Role of Preoperative Albumin Quotient in Surgical Planning for Posttraumatic Syringomyelia: A Comparative Cohort Study
Article information
Abstract
Objective
Surgical procedures for patients with posttraumatic syringomyelia (PTS) remain controversial. Until now, there have been no effective quantitative evaluation methods to assist in selecting appropriate surgical plans before surgery.
Methods
We consecutively enrolled PTS patients (arachnoid lysis group, n = 42; shunting group, n = 14) from 2003 to 2023. Additionally, 19 intrathecal anesthesia patients were included in the control group. All patients with PTS underwent physical and neurological examinations and spinal magnetic resonance imaging preoperatively, 3–12 months postoperatively and during the last follow-up. Preoperative lumbar puncture was performed and blood-spinal cord barrier disruption was detected by quotient of albumin (Qalb, cerebrospinal fluid/serum).
Results
The ages (p = 0.324) and sex (p = 0.065) of the PTS and control groups did not differ significantly. There were also no significant differences in age (p = 0.216), routine blood data and prognosis (p = 0.399) between the arachnoid lysis and shunting groups. But the QAlb level of PTS patients was significantly higher than that of the control group (p < 0.001), and the shunting group had a significantly higher QAlb (p < 0.001) than the arachnoid lysis group. A high preoperative QAlb (odds ratio, 1.091; 95% confidence interval, 1.004–1.187; p = 0.041) was identified as the predictive factor for the shunting procedure, with the receiver operating characteristic curve showing 100% specificity and 80.95% sensitivity for patients with a QAlb > 12.67.
Conclusion
Preoperative QAlb is a significant predictive factor for the types of surgery. For PTS patients with a QAlb > 12.67, shunting represents the final recourse, necessitating the exploration and development of novel treatments for these patients.
INTRODUCTION
Syringomyelia is a heterogeneous group of diseases with major causes including but not limited to Chiari malformation (CM), trauma and tumor. Posttraumatic syringomyelia (PTS) is a disorder characterized by progressive neurological deterioration as the result of cerebrospinal fluid (CSF)-filled cysts expansion that occurs following spinal cord injury (SCI), accounting for approximately 20% of patients with traumatic SCI, causing pain and neurological deficits in addition to those incurred with the original injury [1-4]. Most research suggested that PTS originates from a CSF circulation disorder caused by intradural adhesions [5-7]. In clinical practice, magnetic resonance imaging (MRI) is usually used to determine the morphology and levels of the syrinx, whereas myelography is usually used to show the exact location of intradural adhesions [4,8]; however, myelography is too subjective in determining the degree of arachnoid adhesions. Typically, neurosurgeons select surgical approaches based on intraoperative assessment of intradural adhesions, and arachnoid lysis is often chosen for patients with mild adhesions. For patients with more severe adhesions, neurosurgeons prefer shunting because arachnoid lysis is more likely to aggravate original SCI. However, determining the optimal surgical approach preoperatively to achieve improved prognostic outcomes remains a challenging issue.
The pathophysiological mechanism of PTS is generally considered to be the transparenchymal flow of CSF from the subarachnoid space to the syrinx [1], while some have suggested that opening of blood-spinal cord barrier (BSCB) may also play an important role in the occurrence and development of syringomyelia [9]. The involvement of BSCB opening in secondary SCI has been reported in previous degenerative cervical myelopathy [10], and we found a positive association between damaged BSCB and symptoms in CM-syringomyelia through our prospective cohort study (NCT04856839) (unpublished). Additionally, PTS is associated with more severe myelopathy and intradural adhesions than CM-syringomyelia according to our previous experience [3]. Moreover, previous researches on PTS animal models have also suggested that BSCB impairment may be a possible contributing mechanism [9,11]. It is reasonable to speculate that the presence of BSCB impairment in PTS and a severely damaged BSCB may provide a pathway for more fluid to flow from the blood into the syrinx, leading to further development of PTS [9,12].
There are 2 main surgical procedures commonly used for patients with PTS: arachnoid adhesion lysis, aimed to reconstruct intradural CSF circulation, is usually the first option for most patients [13,14], while shunting usually serve as the secondary choice, to construct a new drainage pathway based on the original physiological structures. Although several studies have concluded that arachnoid lysis is an effective etiological treatment for conditions like CM-syringomyelia or PTS [3]. However, intradural decompression often improves myelopathy in less than 50% of PTS patients [1,3,14,15], whereas it improves 80% in CM-syringomyelia [16]. Moreover, based on our clinical observations, we noticed a correlation between higher QAlb levels and a poorer prognosis in patients during follow-up examinations. Hence, it is reasonable to hypothesize that, in addition to abnormal intradural CSF hydrodynamics, damage to the BSCB may be a contributing factor in the pathophysiological mechanisms of PTS. Nonetheless, the connection between the extent of BSCB damage and the choice of surgical procedure remains to be elucidated.
We propose the following hypotheses for our study. Firstly, we hypothesize that the degree of BSCB damage, as indicated by QAlb, will be elevated in the PTS group compared to the control group. Secondly, we postulate that the selection of the surgical approach might be predicted not solely based on the degree of intradural adhesions but also by the extent of damage to the BSCB damage (QAlb). Lastly, we hypothesize that the prognostic differences between the arachnoid lysis and shunting groups will be comparable, indicating the efficacy of shunting in patients with severe PTS.
MATERIALS AND METHODS
1. Patients
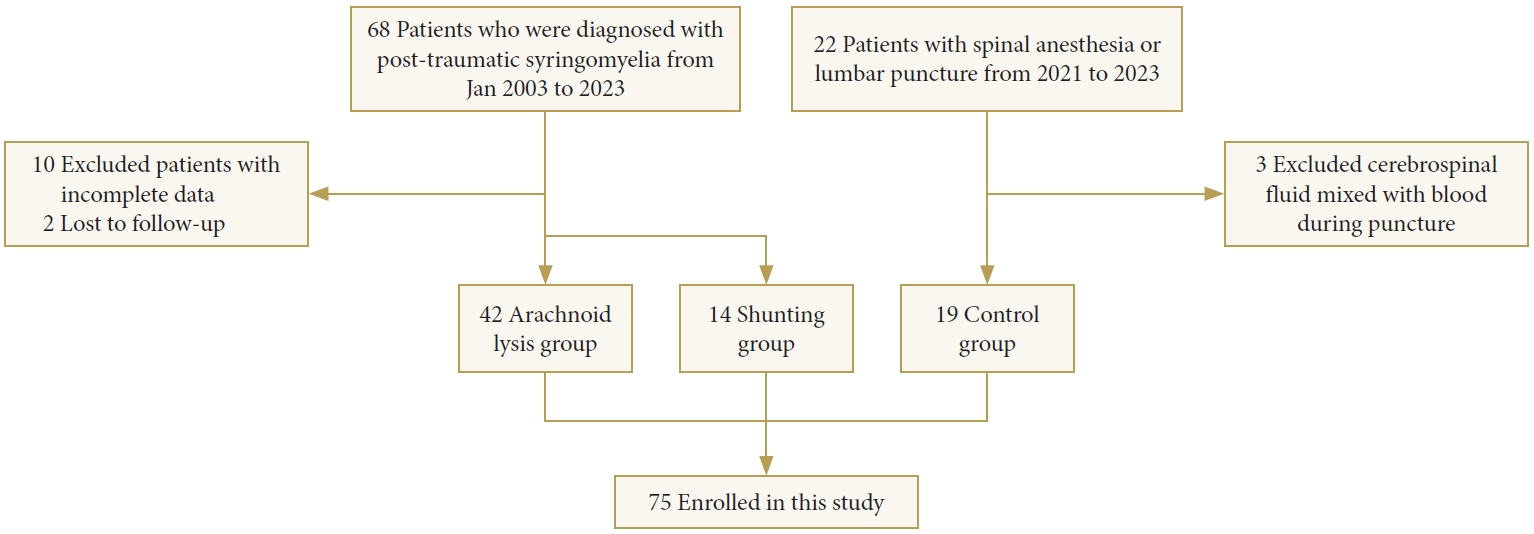
Participants were recruited from January 2003 and 2023 at Xuanwu Hospital. The PTS patient cohort was part of the XWCOPSM study (NCT04856839), which is extensively described elsewhere. Inclusion criteria for patients were as follows: (1) confirmed diagnosis of PTS by MRI; (2) willingness and ability to participate in the study. Exclusion criteria encompassed: (1) incomplete data; (2) prior shunting intervention; (3) pregnancy; (4) inability to complete follow-up; (5) presence of other concomitant spinal lesions; (6) comorbidity with other central nervous system disorders that could potentially impact the central nervous system, such as multiple sclerosis and neuromyelitis optica. The control group consisted of osteoarthritis patients who underwent intrathecal anesthesia for artificial joint replacement surgery. These individuals exhibited normal neuraxial imaging, had normal physical examinations, and possessed no history of neurological disease.
At our center, neurosurgeons determine the surgical approach based on intraoperative assessment of the degree of arachnoid lesions. Arachnoid pathology in all surgically treated patients were graded according to previously proposed criteria. The severity of adhesions was categorized as follows: grade 0 indicated no arachnoid pathology detected, grade 1 represented minor arachnoid adhesions to the spinal cord with arachnoid translucency, and grade 2 denoted severe arachnoid scarring with arachnoid nontranslucency [17]. The extent of adhesions was assessed in terms of length: limited adhesions were defined as those with lengths less than or equal to 3 spinal segments, while adhesions with lengths of at least 4 spinal segments were considered extensive [18]. Due to the challenging nature of achieving thorough arachnoid lysis, patients with grade 2 adhesions with lengths of at least 4 spinal segments received shunting to either the pleural or peritoneal cavities, while arachnoid adhesion lysis were performed in other cases.
Our study, which was registered in the prospective database, received ethical approval from the Ethics Committee of our hospital, and all participants provided informed consent by signing the requisite informed consent forms.
2. Surgery Procedure
As mentioned above, patients with milder PTS underwent arachnoid lysis [19]. The patient was placed in a prone position, a vertical incision was made at the incision determined by the location of the cavity, the vertebral plate were exposed and removed and the dura mater was incised and suspended. The adhesions between the arachnoid and the spinal cord are fully released and the severely thickened arachnoid is excised until the spinal cord is clearly visible or the adhesions can no longer be peeled away. Sutures are applied after tight haemostasis.
Shunting was conducted in patients with more severe PTS who met the previously outlined criteria. The procedure involved making a 5- to 6-cm-long incision along the posterior median skin, centered on the intended drainage site. Subsequently, the vertebral plate was exposed and removed. Incisions were made in the dura mater and arachnoid, longitudinally suspending them to reveal the distended spinal cord. A small-diameter silicone catheter was carefully inserted into the syrinx through a conventional midline myelotomy at the thinnest part of the cord. This catheter was then secured to the pial layer and connected to a low-pressure catheter inserted into either the peritoneal or pleural cavity. Sutures are applied after tight haemostasis.
3. Data Collection
Neurological function assessment was conducted before the surgery and at 3–12 months postoperatively using the American Spinal Cord Injury Association Impairment Scale (AIS) and the modified Japanese Orthopaedic Association score (mJOA) [20]. Additionally, we collected data on the duration between trauma exposure and the onset of myelopathy symptoms, as well as the time of the preoperative clinical visit. For each patient, MRI was employed to determine the location and segments of spinal cord lesions, while myelography aided in identifying the site of adhesion at the spinal level. Patients were followed up through outpatient visits or telephone interviews to evaluate the effectiveness of the surgical procedure in alleviating main symptoms and improving spinal cord lesions. The effect of surgery was evaluated based on the development of symptoms in PTS patients before, after, and during follow-up, categorized as “worsened,” “stable,” and “improved,” [21] while “improved” was characterized by a reduction in neurological deficits or an improvement in the patient’s subjective experience [22].
At our center, CSF samples were routinely collected by myelography before surgery in the PTS group. Meanwhile, serum samples were also collected and transported to the laboratory for examination. Routine laboratory analysis of the CSF included choloride (mmol/L) and glucose (mg/dL). The concentrations of albumin (mg/dL) in both CSF and serum samples were quantified using the turbidimetric inhibition immune assay (IMMAGE 800, Beckman Coulter, America). The quotients of albumin (CSF/serum) were calculated and evaluated according to the Reiber diagnostic criteria, with all quotients expressed as n × 10-3 [12].
4. Statistical Analysis
Continuous variables are presented as mean ± standard deviation or median (interquartile range) if they are not normally distributed. The comparison of continuous data was conducted using either Student t-test or Mann-Whitney U-test, depending on the data distribution. Categorical variables were compared using the chi-square test or Fisher exact test, as appropriate. A multivariable analysis was performed using logistic regression to explore the independent factors influencing surgical selection. Variables that were considered clinically relevant or showed significant differences between groups were included in the regression model. Additionally, receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic value of QAlb. Statistical analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA) and GraphPad Prism ver. 9.0 (GraphPad Software Inc., La Jolla, CA, USA). All p-values were 2-sided, and a p-value < 0.05 was considered statistically significant.
RESULTS
1. PTS Group vs. Control Group
The PTS group of 56 cases (37 males and 19 females; mean age, 47.8 ± 11.5 years) consisted of 42 cases in the arachnoid lysis group (29 males and 13 females; mean age, 48.9 ± 10.5 years) and 14 cases in the shunting group (8 males and 6 females; mean age, 44.5 ± 13.9 years), along with a total of 19 cases in the control group (8 males and 11 females; mean age, 45.0 ± 7.6 years) were included in the study (Fig. 1).
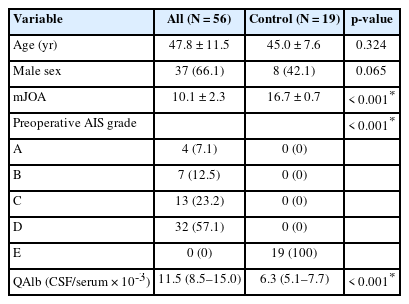
There were no statistically significant differences in age (p = 0.324) or gender distribution (p = 0.065) between the PTS and control groups. However, significant differences were observed in mJOA scores (p < 0.001) and preoperative AIS (p < 0.001) between the PTS group and the control group. Notably, QAlb was significantly higher in the PTS group (median [interquartile range, IQR], 11.5 [8.5–15.0]) compared to the control group (6.3 [5.1–7.7], p < 0.001) (Table 1).
2. Arachnoid Lysis Group vs. Shunting Group
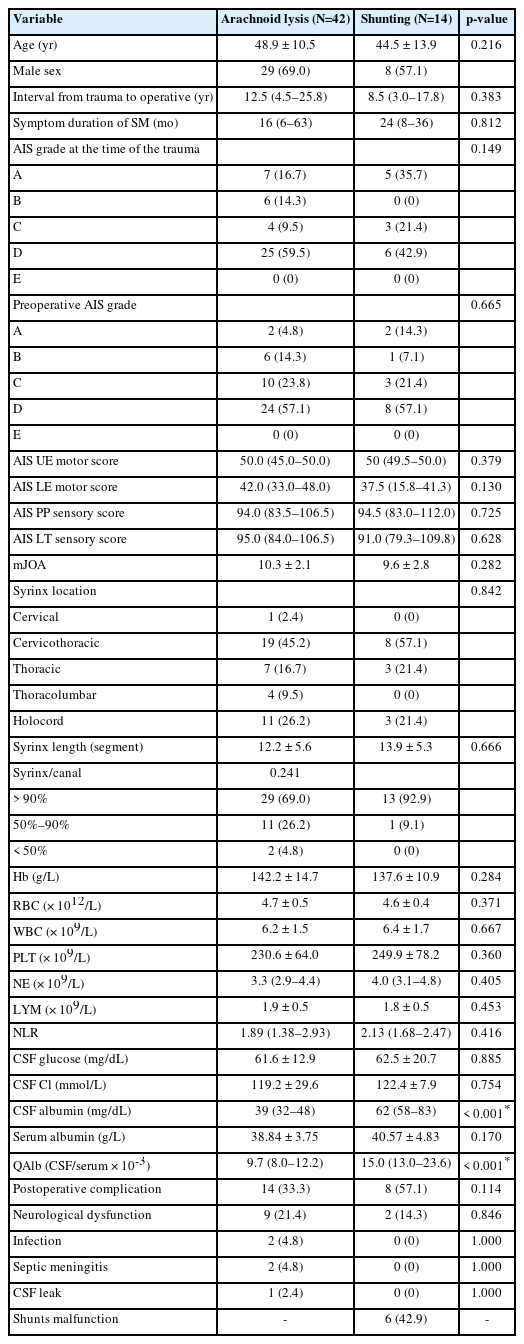
There were no significant statistically differences observed between the arachnoid lysis (Fig. 2) and shunting groups (Figs. 3, 4) in several baseline characteristics, including age (p = 0.216), sex (p = 0.625), time interval between the exposure of trauma (p = 0.383) and spinal symptoms (p = 0.812) to preoperative, mJOA scores (p = 0.282), or syrinx location (p = 0.842) and syrinx length (p = 0.666).
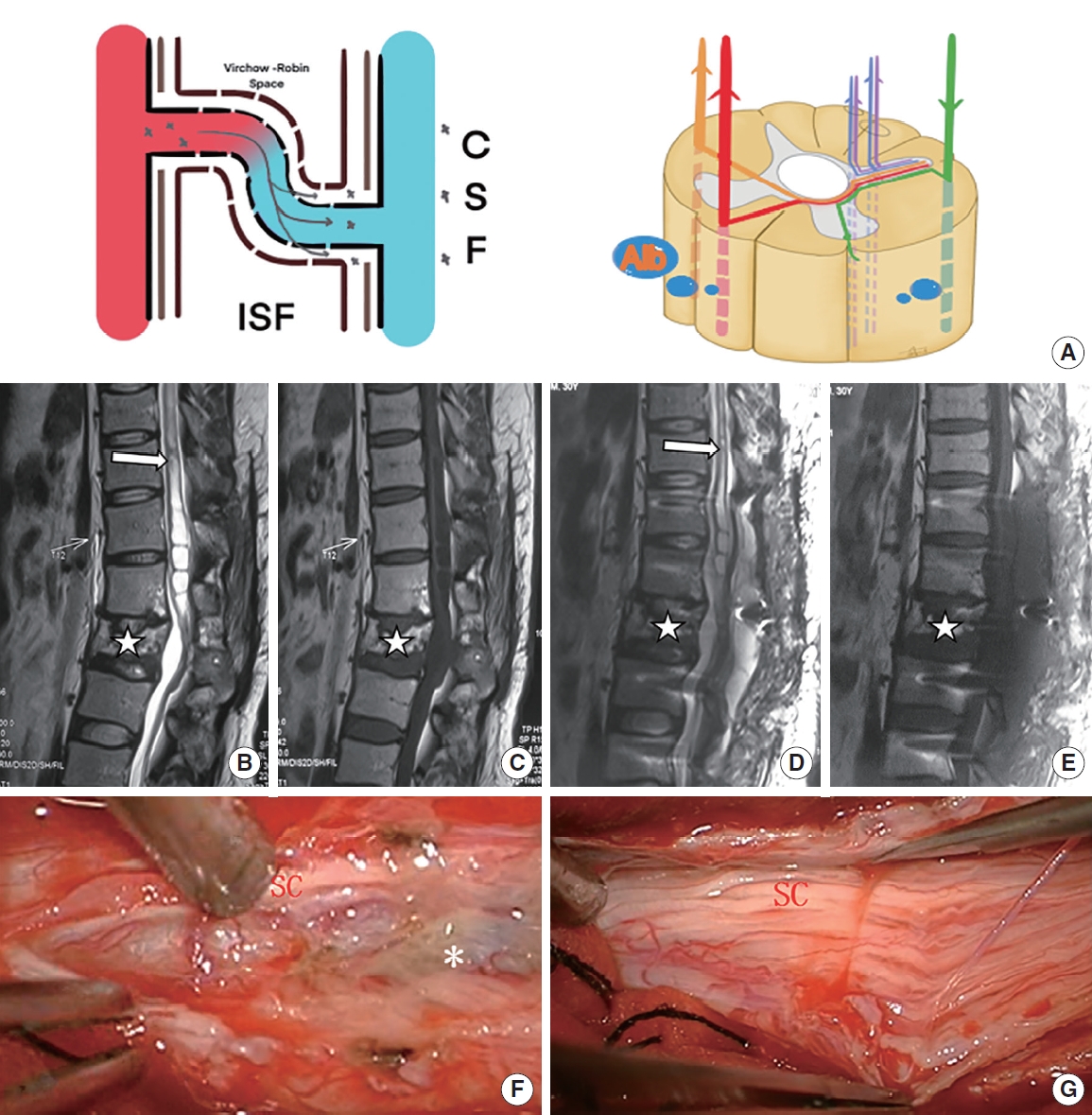
Patient with mild posttraumatic syringomyelia underwent arachnoid lysis. (A) The schematic diagram shows that through damaged blood-spinal cord barrier, a moderate amount of QAlb*10-3 (6.82) was released into the subarachnoid space. (B, C) Preoperative sagittal T2-weighted and T1-weighted magnetic resonance imaging (MRI) scan showed a large syrinx (white arrow) above L1 and a L2 compression fracture (star symbol). (D, E) Postoperative sagittal T2-weighted and T1-weighted MRI scan showed part of the syrinx (above T11, white arrow) obviously reduced. (F, G) Slight adhesion (asterisk, grade 1) around the spinal cord (SC) at L1 were removed intraoperatively. QAlb, quotient of albumin; CSF, cerebral spinal fluid; ISF, interstitial fluid.
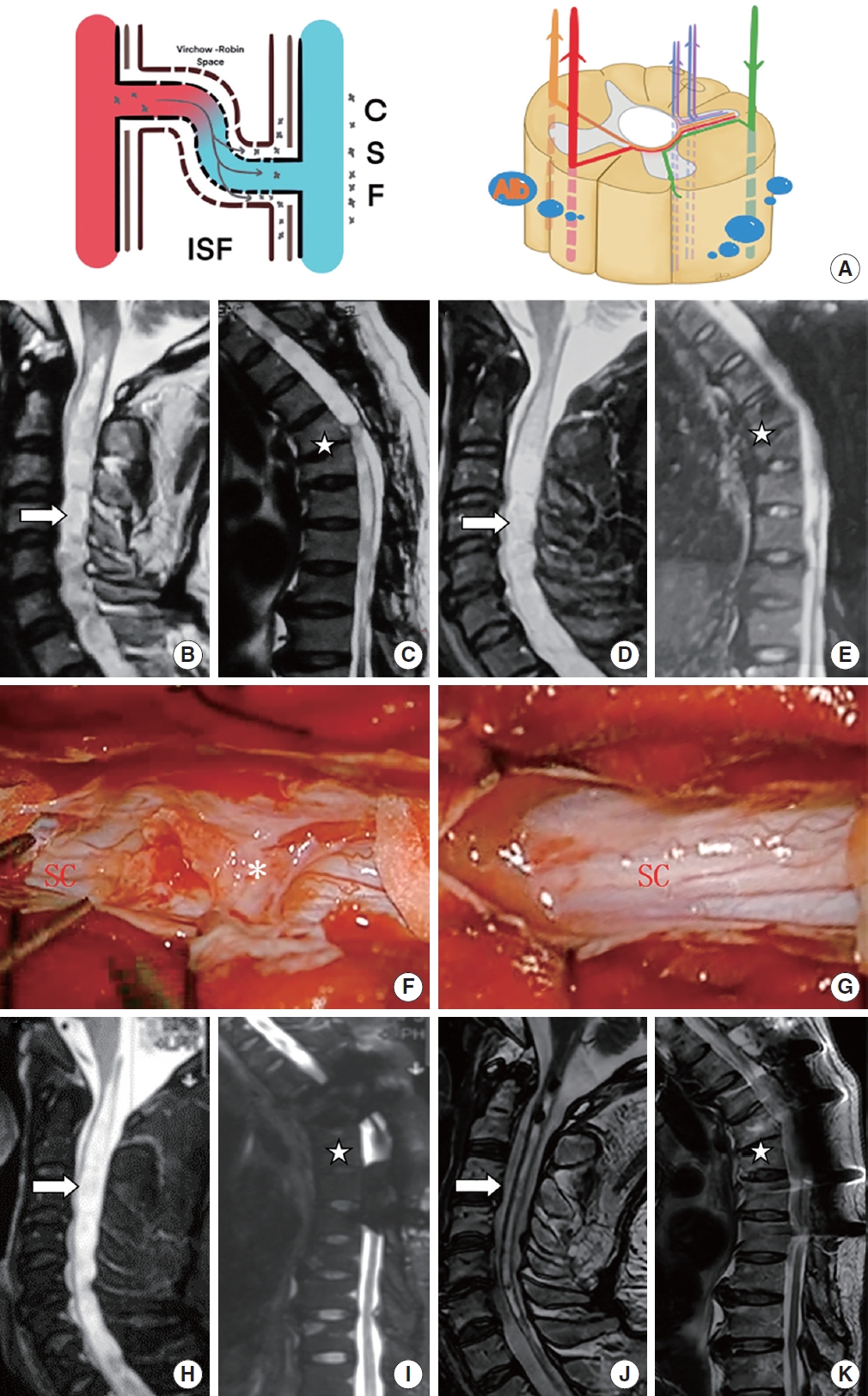
Patient with extremely severe posttraumatic syringomyelia underwent arachnoid lysis and shunting in sequence. (A) The schematic diagram shows that through a severely damaged blood-spinal cord barrier. (B, C) Preoperative sagittal T2-weighted magnetic resonance imaging (MRI) scan showed a large syrinx (up to C1 and down to T8, white arrow) and a T5–6 compression fracture (star symbol), a large amount of QAlb*10-3 (13.07) was released into the subarachnoid space. (D, E) Postoperative sagittal T2-weighted MRI scan 3 years after arachnoid lysis showed almost no change to the syrinx (white arrow). (F, G) Oblivious adhesions (asterisk, grade 2) around the spinal cord (SC) at T4–8 were removed intraoperatively. (H, I) Sagittal T2-weighted MRI scan showed a large syrinx unchanged after arachnoid lysis (white arrow). (J, K) Postoperative sagittal T2-weighted MRI scan showed part of the syrinx (above T1, white arrow) obviously reduced. QAlb, quotient of albumin; CSF, cerebral spinal fluid; ISF, interstitial fluid.

Patient with severe posttraumatic syringomyelia underwent shunting. (A, B) The schematic diagram shows that through a severely damaged blood-spinal cord barrier, a large amount of QAlb*10-3 (13.24) was released into the subarachnoid space. (C) A shunting tube was placed in the syrinx intraoperatively (star symbol). (D, E) Preoperative sagittal T2-weighted and T1-weighted magnetic resonance imaging (MRI) scan showed a large cervical syrinx (white arrow). (F, G) Postoperative sagittal T2-weighted and T1-weighted MRI scan showed that the cervical syrinx was reduced (white arrow). QAlb, quotient of albumin; CSF, cerebral spinal fluid; ISF, interstitial fluid.
Laboratory tests showed no significant differences between the arachnoid lysis and shunting groups in blood routine indexes, including hemoglobin (p = 0.284), leucocytes (p = 0.371), and neutrophil-to-lymphocyte ratio (p = 0.416). The CSF tests revealed no significant differences in glucose (p = 0.885) and chloride (p = 0.754) levels between the 2 groups. However, QAlb levels were significantly higher in the shunting group (median [IQR], 15.0 [13.0–23.6]) compared to the arachnoid lysis group (9.7 [8.0–12.2]), demonstrating a statistically significant difference (p < 0.001).
In terms of the postoperative situations, although there was a slightly higher complication rate in the shunting group (57.1%) compared to the arachnoid lysis group (33.3%), this difference did not reach statistical significance (p = 0.114). If excluding long-term complications resulting from shunt malfunction within the shunting group, the observed complication rate (14.3%) in this group appears slightly lower than that in the arachnoid lysis group. However, this difference does not attain statistical significance as well (p = 0.306) (Table 2).
3. Multivariate Analysis and ROC Curve
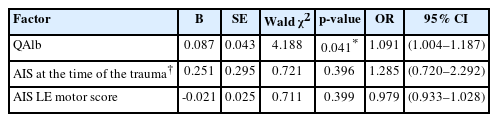
Multivariate logistic regression analysis showed that higher preoperative QAlb (odds ratio [OR], 1.091; 95% confidence interval [CI], 1.004–1.187; p = 0.041) was an independent factor favoring the utilization of shunting in patients. To evaluate the potential of QAlb as a guide for surgical selection, a ROC curve was constructed. The area under the curve for QAlb was 0.8861 (p < 0.001). The results demonstrated a sensitivity of 100% and specificity of 80.95% when the cutoff value of QAlb was set at 12.67 (Table 3, Fig. 5).
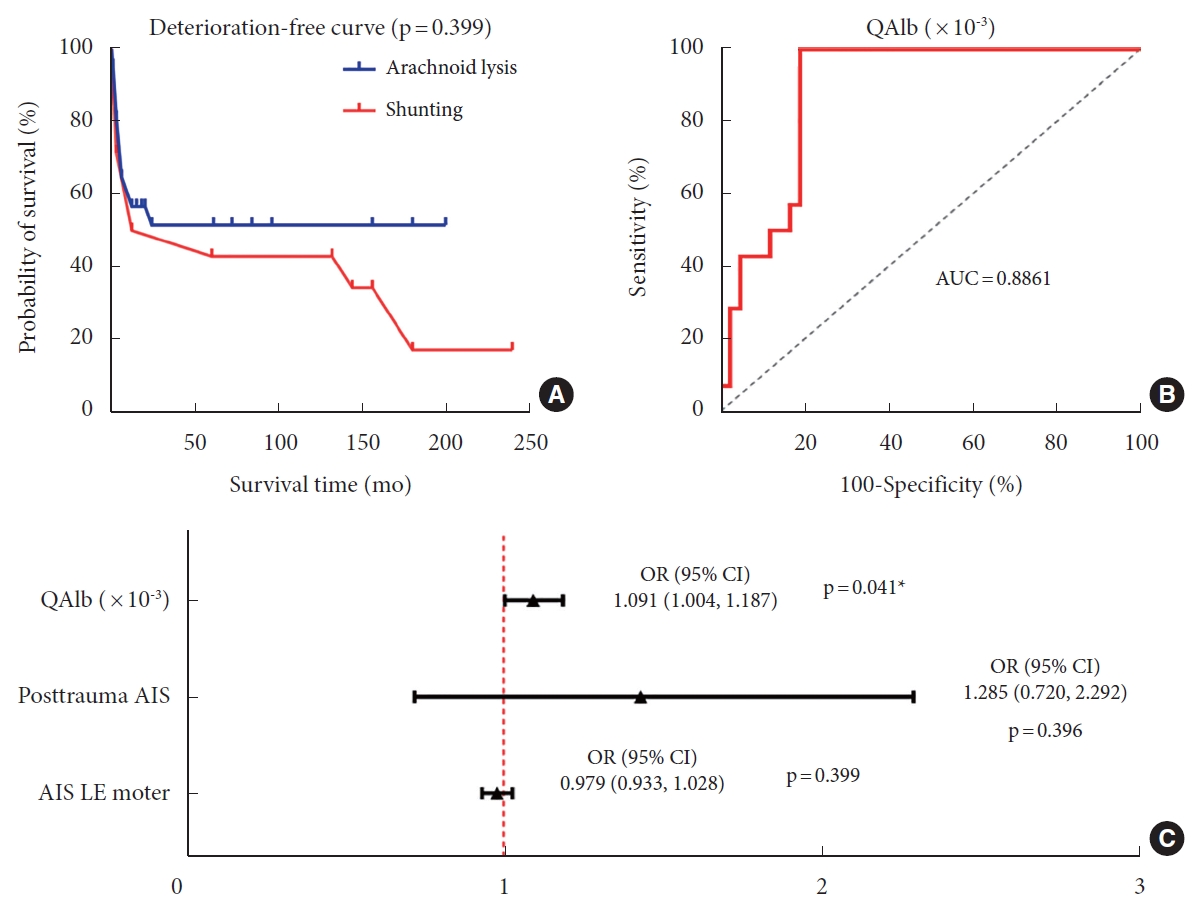
(A) The deterioration-free curve of posttraumatic syringomyelia (PTS) patients underwent different surgical procedures showed that there was no significant difference in prognosis between the 2 groups (p=0.399). (B) The ROC curve for predicting the need for shunting showed 100% specificity and 80.95% sensitivity in PTS patients, and the threshold QAlb*10-3 value is 12.67 (p<0.001). (C) Multivariate logistic analysis in surgical procedure selection of shunting showed that higher preoperative QAlb (odds ratio [OR], 1.091; 95% confidence interval [CI], 1.004–1.187; p=0.041) was an independent indicative factor for the use of shunting in patients. QAlb, quotient of albumin; AUC, area under the curve; AIS, American Spinal Cord Injury Association Impairment Scale; LE, lower extremity.
4. Long-term Outcomes
Kaplan-Meier statistics showed a slightly better prognosis in arachnoid lysis group than in shunting group, but there was no statistically significant difference (p = 0.399) (Table 3, Fig. 5).
Based on the ROC-derived cutoff value of 12.67 for grouping QAlb levels, our observations revealed patients within the arachnoid lysis group with lower QAlb levels exhibited significantly better prognoses than those with higher QAlb levels (p = 0.028). Additionally, it’s noteworthy that the prognosis of patients in the shunting group did not exhibit a statistically significant difference when compared to patients in the arachnoid lysis group with lower QAlb levels (p = 0.140) (Supplementary Fig. 1).
DISCUSSION
There still exist controversial about the choice of surgical procedure for patients with PTS. Aghakhani suggested that patients with progressing PTS should undergo arachnoid lysis promptly and it may offer a more favorable prognosis compared to shunting [13]. The study of Klekamp [2] showed that arachnoid lysis provides good long-term outcomes for patients with AIS grades A, B, E. However, a systematic review by Kleindienst showed similar unsatisfactory outcomes for patients with PTS, suggesting the importance of choosing the appropriate procedure based on the patient’s condition preoperatively [14].
Aimed at removing obstructions in CSF flow and restoring normal circulation, arachnoid adhesion lysis remains the first choice for patients with PTS [4,8,23,24]. However, even when arachnoid adhesions were adequately released and eliminated, some patients with PTS reported a poor prognosis for arachnoid lysis [3]. This may be due to the inability to reconstruct the damaged BSCB, resulting in persistent myelopathy. At the molecular level, BSCB rupture leads to disruption of tight junctions and basal lamina integrity [11,25], resulting in increased self-permeability, which in turn contributes to a range of pathological mechanisms including tissue oedema, inflammation and alterations in blood perfusion [26-29]. This BSCB-induced secondary injury process causes syrinx progression and persistent neurological symptoms of SCI, and may lead to irreversible neuronal apoptosis, thereby reducing the patient’s likelihood of clinical recovery, which cannot be reversible by intradural decompression only.
The effectiveness of shunting for continuous drainage of syrinx fluid through the suction of a low-pressure system and increased compliance of the subarachnoid space has been validated in previous animal studies and case reports in PTS [30,31]. We suggest that for PTS patients with severe BSCB injuries that cannot be alleviated through arachnoid adhesion lysis, symptom relief through supplementary shunting is a viable option. This is supported by the similar outcomes observed in the 2 PTS subgroups in our study.
As a result, the judgement of the extent of BSCB damage is pivotal in determining the preoperative surgical approach of patients with PTS. In clinical practice, for all subtypes of syringomyelia, MRI is usually the critical examination to detect syrinx length, location and diameter. However, the diagnostic ability of MRI, as well as diffusion tensor imaging and somatosensory evoked potentials, remains limited in the diagnosis of myelopathy of PTS patients [32-34]. Myelography is commonly used in the preoperative diagnosis of patients with PTS to determine the exact location of intradural adhesions, but the capability to determine the extent of intradural adhesions remains primarily subjective and qualitative. Hence, we developed a quantitative criterion to assess the extent of BSCB destruction (QAlb), aiding in preoperative decision-making.
As a result of the heightened permeability of BSCB, proteins such as albumin and immunoglobulins enter the spinal cord from blood vessels through the disrupted BSCB, rather than simply diffusing passively through the capillaries. This leads to an elevated CSF/serum quotient for proteins. Albumin, with a molecular mass of 66 kD, has a smaller size compared to IgA (160 kD) and IgM (> 320 kD). Therefore, it is more likely to traverse the compromised BSCB, making QAlb a suitable indicator for assessing the extent of BSCB damage [35,36]. In our study, the significant difference in QAlb between groups is valid in the presence of age and syrinx area matching. Higher QAlb levels in the shunting group than in the arachnoid lysis group suggest a higher degree of BSCB disruption in the shunting group.
This study comes with several limitations. Firstly, the limited number of study samples may somewhat impact the robustness of the multivariate logistic regression findings. However, it is important to recognize the challenges in acquiring CSF samples from PTS patients [37], and our study stands as the largest cohort in the available literature. Secondly, while our findings reveal similar long-term outcomes for the shunting group and the arachnoid lysis group, further investigations with larger sample sizes are essential to establish more refined surgical selection methods. Thirdly, the correlation between the severity and extent of subarachnoid adhesions and QAlb appears inconclusive. This ambiguity might stem from multifaceted influences on QAlb and the subjective, qualitative assessment of subarachnoid adhesions. Lastly, although our study indicates a possible link between BSCB disruption and syringomyelia, the causal relationship between these elements remains uncertain. To gain deeper insights, we plan to conduct further studies aimed at elucidating these causal relationships (NCT06268093).
CONCLUSION
Shunting appears to be a suitable surgical approach for patients with PTS who have severe BSCB injury. The preoperative QAlb is an important determinant of the appropriate choice of surgical approach in patients with PTS and 12.67 is the threshold value at which shunting is recommended. We intend to pursue further investigations aimed at elucidating the causal relationships between these factors and exploring novel treatment modalities (NCT06268093).
Supplementary Material
Supplementary Fig. 1 can be found via https://doi.org/10.14245/ns.2347152.576.
The deterioration-free curve of posttraumatic syringomyelia patients shows patients within the arachnoid lysis group with lower QAlb levels exhibited significantly better prognoses than those with higher QAlb levels (p=0.028). Furthermore, it’s noteworthy that the prognosis of patients in the shunting group did not exhibit a statistically significant difference when compared to patients in the arachnoid lysis group with lower QAlb levels (p=0.140). QAlb, quotient of albumin.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study was sponsored by Beijing Municipal Education Commission (KZ202010025043 and 11920 70315) and Beijing Municipal Science and Technology Commission (L212007).
Author Contribution
Conceptualization: YD; Data curation: PX, CY, HL; Formal analysis: WD, CZ, ZL, KW, ZW, XW, HW, ZC; Funding acquisition: FJ; Methodology: ZL, FJ; Project administration: FJ; Visualization: JW, JG; Writing - original draft: PX, CY, HL, YD; Writing - review & editing: JG, FJ.
Acknowledgements
We would like to thank John D Heiss (NIH) for the constructive suggestion. We would like to thank Hui Zhan (The Chinese University of Hong Kong) for the selfless assistance. Ane we would like to thank Zhang Xiangyu from Xuanwu Hospital for his invaluable support and assistance throughout the research process.