Does Ossification of the Posterior Longitudinal Ligament Progress After Fusion?
Article information
Abstract
Starting in the 1960s, ossification of the posterior longitudinal ligament (OPLL) became more commonly diagnosed in Japan. The disease is characterized by a gradual increase in calcification of the posterior longitudinal ligament with the eventual sequelae of cervical canal stenosis and myelopathy. Surgical interventions to relieve stenosis and neurologic symptoms are performed to decompress the cervical canal. Studies demonstrate continued ossification of the OPLL in both nonsurgical and surgically treated patients. In this review, the authors evaluate the epidemiology, pathophysiology, and literature regarding disease progression in OPLL after cervical fusion.
INTRODUCTION
Since the 1960s, there has been greater awareness of the condition of ossification of the posterior longitudinal ligament (OPLL) most notably in Japan. Radiological studies demonstrate a prevalence of 6.3% in the Japanese population,1 and one-quarter of Americans with cervical myelopathy have evidence of calcification along the posterior longitudinal ligament [2,3]. Clinically, OPLL can result in neurological compromise through gradual increase of calcium deposition within the posterior longitudinal ligament (PLL) in a transverse or longitudinal pattern resulting in canal stenosis, spinal cord compression, and myelopathy. Principally, the goal of surgery for OPLL is to decompress the cervical spinal cord, however the optimal surgical approach is often debated [4-12]. Posterior surgery, such as cervical laminectomy (with or without fusion) or laminoplasty, provides indirect decompression of the cord. Anterior surgery (e.g., cervical discectomy and fusion [ACDF] or corpectomy) involves resection of the calcified ligament and directly addresses the offending pathology.
Motion-preserving laminoplasty has been the most studied treatment of OPLL [13]. Laminoplasty, however, may not be appropriate in select indications such as patients with severe, focal segmental OPLL or kyphosis [14,15]. Additionally, long-term follow-up of patients after laminoplasty has demonstrated radiographic progression of OPLL, though neurologic deterioration and reoperation for symptomatic progression is rare [5,7,9,16-19]. Given these potential limitations of laminoplasty, there is interest in understanding if cervical fusion surgery may inhibit further OPLL growth through restriction of motion. This article reviews the pathophysiology of OPLL progression, and discusses the existing literature for OPLL progression and outcomes after cervical fusion.
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
In Japan, the prevalence of OPLL is approximately 6.3% in adults with other Asian countries demonstrating comparable rates [1,2,20,21]. The prevalence of OPLL or OPLL variants in the North American population has been estimated to be as high as 25% in patients with cervical myelopathy [2]. In epidemiological studies, risk factors for OPLL include diabetes, hypoparathyroidism, obesity and a high sodium diet [22-24]. In addition to these metabolic factors, there appears to be a genetic component as evidenced through familial inheritance studies and genetic analysis [25-27]. Moreover, elevated growth factors that result in osteogenesis such as bone morphogenetic protein have been identified in OPLL specimens [28]. OPLL has also been associated with other progressive calcification disorders of the spine including diffuse idiopathic skeletal hyperostosis and ossification of the ligamentum flavum [29].
DIAGNOSIS OF OPLL
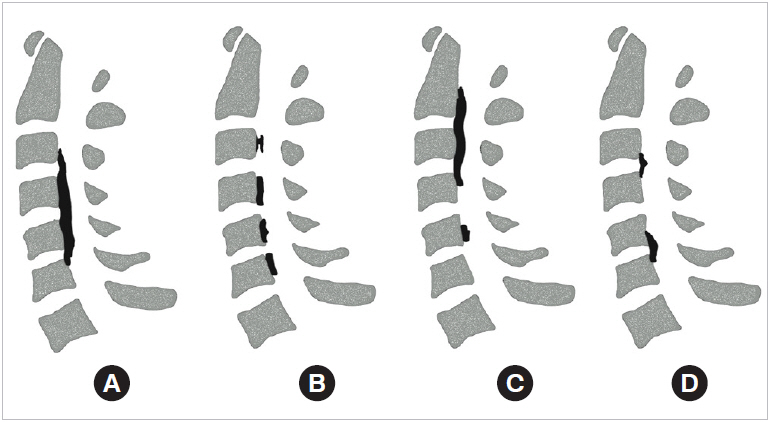
The pathognomonic finding of OPLL is the presence of an ossified posterior longitudinal ligament. This is best demonstrated on computed tomography (CT), but can often be visualized on lateral X-ray [2,30]. OPLL morphology is typically described as one of 4 variants: (1) segmental, (2) continuous, (3) mixed, and (4) localized (Fig. 1) [4]. The continuous type extends across vertebrae and disc spaces whereas the segmental type does not cross disc spaces. Mixed is a combination of segmental and continuous. Last, the localized variant of OPLL is confined to the disc space [2,4,24,30]. OPLL most commonly presents in the cervical spine (70%), with the remaining 30% of cases equally distributed between thoracic and lumbar regions [2,4]. Patients presenting with myelopathy or radicular symptoms should be evaluated with magnetic resonance imaging with close attention to the PLL. Hypertrophied PLL with or without heterogeneous signal may warrant additional imaging with CT scan if not previously obtained. A radiographic predictor of symptomatic OPLL is the space available for the spinal cord (SAC), as measured on a midline sagittal image. The SAC is defined as the anterior-posterior dimension at the most stenotic area, and is a useful tool for predicting neurologic progression. In multiple studies, a SAC less than 6 mm (or an OPLL occupying ratio of 60% or greater) consistently was associated with myelopathy on presentation, whereas a SAC greater than 14 mm was typically nonmyelopathic [31,32].
NATURAL HISTORY: CLINICAL AND RADIOGRAPHIC PROGRESSION
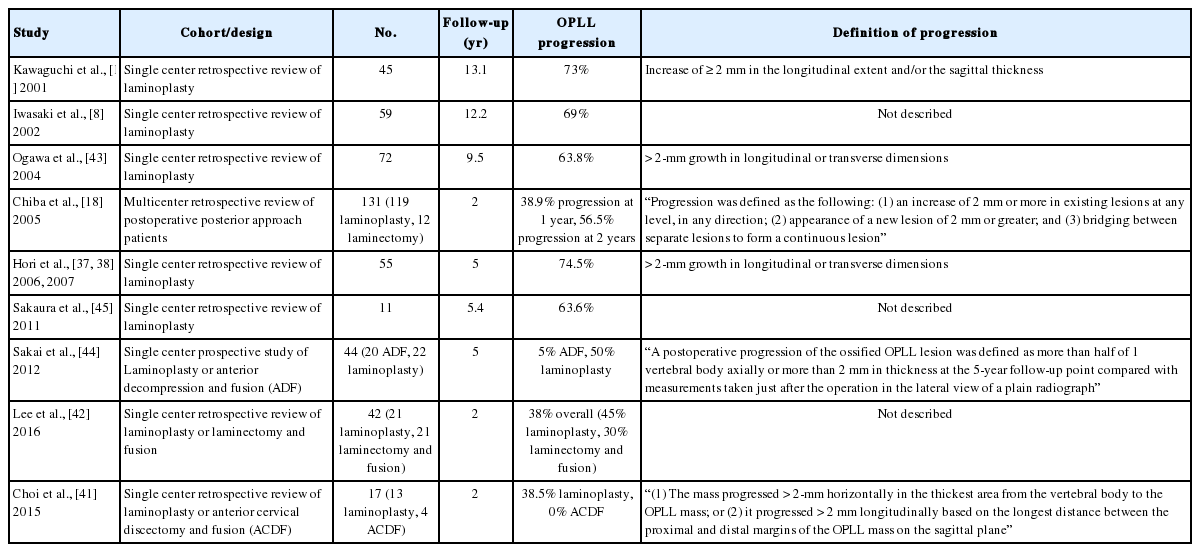
The long-term natural history of OPLL has been well described in both symptomatic and asymptomatic individuals. There is no clear universal definition of OPLL growth as it may be either clinical or radiologic progression. Several previous studies generally define radiologic progression as 2-mm growth in either longitudinal or transverse plane of an existing lesion, or appearance of a new adjacent lesion. In a longitudinal study of 359 patients and average follow-up of 17.6 years, Matsunaga et al. [31], reported 17% of patients that presented without myelopathy at time of diagnosis became symptomatic during the follow-up period, whereas 64% of patients with myelopathy experienced symptomatic exacerbation. In a longitudinal study of 167 conservatively managed patients, 42% of patients demonstrated radiographic OPLL progression with a 2 mm or more increase in ossification observed in 19% of subjects [33]. Chiba et al. [18] performed a multicenter retrospective review measuring radiographic progression of OPLL in patients following posterior decompressive surgery at one and 2 years postoperatively. Using computer assisted standardized measurements, they found radiographic progression of OPLL in approximately 39% of patients at 1 year, and 57% at 2 years. Age less than 60 and mixed and continuous type OPLL were also found to be predictive of radiographic progression. The mean growth in superior extension was 1.5 mm and 2.4 mm at one and 2 years respectively, and the mean growth in the inferior extension was 1.3 mm and 2.4 mm at 1 and 2 years respectively. Mean increase in anteroposterior thickness was 1.1 mm at one year and 1.4 mm at 2 years. Given these findings and the slow rate of growth, asymptomatic patients are often treated conservatively without a clear necessity for prophylactic decompression.
OPLL GROWTH AND BIOMECHANICAL STRESS
One hypothesis for OPLL progression is that it is related to biomechanical strain. Takatsu et al. [34] studied 97 patients with OPLL treated conservatively or surgically with either laminoplasty or laminectomy, and followed with serial radiographs. They found that growth of the OPLL occurred more rapidly in the surgical group. Takatsu theorized that increased mechanical forces by postoperative structural changes, or perhaps the inflammatory postsurgical milieu may lead to increased OPLL. These conclusions, however, must be considered with the possibility of selection bias. Symptomatic individuals with more aggressive OPLL may have more likely undergone surgery.
Iwasaki et al. [8] found age at presentation to be a significant predictor of OPLL progression with younger patients more likely to progress during a minimum 10-year follow-up compared to older patients. Given the disparity in disease severity by age, Iwasaki et al. [8] indirectly supports the hypothesis that proliferation of OPLL is stimulated by biomechanical loading and increased range of motion. Specifically, younger subjects experience increased range of motion and thereby increasing strain on the PLL. Older subjects in turn have relatively less range of motion due to degenerative changes, which ultimately may be protective against biomechanical stress. Matsunaga et al. [33] have further provided compelling evidence for a correlation between increased range of motion on flexion-extension films and myelopathy in patients with OPLL hinting at possible further OPLL progression being the causative factor.
Biomechanical principles in the context of OPLL are consistent with clinical findings. Continuous type OPLL and mixed type OPLL have been found to progress at a faster rate compared to segmental type [18,32]. This may be due to increased stress and loading superior and inferior to the areas of ossified PLL as compared to load sharing demonstrated by segmental OPLL. Long-term follow-up of patients postlaminoplasty have demonstrated delayed decreased range of motion thought to be secondary to spontaneous arthrodesis along the lateral margins of the laminoplasty [35]. Several studies have found growth of the OPLL continues in the first 2–5 years after laminoplasty followed by gradual termination of growth [18,36]. This late slowing of OPLL growth after laminoplasty potentially supports the theory that eventual decrease in range of motion may inhibit OPLL progression. In contrast, findings of continued OPLL growth up to 10 years after laminoplasty argue against a relationship between OPLL progression and changes in biomechanical stress [8,37,38]. This has led some investigators to conclude that progression is likely more related to OPLL subtype with continuous or mixed patterns demonstrating continued growth.
OPLL GROWTH AND FUSION SURGERY
The goal of OPLL surgery is to decompress the cervical spine, however the surgical approach continues to be debated [4-12]. Posterior approaches such as cervical laminectomy (with or without fusion) or laminoplasty indirectly decompress the spine by allowing dorsal migration of the spinal cord away from the OPLL, and are associated with reduced rate of complications. Figs. 2 and 3 demonstrate posterior decompression via laminoplasty and laminectomy and fusion respectively. Alternatively, anterior surgical approaches like cervical discectomy and fusion (ACDF) or corpectomy (Fig. 4) involve resection of the calcified ligament with direct ventral decompression, potentially resulting in earlier symptomatic improvement, but with higher incidence of complications. Complications specifically related to anterior surgery include difficulty swallowing, hoarse voice, injury to the spinal cord resulting in neurologic deficit, and durotomy with cerebrospinal fluid fistula [2,3,5,18,30,39,40].
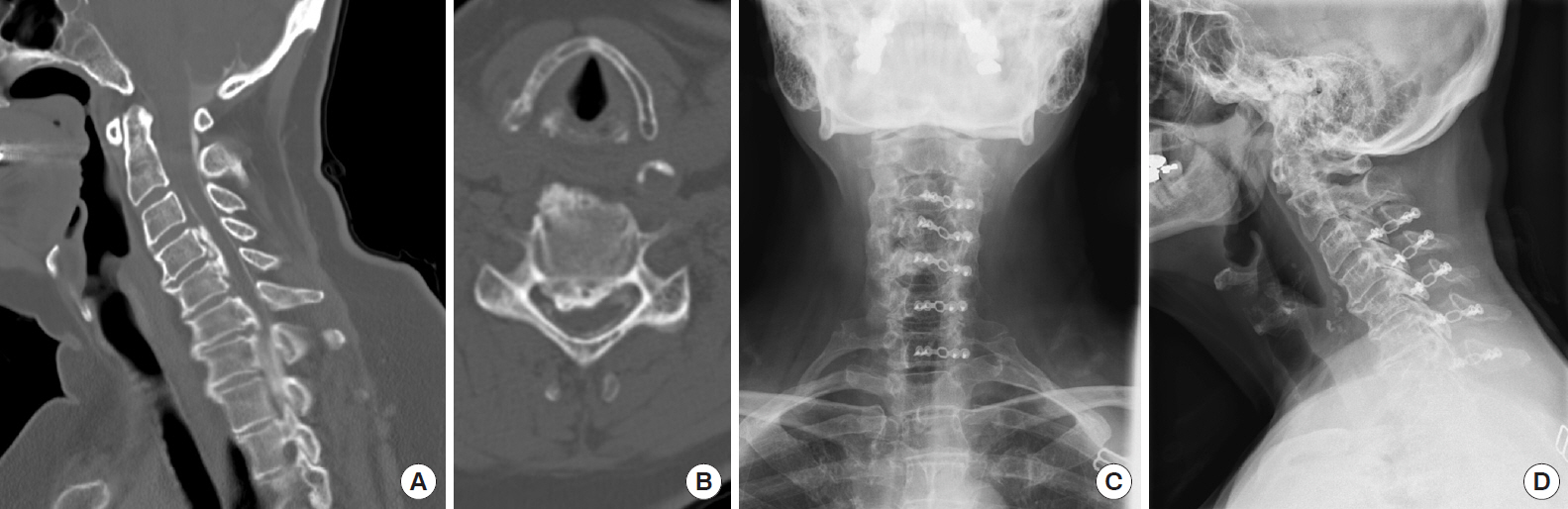
Preoperative computed tomography (CT) myelogram of a 69-year-old female who presented with cervical myelopathy. (A) Sagittal imaging demonstrating mixed type ossification of the posterior longitudinal ligament (OPLL) from C3–7. (B) Axial CT imaging demonstrating OPLL causing spinal cord compression at the level of the C4–5 disc space. Posterior decompression was performed via C3–7 open door expansile laminoplasty with plate reconstruction of the posterior elements. (C, D) Postoperative anteroposterior and lateral plain radiographs.
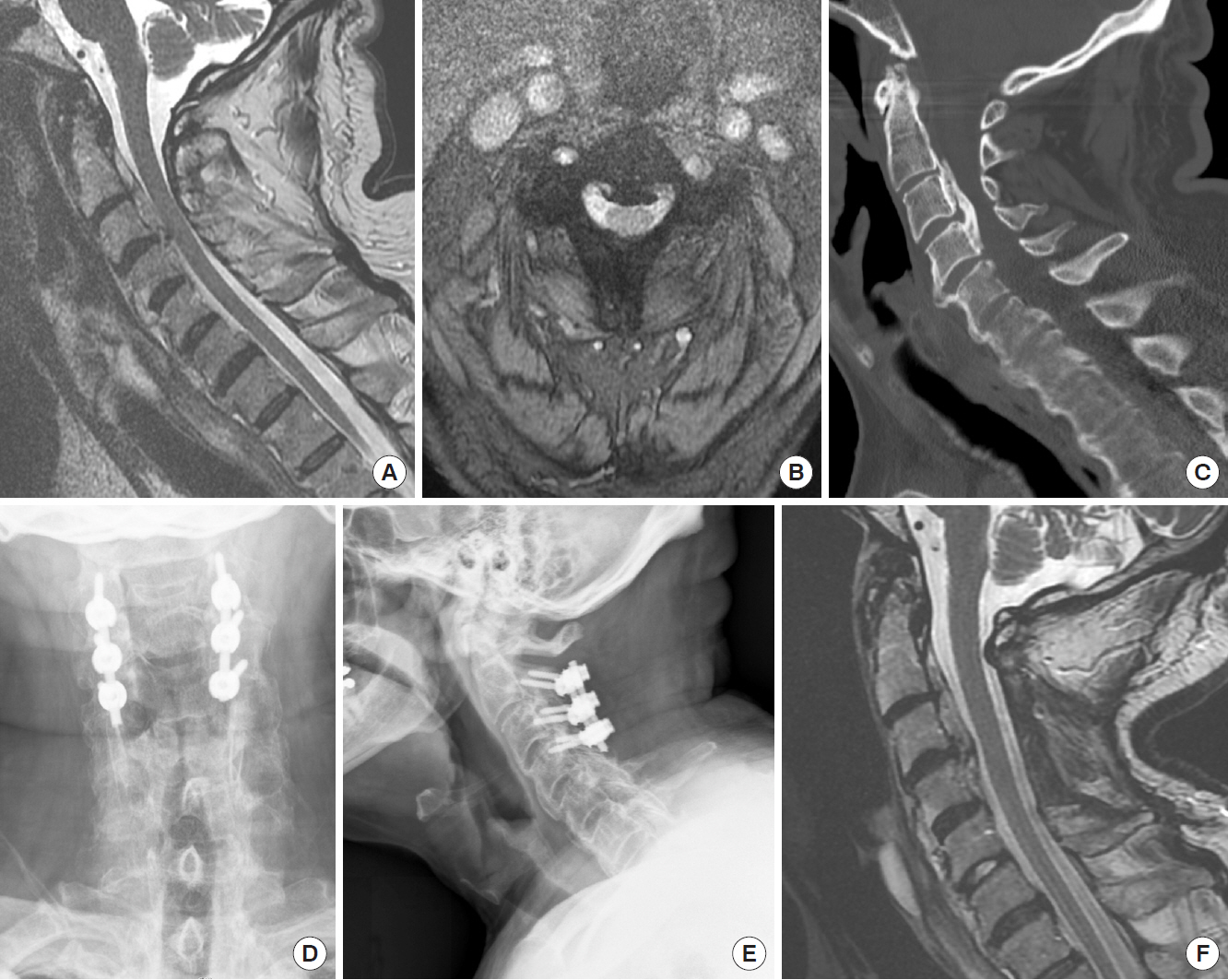
A 70-year-old male presented with progressive cervical myelo-radiculopathy. He had a known history of OPLL and was initially managed conservatively until symptom progression 5 years later. (A) Midsagittal T2 magnetic resonance imaging (MRI) demonstrating spinal canal stenosis with spinal cord compression from C2–4. (B) Preoperative axial MRI demonstrating spinal cord compression at the level of C3. (C) Preoperative sagittal computed tomography demonstrating continuous type OPLL from C2–4. (D, E) Six-week postoperative anteroposterior and lateral plain radiographs demonstrating C2–4 laminectomy and posterior instrumented fusion. (F) Postoperative MRI demonstrating decompression of the spinal cord.

A 37-year-old athlete male with acute posttraumatic severe myelopathy. (A) Magnetic resonance imaging demonstrating sagittal and axial severe spinal cord compression at the levels of C4–5. (B, C) Preoperative computed tomography (CT) confirming segmental type OPLL. The patient underwent a multilevel corpectomy (C4 and C5) and OPLL resection with a C3–6 instrumented fusion. (D) Postoperative sagittal CT demonstrating corpectomy and OPLL resection, anterior column reconstruction with a fibular strut graft, and plate and screws.
The predominance of literature has evaluated OPLL progression in the context of laminoplasty, a motion-preserving procedure. Given the possibility that further OPLL growth may be related to cervical motion and biomechanical stress, several studies have investigated whether OPLL continues to progress after anterior or posterior decompression and fusion surgery. Table 1 summarizes several of these studies with respect to postoperative OPLL growth rates [8,18,19,37,38,41-45].
1. Anterior Surgery
Several studies have evaluated anterior surgical decompression and fusion for OPLL. Sakai et al. [44] prospectively evaluated 20 patients that underwent ACDF compared to 22 patients that underwent laminoplasty with 5-year follow-up. The ACDF patients had significantly longer surgical times (300.3 minutes vs. 183.2 minutes), 5 complications including 3 reoperations compared to zero complications in the laminoplasty group. The Japanese Orthopedic Association scale (JOA) recovery rate was better in the ACDF group (71.4% vs. 55.3%). Continued ossification of the PLL was observed in 5.0% of the ACDF group compared to 50% of the laminoplasty group with similar neurologic recovery at the 3-year follow-up period, although the anterior cohort included patients in whom the OPLL was resected. At 4 years, however, neurologic recovery of the ACDF group was statistically superior compared to the laminoplasty group, particularly in patients with extensive OPLL and kyphotic alignment. At 5-year follow-up, neurologic deterioration was not observed in the ACDF group, but 5 cases of late neurologic deterioration were identified in the laminoplasty group. Two of these 5 cases of late neurologic deterioration were attributed to postoperative progression of the OPLL at the surgical level or adjacent segment.
Matsuoka et al. [46] evaluated 63 patients that underwent anterior corpectomy and fusion for OPLL using the anterior floating method with average 13-year follow-up. An important distinction of this surgical technique is that the OPLL is not directly resected (i.e., circumferentially released and left “floating”), but the involved levels are fused anteriorly. Thirteen of the 63 patients experienced recurrence of myelopathy. Three patients experienced myelopathy recurrence due to OPLL progression adjacent to the surgical level, 4 patients experienced recurrence of symptoms due to inadequate decompression, and 6 cases were due to expansion of ossification at levels that were not adjacent to the operative levels (e.g., thoracolumbar). Reoperation typically occurred 8 years after the index operation. Postoperative progression of OPLL at the same surgical level was present in only 3 of 63 patients secondary to ligament hyperplasia of 1 to 1.5 mm. Radiographically, 36 patients demonstrated progression of ossification with 9 patients demonstrating greater than 2-mm ossification at the levels adjacent to the surgical field [46].
2. Posterior Surgery
Lee et al. [42] compared laminoplasty, laminectomy alone and laminectomy fusion in 57 patients with minimum 24-month follow-up. Twenty-one patients underwent laminoplasty, 21 patients laminectomy with fusion, and 15 patients underwent laminectomy alone. In all 3 groups, quality of life measures similarly improved postoperatively. Progression of ossification of OPLL was observed in 45.5% in the laminoplasty group, 62.5% in the laminectomy group and only 30% in the laminectomy and fusion group [42]. These results indicate that fusion is associated with the lowest incidence of OPLL progression. The lower risk of OPLL progression with laminoplasty compared to laminectomy alone also suggests that preservation of the dorsal tension band with potentially better stability may also reduce OPLL growth.
Ota et al. [47] studied 50 patients with OPLL (46% laminoplasty and 54% posterior decompression and fusion). Preoperative CT scans demonstrated no significant radiographic differences between the 2 groups. In the laminoplasty group, 4 of 23 patients eventually had spontaneous fusion postoperatively compared to 23 of 27 patients in the posterior decompression and fusion group. The progression of thickness in the posterior decompression and fusion group was significantly smaller (-0.1±0.4 mm) compared to the laminoplasty group (0.6±0.7 mm). Interestingly, several patients in the posterior decompression and fusion group not only demonstrated growth arrest of the ossified ligament but decreased thickness by more than 0.5 mm was observed as well. The investigators postulate that OPLL progression can occur from segmental motion and instrumented fusion may suppress OPLL progression by reducing cervical range of motion [47].
Similarly, Katsumi et al. [9] performed a comparative study of OPLL and laminoplasty vs. posterior decompression and fusion. They compared 19 patients in the posterior laminectomy and fusion group to 22 patients in the laminoplasty group. The patients that underwent posterior decompression and fusion demonstrated a significantly reduced rate of OPLL progression (2.0±1.7%/yr) compared to the laminoplasty group (7.5±5.6 %/yr). Interestingly, the annual growth rate decreased with later follow-up, suggesting that OPLL growth arrest may occur as the surgical levels fused [9].
Lin et al. [40] compared 26 patients that underwent anterior cervical corpectomy, and 30 patients that underwent posterior laminectomy and fusion with a minimum of 2-year follow-up. The 2 groups were similar in baseline radiographic and demographic characteristics. The anterior surgery cohort had significantly greater blood loss (763 mL compared to 516 mL) and longer surgical time (161 minutes compared to 125). The average number of corpectomy levels was 1.92 (range, 1–3 vertebrae) and number of levels fused was 3.92 (range 3–5 vertebrae). The posterior surgery cohort averaged 3.67 (range, 3–5) laminectomy levels and 4.27 fused segments (range, 3–6). The JOA recovery rate was similar between anterior (58.6%) and posterior (54.8%) surgery. Only 1 patient that underwent posterior fusion had OPLL progression compared to none in the anterior corpectomy and fusion cohort.
In contrast to these studies, Choi et al. [41] retrospectively evaluated 60 cervical OPLL patients with greater than 24-month follow-up (average, 29.6 months) with preoperative and postoperative CT scans. Criteria for progression was at least 2-mm growth horizontally or longitudinally during the follow-up period. Patients were divided into 2 groups based on OPLL progression at most recent follow-up (14 patients with OPLL progression and 48 patients without OPLL progression). Surgical intervention was performed in 38 patients and conservative management in 22 patients with similar OPLL progression rates of 23% and 22% in the surgical and nonsurgical groups. Patients were controlled for surgical approach (anterior vs. posterior and fusion vs. nonfusion). No statistically significant difference in OPLL progression was found between cohorts, albeit with a relatively small overall study population [41].
Finally, in a meta-analysis of laminoplasty versus laminectomy and fusion studies, Lee et al. [48] reviewed 11 studies totaling 530 patients with OPLL who underwent posterior cervical decompression. Four hundred and 29 patients underwent laminoplasty and 101 underwent posterior decompression and fusion. Radiographic progression of OPLL in the laminoplasty group was 62.5% and only 7.6% in the decompression and fusion group. Laminoplasty patients exhibited a ~60% prevalence of progression of OPLL at 10-year follow-up. Despite this, no statistically significant difference was observed in incidence of late neurologic decline (defined as a decrease of 2 or more points on the Japanese Orthopedic Association scale) for laminoplasty (8.3%) vs. laminectomy and fusion (3.8%). This meta-analysis should be interpreted with caution, however, as potential biases (e.g., selection or publication bias) and other confounding factors (e.g., clinical outcome measures, radiographic assessment) may have had varying impact on the reported results. Nonetheless, this meta-analysis demonstrates that posterior decompression with or without fusion results in improved clinical outcomes, with an indication that the addition of fusion may decrease risk of radiographic OPLL progression.
CONCLUSION
OPLL is a progressive disease with evidence of slow growth over time. Surgical treatment for decompression of the neural elements is effective at improving neurologic function, and has been demonstrated to have durable long-term benefit. Despite surgery, OPLL demonstrates continued growth particularly after laminoplasty. The addition of fusion to surgical treatment is associated with a lower incidence of OPLL growth, and some studies even suggest potential for growth arrest once arthrodesis occurs. These findings indicate a potential relationship between OPLL growth and biomechanical factors such as motion with strain on the involved segments. Future studies are needed to better elucidate these pathophysiologic mechanisms, and how they may better predict need for surgery and subsequent long-term outcome.
Notes
The authors have nothing to disclose.