Endoscopic and Nonendoscopic Approaches to Single-Level Lumbar Spine Decompression: Propensity Score-Matched Comparative Analysis and Frailty-Driven Predictive Model
Article information
Abstract
Objective
The endoscopic spine surgery (ESS) approach is associated with high levels of patient satisfaction, shorter recovery time, and reduced complications. The present study reports multicenter, international data, comparing ESS and non-ESS approaches for single-level lumbar decompression, and proposes a frailty-driven predictive model for nonhome discharge (NHD) disposition.
Methods
Cases of ESS and non-ESS lumbar spine decompression were queried from the American College of Surgeons National Surgical Quality Improvement Program database (2017–2020). Propensity score matching was performed on baseline characteristics frailty score (measured by risk analysis index [RAI] and modified frailty index-5 [mFI-5]). The primary outcome of interest was NHD disposition. A predictive model was built using logistic regression with RAI as the primary driver.
Results
Single-level nonfusion spine lumbar decompression surgery was performed in 38,686 patients. Frailty, as measured by RAI, was a reliable predictor of NHD with excellent discriminatory accuracy in receiver operating characteristic (ROC) curve analysis: C-statistic: 0.80 (95% confidence interval [CI], 0.65–0.94) in ESS cohort, C-statistic: 0.75 (95% CI, 0.73–0.76) overall cohort. After propensity score matching, there was a reduction in total operative time (89 minutes vs. 103 minutes, p = 0.049) and hospital length of stay (LOS) (0.82 days vs. 1.37 days, p < 0.001) in patients treated endoscopically. In ROC curve analysis, the frailty-driven predictive model performed with excellent diagnostic accuracy for the primary outcome of NHD (C-statistic: 0.87; 95% CI, 0.85–0.88).
Conclusion
After frailty-based propensity matching, ESS is associated with reduced operative time, shorter hospital LOS, and decreased NHD. The RAI frailty-driven model predicts NHD with excellent diagnostic accuracy and may be applied to preoperative decision-making with a user-friendly calculator: nsgyfrailtyoutcomeslab.shinyapps.io/lumbar_decompression_dischargedispo.
INTRODUCTION
Minimally invasive surgery (MIS) techniques have improved patient and surgeon satisfaction across the spectrum of spine pathologies [1,2]. This group of techniques has minimized soft tissue manipulation, blood loss, and infection rates while allowing for expeditious recovery time [3,4]. More recently, endoscopic spine surgery (ESS) was introduced as a minimally invasive treatment option for lumbar spine pathologies [5]. ESS is defined by endoscope utilization for visualization in adjunct with tubular instruments through small incisions. This approach holds promise for minimizing tissue disruption and associated postoperative pain, further accelerating recovery [6].
ESS has been previously shown to decrease the risk of common surgical complications such as muscle crush injury from protractors, soft tissue stripping, and excessive bone loss [7,8]. While the literature regarding ESS versus non-ESS (open or other MIS) spinal surgery is sparse, several studies have suggested MIS superiority [8-13]. When comparing ESS to other MIS techniques, recent literature suggests ESS is better with the appropriate surgical indications [14,15]. Of note, one recent study found no difference in early postoperative outcomes between endoscopic guided approaches and open approaches to single-level lumbar decompression [16]. However, the significance of the study is questionable as the sample size was low and there was no adjustment for baseline measured differences.
Frailty, as measured by scales such as modified frailty index-5 (mFI-5) and risk analysis index (RAI) administrative-revised, have been shown to predict neurosurgical outcomes across the spectrum of neurosurgical subspecialties in the recent literature, and frailty assessment provides a reliable baseline of physiological reserve [17-21]. Herein, the authors sought to supply data to support preoperative decision-making for minimally invasive spine surgery by analyzing outcomes across propensity score-matched ESS and non-ESS groups using data derived from a large, multicenter, surgical database. The intention was to identify whether any ESS benefits were present, with an emphasis on the hospital course. Furthermore, the authors sought to describe the impact of baseline frailty on patient outcomes using predictive analytics.
MATERIALS AND METHODS
1. Study Design
The present study was a retrospective observational analysis of a prospectively maintained, multicenter, international (49 USA, 11 countries), database. This manuscript was formatted in accordance with standardized reporting guidelines from the Equator Network: The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.
2. Data Source and Setting
The data source was the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, 2017–2020. Characteristics of the ACS-NSQIPs database have been described previously [22,23]. The study was considered exempt from continuing review by our Institutional Review Board (IRB-21-315) and conducted under the data user agreement between ACS and our institution.
3. Participants
Study participants included patients aged 18 or greater who underwent nonfusion single-level spine decompression at an NSQIP-participating institution. Cases were selected using Current Procedural Terminology (CPT) codes 62380 (endoscopic decompression of neural elements and/or excision of herniated disc) and 63030 (simple single-level lumbar decompression of neural elements and/or excision of a herniated disc, may include partial facetectomy, foraminotomy). Patients were excluded from the study if their age or discharge disposition were not reported.
4. Variables
Preoperative frailty, as measured by the mFI-5 and RAI, was the primary predictor variable. The RAI was computed using methodology previously described by Arya and Hall et al in the recalibration and external validation of the RAI for utilization with the ACS-NSQIP database (RAI-Rev) [24,25]. Demographic information (age, sex, race, ethnicity, body mass index [BMI]) and elective surgery status were also considered. The primary outcome was nonhome discharge disposition (NHD). “Home” included discharge home or facility which was home. Secondary outcomes included major complications (intubation over 48 hours, unplanned intubation, deep vein thrombosis/thrombophlebitis, pulmonary embolism, cerebrovascular accident/stroke, myocardial infarction, wound disruption, cardiac arrest requiring cardiopulmonary resuscitation), total operative time, unplanned readmission and reoperation, and 30-day mortality. Complications present at the time of admission (PATOS) were not considered to be surgical complications.
5. Statistical Analysis
Statistical analysis was performed with the open-source R ver. 2022.07.0+548 (R Foundation for Statistical Computing, Vienna, Austria) with adjunctive assistance from IBM SPSS Statistics ver. 28.0 (IBM Co., Armonk, NY, USA). Alpha was designated at 0.05, where p < 0.05 was considered statistically significant. Baseline demographics, preoperative clinical characteristics, and outcomes were derived from the NSQIP database. Continuous variables were reported as mean with standard deviation (standard deviation). Proportions were reported as frequencies with a percentage of the cohort total. The Pearson chi-square test was used for categorical variables and the independent-samples t-test or Mann-Whitney U-test for the comparison of continuous variables. A predictive model was built using logistic regression for the primary outcome of NHD after single-level lumbar spine surgery. Discriminatory ability was assessed with receiver operating characteristic (ROC) curve analysis with computation of C-statistics (95% confidence intervals [CIs]) and interpreted using established epidemiological criteria per Hosmer-Lemeshow: outstanding (0.9–1.0), excellent (0.8–0.89), acceptable (0.7–0.79), poor (0.6–0.69), and no discrimination (0.5–0.59) [26]. The DeLong test assessed whether the area under the curve for RAI was statistically significantly different from that for chronological age and the mFI-5 score. The R packages rms and shiny were used to generate an interactive calculator [27-29].
RESULTS
1. Participants
The study cohort included 38,686 patient cases with 174 (0.4%) treated ESS and 38,512 treated non-ESS. The study cohort was 44% female with median age, in years, of 51 (interquartile range [IQR], 38–64 years). The overall cohort was stratified by RAI frailty scoring into robust (RAI 0–20: N = 28,288 [73.1%]), normal (RAI 21–30: N = 9,923 [25.7%]), frail (RAI 31–40: N = 452 [1.2%]), and severely frail (RAI ≥ 41: N = 1 [ 0.1%]) (Table 1).
2. Descriptive Data
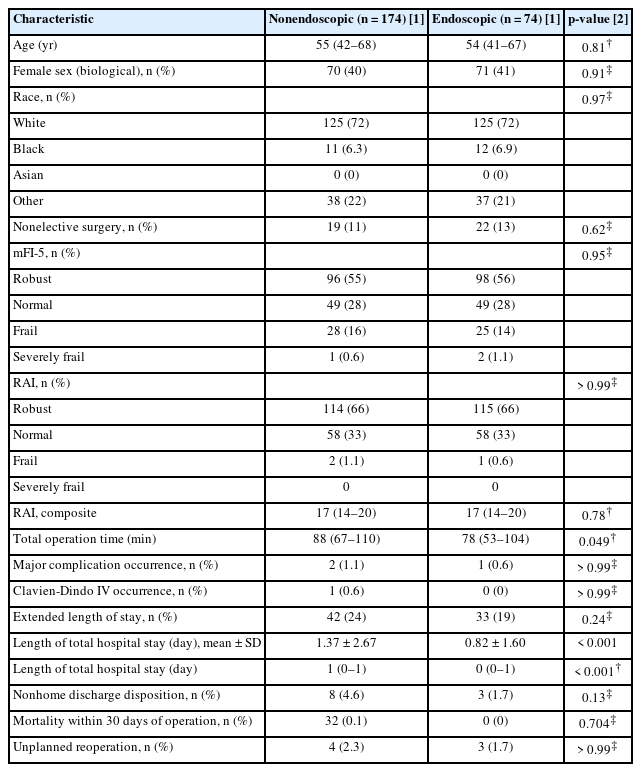
At baseline, the ESS cohort was chronologically older (54 years vs. 51 years, p = 0.019) and frailer (RAI average 17 vs. 16, p = 0.016) compared to the non-ESS cohort. Before propensity score matching, hospital length of stay (LOS) was shorter in ESS (0.8 days) versus non-ESS (1.3 days) surgery with other postoperative outcomes equivocal between cohorts (Table 1).
3. Outcome Data
Postoperative outcomes within 30 days for both cohorts were reported before and after propensity matching. Prior to matching, major postoperative complications were seen in 0.6% of ESS patients and 0.9% of non-ESS patients (p = 0.678). Clavien-Dindo IV complications were seen in 0.0% and 0.7%, respectively (p = 0.272). The average LOS for ESS patients was 0.8 days compared to 1.3 days in the non-ESS cohort (p < 0.001). NHD was reported in 1.7% of the ESS cohort compared to 3.3% of the non-ESS patients (p = 0.249). Unplanned readmission was reported in 4.0% and 3.1%, respectively (p = 0.498), while unplanned reoperation was reported in 2.3% and 2.7%, respectively (p = 0.752). There were no fatalities in the ESS cohort, and 11 patients expired in the non-ESS cohort (p = 0.824). Complete postoperative complication data prior to matching can be found in Table 1.
After propensity score matching (1:1 nearest neighbor method, 0.1 caliper), a non-ESS cohort of 174 similar patients was compared to the original ESS cohort. Propensity matching calibration can be found in Fig. 1. There was a statistically significant reduction in total operative time (89 minutes vs. 103 minutes, p = 0.049) and hospital LOS (0.82 days vs. 1.37 days, p < 0.001) in patients treated endoscopically (Table 2). Other outcomes were extremely rare in the ESS cohort and thus limited statistical comparison: Clavien-Dindo IV complication (N = 0), unplanned reoperation (N = 3), and mortality within 30 days of operation (N = 0) (Table 2).

Propensity score matching (1:1 nearest neighbor, caliper 0.1) diagnostics of endoscopic and nonendoscopic study cohorts. mFI-5, modified frailty index-5; RAI, risk analysis index; eCDF, empirical cumulative distribution function.
4. Main Results – Frailty-Driven Predictive Model
Frailty, as measured by RAI, was a reliable predictor of the primary outcome of NHD with excellent discriminatory accuracy in ROC analysis: C-statistic: 0.80 (0.65–0.94) in ESS, C-statistic: 0.75 (0.73–0.76) overall cohort.
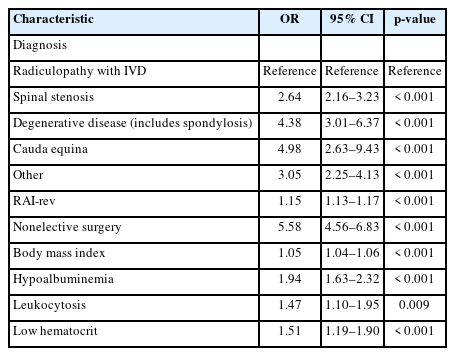
In the overall study cohort (ESS and non-ESS), a predictive model was built for the primary outcome of NHD disposition (Table 3). In the model, the independent predictors of NHD included indication for lumbar decompression, RAI score, nonelective surgery, BMI, and several abnormal preoperative labs (hypoalbuminemia, leukocytosis, low hematocrit). In ROC analysis, the frailty-driven model predicted the primary outcome of NHD with excellent discriminatory accuracy as displayed in Fig. 2, C-statistic: 0.87; 95% CI, 0.85–0.88). The predictive model was deployed into a web application: nsgyfrailtyoutcomeslab.shinyapps.io/lumbar_decompression_dischargedispo.
Logistic regression predictive model for nonhome discharge after single-level lumbar decompression surgery (endoscopic and nonendoscopic)
DISCUSSION
The present study analyzes a large modern series of 38,686 patients undergoing minimally invasive lumbar spine surgery in the 2017–2020 ACS-NSQIP database. In propensity-matched cohorts, ESS (vs. non-ESS) surgery reduced operative time and hospital LOS. Furthermore, the RAI frailty index predicted NHD destination with excellent diagnostic accuracy (0.75). A predictive model for NHD destination with RAI as the core predictor was proposed and enhanced with consideration of surgical indication, BMI, and several preoperative lab values. By contrast, a prior study of 34 patients undergoing single-level endoscopic lumbar surgery (ACS-NSQIP 2017) reported no differences in the rate of mortality, reoperation, readmission, complications, operative time, or LOS [16]. The low sample size and lack of adjustment for baseline frailty in the prior paper may have contributed to the equivocal outcomes.
1. Interpretation
Here, the RAI was applied to effectively match 2 surgical cohorts on baseline characteristics, which further demonstrates its versatility. Recent literature suggests that RAI, as a metric of frailty, is a reliable, easily utilizable metric with benefits in preoperative decision-making [18,21,24]. The disparity in results before and after propensity score matching highlights the importance of adjusting for baseline frailty for comparative analyses. The findings further underscore the importance of the continued study of ESS research with the design of high-powered randomized controlled trials to minimize confounders attributable to unmeasurable baseline differences. Despite the non-randomized study design, the results demonstrate that ESS for lumbar decompression is exceptionally safe and abbreviates patient recovery.
As discussed in the NSQIP series and systematic review by Chiu et al. [16], the ESS literature is controversial regarding the safety and efficacy of ESS vs. non-ESS approaches to lumbar spine surgery. The literature suggests that ESS is associated with reduced patient time returning to work, increased recovery speed, and preservation of paraspinal muscles, reduced infection, and need for supportive care while also noting ESS to be associated with increased rates of incomplete decompression [30-33]. Some studies report ESS as superior, inferior, or not statistically different than non-ESS techniques, resulting in unclear for any one method of preoperative decision-making [30,33,34]. The limited sample size in most prior studies may explain some degree of ambiguity. As the present study found complications in both single-level non-ESS and ESS to be exceedingly rare, these previous studies may have been similarly unable to capture the granular differences in outcomes. The minuscule complication rate observed in the present cohort supports the trend in literature towards safe, minimally invasive, approaches to lumbar spine surgery [10,35].
While most patients rapidly recover from single-level lumbar decompression, there are certainly a group of patients with a complicated postoperative course warranting attention in the preoperative setting. The early identification of patients at high risk for delayed recovery is critical for the implementation of targeted interventions such as “enhanced recovery after surgery.” Thus, a predictive model was proposed that predicted NHD destination with excellent discriminatory accuracy. The C-statistic of 0.87 suggests most NHD can be anticipated preoperatively by considering RAI frailty score, surgical indication, the timing of surgery (elective vs. nonelective), BMI, and several key lab values (serum albumin, leukocyte count, and hematocrit). A model with this level of diagnostic accuracy is superior compared to similar models in previous literature [36,37]. The predictive model bears clinically translatable knowledge that may be used to reduce poor outcomes among spine surgery patients with augmentation of surgical decision-making or perioperative care.
2. Limitations
The endoscopic spine CPT code, introduced in 2017, was the first CPT code unique to minimally invasive spine surgery. Thus, the code is likely still underutilized and thus the present study may underestimate the total number of ESS (N = 174) performed at NSQIP-participating hospitals during the study period. Nationwide databases provide statistical power to enable complex analyses with widely generalizable results but are not without limitations. Database studies may include observer bias and data quality discrepancies. Patient case information such as the severity of disease, chronicity of disease, and unmeasured comorbidities or risk factors that may affect outcomes are omitted. Coding bias among the ICD and CPT systems may further influence the fidelity of the data. The coding systems reduce the granularity at which analysis may occur, for example, specific nonendoscopic techniques were not differentiated within the study cohort. Furthermore, The NSQIP does not include data beyond 30 days postoperatively, resulting in an inability to assess long-term outcomes.
3. Generalizability
The study population was derived from a multicenter, international (49 USA, 11 countries) database which significantly increases the generalizability of results. Although the specific approach for the nonendoscopic cases was not known, we expect the majority of single-level nonfusion decompression procedures from 2017–2020 to be minimally invasive [8,9,38]. Clinically, the findings suggest that patients flagged as high risk for delayed recovery may benefit from minimally invasive approaches, which may include but are not limited to ESS. However, the present study was limited in granularity by available CPT codes which do not uniquely identify other types of MIS and thus require further in-depth analysis in a different study design.
CONCLUSION
The present study suggests that ESS is a safe and effective type of minimally invasive spine surgery in a large multicenter analysis from 2017–2020. After propensity score matching on baseline characteristics (particularly frailty measured by RAI-rev), endoscopic surgery was associated with reduced operative time, hospital LOS, and NHD disposition. Overall, the rates of delayed recovery and postoperative complications/morbidity after single-level lumbar decompression surgery were exceptionally rare. The RAI frailty index enhances preoperative risk stratification by predicting NHD with excellent diagnostic accuracy and may be translated clinically with a user-friendly calculator: nsgyfrailtyoutcomeslab.shinyapps.io/lumbar_decompression_dischargedispo. The early identification of patients at high risk for delayed recovery is critical for the implementation of targeted interventions and anticipatory guidance.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: AJK, KR, SFK, MHS, PCS, CAB; Data curation: AJK, KR, MHS, PCS; Formal analysis: AJK, KR, ACS; Methodology: AJK, KR, SFK, JV; Project administration: KR, SFK, MHS, PCS, CAB; Visualization: AJK; Writing - original draft: AJK, ACS, JV; Writing - review & editing: AJK, KR, ACS, SFK, JV, MHS, PCS, CAB