Evolution of Spinal Endoscopic Surgery
Article information
Abstract
Innovations in the development of endoscopic spinal surgery were classified into different generations and reviewed. Future developments and directions for endoscopic spinal surgery were discussed. Surgical therapy for spinal disease has been gradually changing from traditional open surgery to minimally invasive spinal surgery. Recently, endoscopic spinal surgery, which initially was limited to the treatment of soft tissue lesions, has expanded to include other aspects of spinal disease and good clinical results have been reported. As the paradigm of spinal surgery shifts from open surgery to endoscopic surgery, we discussed the evolution of endoscopic spine surgery in our literature review. Through this description, we presented possibilities of future developments and directions in endoscopic spine surgery.
INTRODUCTION
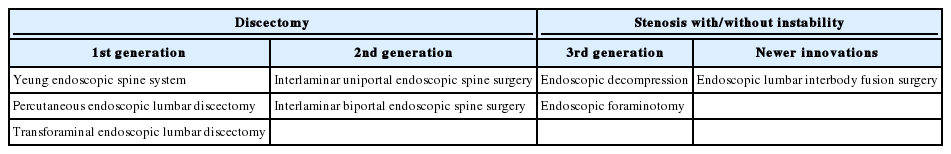
With the increase in life expectancy, the number of patients with spinal disease is on rise [1]. Due to the increased patient age, surgeons now have to manage patients with increased medical comorbidities such as lung, heart and kidney dysfunction. Consequently, more spinal surgeries are being performed as minimally invasive spine surgery. Moreover, endoscopic surgery is a subset of minimally invasive spine surgery that has been evolving rapidly and continuously to help manage older and sicker patients [2,3]. Endoscopic surgery has advantages such minimal muscle and bone damage, less pain, early rehabilitation, reduced hospital stay and early return to work [4-6]. Historically, the use of endoscopic spinal surgery was limited to discectomies but recently its indications have widened to include lumbar spinal stenosis. In this review we classify the evolution of endoscopic spinal surgery into different generations (Table 1). The direction in which endoscopic spinal surgery might develop in future is also discussed.
BRIEF HISTORY OF ENDOSCOPIC SPINAL SURGERY
Endoscopic spinal surgery began as percutaneous endoscopic discectomy. Kambin (1973) and Hijikata et al. (1975) had attempted the earliest endoscopic surgery in 1970’s [7]. However, at that time, removal of lesion was not under direct visualization and therefore spinal canal decompression was indirect. Therefore, the technique of that time can be regarded as percutaneous endoscopic spinal procedure before percutaneous endoscopic spinal surgery. Later Kambin and colleagues [8,9] introduced a method to remove nucleus pulposus using a 5-mm diameter working cannula and flexible forceps in 1986 and 1987. Subsequently, other authors reported improvement of percutaneous endoscopic discectomy techniques [10-12]. In 1990, Kambin [13] introduced the anatomical understanding of the transforaminal approach and triangular safe zone where a lesion can be approached without neural damage. This became a catalyst for endoscopic spinal surgery to develop rapidly with the use of slightly larger instruments and working channels.
FIRST GENERATION OF ENDOSCOPIC SPINAL SURGERY
The first generation of endoscopic spinal surgery can be epitomized by transforaminal endoscopic spine surgery. Yeung [14] introduced minimally invasive disc surgery using the Yeung endoscopic spine system in 1999. This method substituted the already existing indirect percutaneous endoscopic procedure. As such, visualized endoscopic spine surgery began from this point. Percutaneous endoscopic lumbar discectomy was introduced in 1993 [15]. Afterwards, percutaneous endoscopic transforaminal discectomy approaching intervertebral foramen through posterolateral side of back was performed mainly as endoscopic spine surgery [16-18]. Contrary to the conventional open microscopic discectomy, transforaminal endoscopic lumbar discectomy had the advantages of early recovery and ambulation, less anesthetic requirement, minimal damage to anatomical structures, preservation of motion segments and neurological function consequently early return to normal life [4-6].
The Yeung endoscopic spine system at this time was based on inside-out approach which had limited surgical indication. Epidural approach was attempted to address the limitations of the transforaminal approach that initially targeted the intradiscal space [19]. Outside-in approach was first introduced by Schubert and Hoogland [20] in transforaminal approach allowing wider range of transforaminal approach. The transforaminal approach became an epidural approach with direct access to most of the lesion sites through the half-and half approach, a transforaminal approach introduced in 2007 [21].
The transforaminal approach has 2 anatomical limitations. First, there are bony limitations including the facet joint in high canal compromise and pedicle in a highly inferiorly migrated disc. Second, there is the neurological limitation of exiting root. The exiting root include the dorsal root ganglion in relation with a foraminal disc [22]. In later iterations of this technology, the bony limitations were overcome with introduction of endoscopic drills by Choi et al. [23] in 2008. Most of the endoscopic surgeries were performed through the transforaminal route up to this time and focused on surgical development. However, overcoming complications and surgical limitations associated with endoscopic spinal surgery was an important part of generalizing endoscopic spinal surgery. Choi et al. [24] presented exiting root injury in 2013, which was a step forward in endoscopic spinal surgery.
Early endoscopic spinal surgery had limited surgical exposure and instruments. Kambin [13] introduced the triangular safe zone however it had limitations due to neurological and anatomical barriers (Fig. 1). There has been continuous evolution of the approach such as outside-in, inside-out and mobile outside-in techniques. Surgical techniques have also improved with newer developments (Figs. 2, 3) [17,20,25]. Currently many of the earlier limitations of transforaminal endoscopic lumbar discectomy have been overcome [26]. Recent studies have reported the clinical results of transforaminal endoscopy are as good as traditional open surgery [27,28].
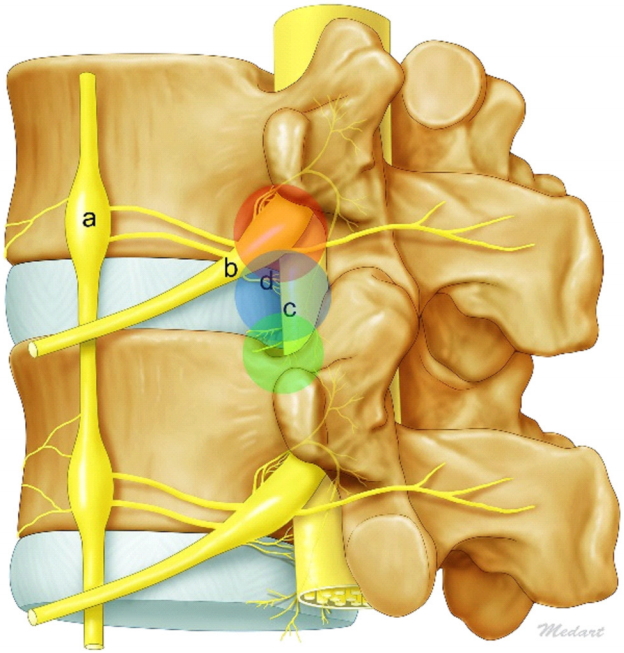
Illustration of 3 anatomical barriers. Sympathetic trunk and ganglia (a), exiting nerve and ganglia (b), traversing nerve (c), and sinuvertebral nerves (d).

Illustrations of mobile inside-out technique of percutaneous endoscopic transforamial discectomy. (A) Preparation for approach with bone trimming. Levering the cannula against the ventral facet to direct the cannula trajectory to the ventral (B) or dorsal (C) disc cavity.

Illustrations of mobile outside-in technique of percutaneous endoscopic transforamial discectomy. (A) This panel shows the initial placement of the cannula in the transforaminal approach. (B) This panel shows the levering of the working cannula. (C) This panel shows the suprapedicular route. (D) This panel shows the intervertebral route. (E) This panel shows the foraminal route. (F) This panel shows the round cannula placement for the far lateral disc.
SECOND GENERATION OF ENDOSCOPIC SPINAL SURGERY
Transforaminal endoscopic lumbar discectomy was developed as a common surgical treatment for disc herniations. However, inserting a cannula safely into the foramen at the L5–S1 level is difficult due to high iliac crest and narrow foramen. In addition, transforaminal endoscopic lumbar discectomy had longer surgery time and increased radiation exposure due to repetitive approach attempts [29,30]. For this reason, the interlaminar approach was developed and introduced [31,32]. As such, the second generation of endoscopic spine surgery can be epitomized by interlaminar endoscopic spine surgery. A discectomy performed through an interlaminar approach is referred to as interlaminar endoscopic lumbar discectomy. For this reason, interlaminar endoscopic lumbar discectomy was initially not included in the mainstream of endoscopic spine surgery and was considered to be an advanced form of microscopic lumbar discectomy. A recent meta-analysis has reported better results with interlaminar endoscopic lumbar discectomy than transforaminal endoscopic lumbar discectomy in L5–S1 disc herniation due to the wide interlaminar space [33].
Interlaminar endoscopic lumbar discectomy was conducted in 2 main ways. They are the uniportal technique using one portal and the biportal technique using 2 ipsilateral portal [31,34]. Despite there is a technical difference between the 2 methods, both methods have shown good clinical results and now the method is determined by the preference and proficiency of the operator.
Interlaminar endoscopic lumbar discectomy as a treatment option for L5–S1 disc herniation [35-37] has technical issues such as the requirement to cut or split the ligamentum flavum. The ligamentum flavum has an important role in structural integrity and prevention of recurrence [38,39]. To overcome these challenges, few authors have recently reported that the rate of relapse could be reduced by sequential insertion of serial dilators and performing annular fissure sealing by coagulation after discectomy to minimize the damage to ligamentum flavum (Fig. 4) [40]. Interlaminar endoscopic lumbar discectomy is continuously evolving. Recently, interlaminar endoscopic lumbar discectomy for bilateral lesions at L5–S1 level and for cervical lesions has been reported [41,42].
THIRD GENERATION OF ENDOSCOPIC SPINAL SURGERY
The third generation is epitomized by endoscopic decompression, which refers to endoscopic lumbar decompression for stenosis. Previously spinal stenosis was a contraindication for endoscopic spine surgery [43]. Now it has become an indication for endoscopic spine surgery due to rapid development in techniques and equipment [2,43]. Endoscopic decompression requires less bone resection, muscle damage and can yield sufficient decompression despite the minimal neural retraction [2,44]. Lumbar spinal stenosis is classified as central, lateral recess, or foraminal stenosis depending on the area of stenosis [45,46]. General perception in endoscopic spinal surgery is that interlaminar approach is appropriate for central stenosis, both interlaminal and transforaminal approaches are appropriate for lateral recess stenosis, foraminal or extraforaminal stenosis [2]. In addition to decompression of central and lateral recess stenosis by the interlaminar approach [47], decompression of foraminal or extraforaminal stenosis is now possible through foraminotomy [48].
Lesions at multiple locations are more common in clinical practice and in such cases recently introduced percutaneous endoscopic contralateral interlaminar lumbar foraminotomy is highly effective (Figs. 5, 6) [48]. The clinical results reported until now are divided on the issue of the location of spinal stenosis amenable with interlaminar or transforaminal approach depending on the region of stenosis. However, there utilizing either an interlaminar or transforaminal approach has equivocally demonstrated satisfactory results comparable with traditional open surgery in stenosis [2,48-50]. The advantage of endoscopic spine surgery is that it not only provides sufficient decompression, but also effectively treats multiple lesions and preserves both the facet joint as well as the paraspinal muscle (Fig. 7).
In the C-arm image, it shows the approach from ipsilateral to contralateral foraminal to extraforaminal.
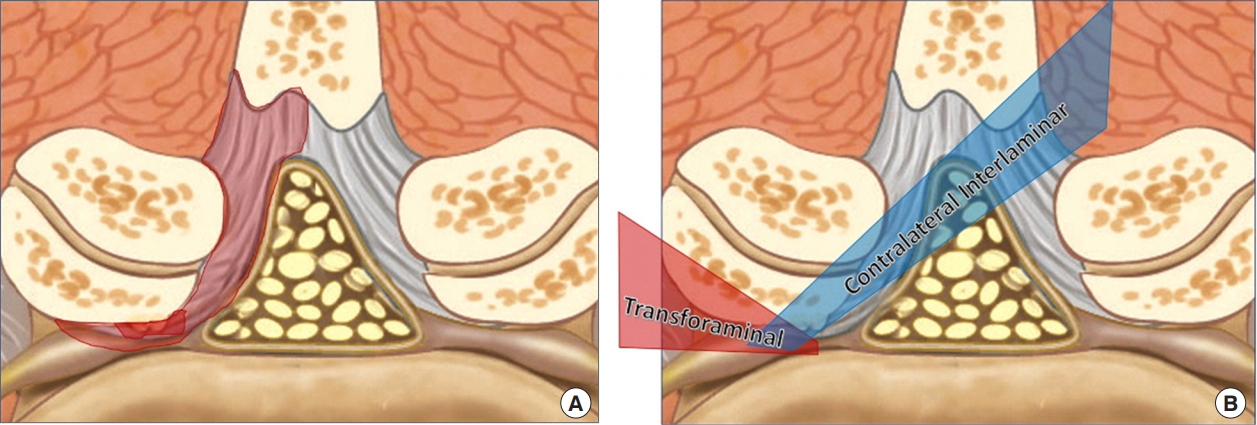
(A) A schematic diagram of spinal stenosis with combined lesions. (B) A schematic diagram comparing the transforaminal and interlaminar approaches.
NEWER INNOVATIONS OF ENDOSCOPIC SPINAL SURGERY
Maybe the fourth generation will be epitomized by lumbar interbody fusion, namely endoscopic lumbar interbody fusion (Figs. 8, 9). Lumbar fusion surgery has been performed as a treatment for various lumbar spinal disorders [51]. Although conventional open fusion surgeries allow wide decompression of neural structures and stabilization of the surgical site, it is also associated with widespread damage to posterior anatomical structures requiring a long recovery period. Recently interbody fusion using a minimally invasive technique has been reported to solve such problems [52-54]. However, these surgeries still required open incision, laminectomy, facetctomy and ligament flavum dissection. Supported by recent developments in surgical equipment, endoscopic spinal surgery can now be used for interbody fusion [55-57]. However, fusion surgery using endoscope has still been associated with many complications such as subsidence and low fusion rate, and has not been shown to have superior results compared to conventional fusion surgery. Nevertheless, attempts to solve these problems are still continuing [58,59].

Intraoperative simple X-ray and endoscopic images obtained during endoscopic lumbar interbody fusion.
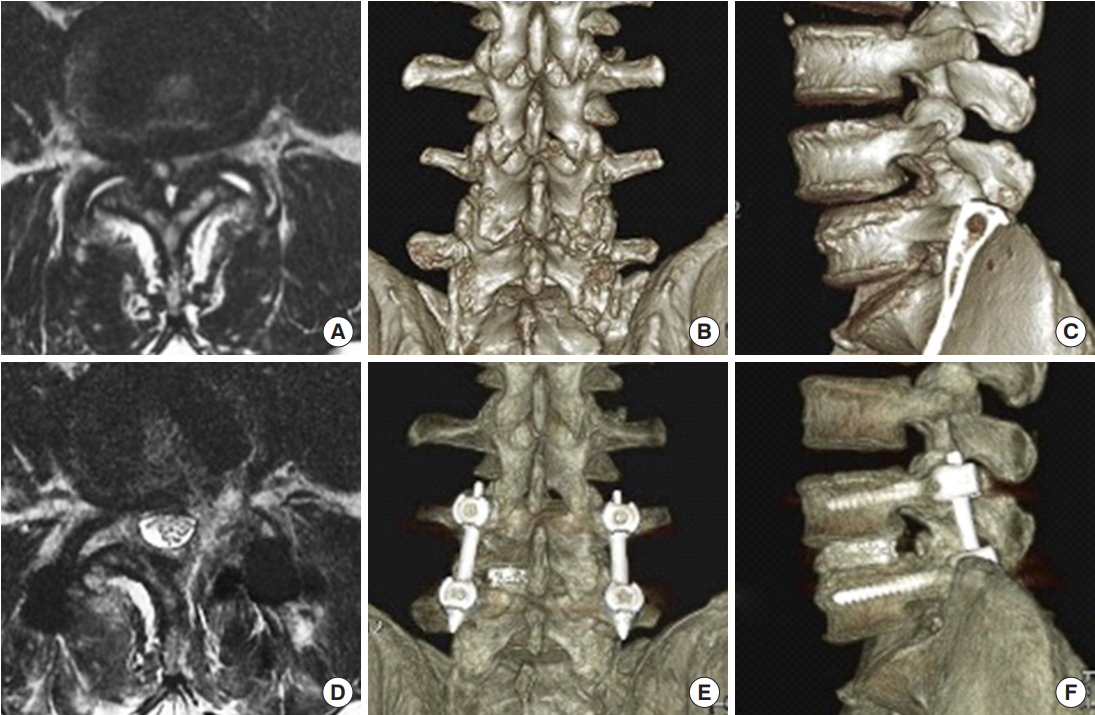
Preoperative magnetic resonance imaging (MRI) (A), 3-dimensional computed tomography (3D CT) posterior (B) and lateral (C) views showing the severe spinal stenosis combined with facet cyst and degenerative spondylolisthesis. The patient received endoscopic transforaminal lumbar interbody fusion. Postoperative MRI (D), 3D CT posterior (E) and lateral (F) views demonstrating the adequate decompression of stenosis and reduction of spondylolisthesis.
FUTURE PERSPECTIVES
Historically, the drawbacks of endoscopic spinal surgery are incomplete surgery and dural tears. However, new innovations in endoscopic surgery have overcome the issue of incomplete surgery, and new methods of endoscopic dural repair have been reported [43,60]. Recent reports show that endoscopic treatment is being extended to more advanced lesions such as intradural lesions [61,62]. At the rate of current development, it is reasonable to predict that endoscopy will be an option to treat all spine disorders.
CONCLUSION
As described, endoscopic spinal surgery has many advantages and is rapidly replacing conventional spinal surgery. The next generation of endoscopic spinal surgery needs to be prepared to meet the needs of patients and show good clinical results in all areas of spinal disease.
Notes
The authors have nothing to disclose.