 5th fingers. He had severe disability of the right hand (gross motor grade III, especially 4th-5th finger grade II). Physical examination revealed marked atrophy of the hypothenar and interosseous muscles of right hand. Full abduction, adduction of the digits, opposition of the thumbs and palmar grasps were impaired. An electromyography/nerve conduction velocity study revealed active denervation change in the atrophied muscles as monomelic amyotrophy. MRI was done in the neutral position and neck flexion in this case. We found straightening of the cervical cord, localized atrophy at C5-C7 level, T2-weighted hyper intensity due to myelomalacia (Fig. 3). A Philadelphia neck brace was placed, and the patient was doing well, with no further progression of symptoms, at follow-up study.
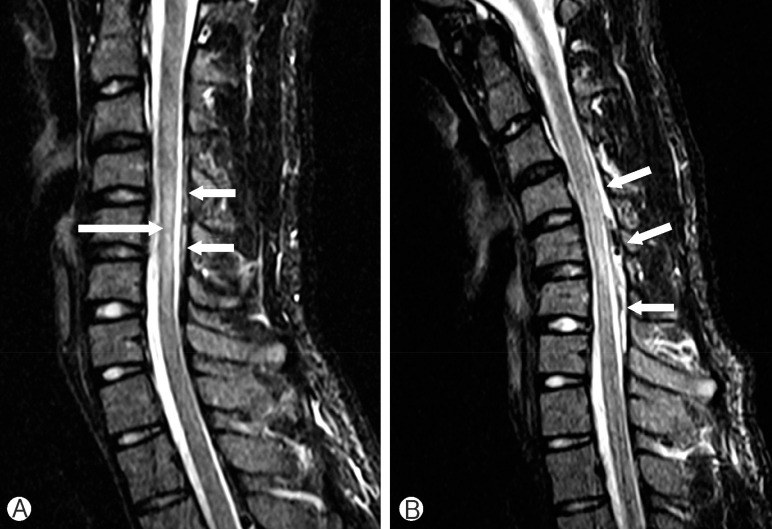
(A) T2-weighted sagittal image obtained at neutral position shows a high intensity lesion and segmental atrophy of the cervical spinal cord (long arrow). There is also a small heterogeneous intensity lesion in the posterior epidural space of the cervical spinal canal (short arrows). (B) T2-weighted image of flexion neck position reveals increase of the posterior epidural space lesion (short arrows).
DISCUSSION
Hirayama disease was first described by Hirayama et al.5). in 1959 and approximately 200 cases have been reported in the literature. This disease is different from the known types of motor neuron diseases because of its nonprogressive behavior and pathologic findings of focal ischemic changes in the anterior horn of the lower cervical cord5). So, the disease has also been described in the literature as juvenile muscular atrophy of the distal upper extremity, juvenile muscular atrophy of a unilateral upper extremity, juvenile asymmetric segmental spinal muscular atrophy, benign focal amyotrophy, or monomelic amyotrophy10).
The exact pathogenesis of the disease is still uncertain. However, the pathologic finding of focal ischemia prompted neurologic imaging investigations, which have revealed dynamic changes in the cervical dural sac induced neck flexion23121314). The relatively short and tight dura mater seen in patients with Hirayama disease is unable to compensate for the increased length of the vertebral canal during neck flexion, as a result of which the dural canal tightens up during neck flexion, resulting in an anterior shift of the posterior dural wall leading to spinal cord compression23478121314). Repeated neck flexion result in multiple episodes of ischemia and chronic trauma to the spinal cord, which eventually leads to myelopathy, as evident by asymmetric lower cervical cord thining235781014). There is another hypothesis that Hirayama disease is a form of intrinsic motor neuron disease rather than flexion-induced amyotrophic cervical myelopathy9).
Although Hirayama disease is self-limiting and application of cervical collar for 3 to 4 years has been generally advocated for the treatment because progression of signs and symptoms is usually expected to cease within several years1), early diagnosis is still necessary. Because avoidance of neck flexion by using neck collars remains the first-line treatment which is also effective in stopping the progression of disease11). But surgical intervention was considered in patients with particularly severe forms of disease, severe spinal cord atrophy, amyotrophy extending to unusual segment (T1), and clinical signs of pyramidal deficit suggestive of severe myelopathy. Surgical interventions such as anterior cervical decompression and fusion have shown some promising results7). The anterior decompressive approach may be better for patients with anterior effacement and severe cervical kyphosis on MRI during neck flexion. Also, cervical duroplasty with tenting sutures via laminoplasty led to spinal cord decompression with the preservation of cervical alignment and local physiological motion in young patients with Hirayama disease without major complications6).
In the first case, he was recommended surgical intervention. Because there are a short duration of disease progression and segmental instability at image work-up, the symptoms are likely to worsen during the conservative treatment. But, he refused surgical treatment and was applied a conservative treatment, a Philadelphia neck brace. Although he presented no signs of disease progression in last follow-up, careful observation and more follow-up periods should be needed considering natural history of disease. In the second case, the patient was prescribed a Philadelphia neck brace to prevent neck flexion other than therapeutic intervention, as disease progression was already in the late stage. To maintain the present functions of the patient, a home exercise program that included joint range of motion exercises was recommended.
All patients were doing well, with no further progression of symptoms, follow-up studies.
CONCLUSION
Hirayama disease has a tendency to be overlooked or misdiagnosed, since Hirayama disease in uncommon and symptoms are similar to other type of motor neuron diseases. Therefore, an early detection and a correct diagnosis may lead to therapeutic opportunity to arrest progression or to improve hand disability of young patients. A high awareness of its natural history, careful physical examination and detailed neurophysiological studies all have a role to play in narrowing down the list of differential diagnoses. Dynamic flexion MRI studies of the cervical spine are key for its accurate diagnosis, as the forward migration of the posterior surface of the dura mater can be demonstrated using this modality.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
