 |
 |
- Search
| Neurospine > Volume 20(3); 2023 > Article |
|
|
Abstract
Objective
This study aimed to clarify the relationship between recurrence and the extent of resection in surgery for intramedullary spinal hemangioblastoma (sHB) and its impact on von Hippel-Lindau (vHL) disease.
Methods
Data on sHB cases followed up for at least 6 months after surgery were extracted from a nationwide registry of 1,033 consecutive spinal intramedullary tumors surgically treated between 2009 and 2020, and were retrospectively categorized into a sporadic or vHL group. The diagnosis of vHL disease was made at each institution based on clinical findings.
Results
A total of 168 patients (sporadic group, 101; vHL group, 67) were included in the study. Compared with the sporadic group, the vHL group had a younger onset (45.4 ± 16.8 years vs. 39.6 ± 14.1 years, p = 0.02), more preoperative motor (47.5% vs. 68.7%, p < 0.01) and gait (37.6% vs. 61.2%, p < 0.01) impairments, and more patients with worsening neurological symptoms at discharge (p = 0.02). The gross total resection (GTR) rates and the recurrence rates were not statistically different between the sporadic and the vHL groups. GTR significantly improved recurrence-free survival compared to non-GTR in all patient analysis (p < 0.01) but this trend was not observed in the sporadic group. Physical functional improvement from discharge to 6 months after surgery was observed in the sporadic group (p < 0.01) but not in the vHL group.
Spinal hemangioblastoma (sHB) is a common spinal intramedullary tumor accounting for 10%–20% of spinal cord tumors [1-3]. sHB is characterized by a highly vascularized and slow-growing tumor with a benign character, often accompanied by cysts and syringomyelia [4,5]; it may occur sporadically or in association with von Hippel-Lindau (vHL) disease in 25%–33% of cases in adults and more frequently in children, and often involves multiple tumors [6-8]. vHL disease is one of the most common hereditary diseases and is responsible for the development of neoplasia in multiple organ systems including not only central nervous hemangioblastomas but renal cell carcinoma, pancreatic tumors, adrenal tumors, and epididymal cystadenomas [6,9]. The multiplicity accounts for the varied natural history of vHL-associated sHBs, complexities in their management, and uncertainties associated with the long-term functional outcome [8].
Although surgical removal of sHB is closely related to recurrence [10] and postoperative functional prognosis [11], the degree of removal varies widely among reports, and its relation to recurrence has not been sufficiently investigated. Recurrence can occur even after achieving gross total resection (GTR) [11,12]. Additionally, there is disagreement in the literature as to whether there is a difference in recurrence between solitary cases and those associated with vHL disease. The outcome of sHB cannot be discussed without ignoring vHL disease, which can adversely affect life expectancy by progressively worsening neurological function and is secondary to hemangioblastoma in the central nervous system (CNS) or other malignancies.
We recently constructed a nationwide registry of spinal intramedullary tumors and reported the clinical data [13]. Although excellent treatment results at a single center are important, collecting data from multiple centers in a national region will reveal real-world treatment results for sHB that can be used in daily clinical practice. Therefore, we extracted and investigated data from this registry for sHBs.
This study aimed to clarify the relationship between recurrence and the extent of resection in surgery for intramedullary sHB and its relation to vHL disease, as well as to reveal the differences in clinical and radiological characteristics and surgical outcomes of sHB between sporadic and vHL diseases by analyzing data from a large number of cases collected from many facilities.
This was a subanalysis of a multicenter cohort study authorized by the Neurospinal Society of Japan. The study protocol was approved by the Institutional Review Board of Tohoku University Hospital (2021-1-130) and the participating centers. As this was a retrospective, noninvasive study, the requirement for written informed consent was waived. Instead, a public notice that provided information on this study was published on individual center’s websites.
This study included patients with pathologically diagnosed sHB, who were extracted from the nationwide registry of 1,033 consecutive spinal intramedullary tumor cases surgically treated at 58 Japanese centers between 2009 and 2020 [1]. Exclusion criteria were insufficient data, surgery for locally recurrent lesions after prior treatment, and a follow-up interval of <6 months.
Clinical characteristics, including age, sex, body mass index (BMI), medical history (including vHL disease, brain tumor, and spinal surgery), clinical presentations, and duration of illness, were anonymously extracted from the patients’ medical records. Modified McCormick scales [11-14] (grade I, normal gait; grade II, mild gait disturbance not requiring support; grade III, gait with support; grade IV, assistance required; and grade V, wheelchair needed) were periodically analyzed, allowing comparisons between patients’ preoperative and postoperative statuses. Radiological data were collected from preoperative and postoperative images, including the tumor location at the spinal level, tumor length measured the long diameter of the enhancing portion on sagittal images, and morphological type classified as cystic, solid or mixed on magnetic resonance imaging (MRI) (Supplementary Fig. 1). The surgical approach, degree of excision, operative time, blood loss, and complications were recorded from the surgical records. Tumor recurrence was defined as the reappearance of the tumor at the same site after GTR or tumor regrowth after partial removal. Information regarding the postoperative clinical course, including adjuvant radiotherapy, the presence of recurrence, and duration to recurrence or final follow-up after surgery was also recorded. To investigate short-term functional improvement after surgery, the change in the modified McCormick scale between the preoperative period and discharge was evaluated in each case: “improved” and “deteriorated” were defined as improvement or deterioration in at least one of the grades, respectively. The diagnosis of vHL disease was made at each institution based on the clinical findings.
The patients were classified into 2 groups, sporadic and vHL, based on the presence or absence of a diagnosis of vHL disease. The diagnosis of vHL disease was made at each institution based on clinical findings. The collected data were retrospectively compared between the 2 groups.
All data were analyzed for completeness and accuracy, and anonymized prior to scrutiny. Recurrence-free survival (RFS) was determined in each case and was defined as the time from surgery to the last follow-up in patients without tumor recurrence; the time from surgery to the first-time recurrence was observed in patients with recurrence.
Continuous variables among the clinical characteristics and radiological variables were compared using the unpaired t-test or Welch t-test based on equal variances in the population. Binary and nominal variables among the clinical characteristics and radiological variables were compared using the Pearson chisquare test. To evaluate the relationship between GTR and recurrence, Kaplan-Meier plots for RFS of all patients stratified by the presence of vHL disease and extent of resection were created and compared using the log-rank test. To investigate whether there were differences between the 2 groups, Kaplan-Meier plots for RFS in each group were created and compared using the log-rank test. To clarify the differences in changes in the physical functional status during the perioperative period, trends in the modified McCormick scales over time were compared in each group using 1-way analysis of variance with repeated measures. Statistical analysis of each recent change and the change from the preoperative period to the time of the last follow-up was performed.
Continuous variables are presented as mean± standard deviation, and categorical variables are presented as numbers and percentages. Statistical significance was defined as p< 0.05. All statistical analyses were performed using IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA).
Of the 1,033 spinal intramedullary tumor registry cases, 195 (18.9%) were diagnosed as sHB. One case with insufficient data, one reoperation case due to local recurrence after prior treatment, and 25 cases with a follow-up duration < 6 months were excluded. Finally, 168 patients treated at 39 centers were included. There were 75 male and 93 female patients aged 8–80 years (43.1± 16.0 years at the time of surgery), and 101 (60.1%) and 67 cases (39.9%) were categorized into the sporadic and vHL groups, respectively.
Detailed clinical characteristics of the patients in each group are presented in Table 1. There were significant differences between the 2 groups in terms of age (45.4± 16.8 years vs. 39.6± 14.1 years, p< 0.02), BMI (22.8± 3.8 kg/m2 vs. 21.4± 3.1 kg/m2, p= 0.02), cigarette smoking (31.7% vs. 14.9%, p= 0.02), history of brain tumor (1.0% vs. 70.1%, p< 0.01), spinal surgery (3.0% vs. 29.9%, p< 0.01), motor weakness (47.5% vs. 68.7%, p< 0.01), and gait disturbance (37.6% vs. 61.2%, p< 0.01). In summary, patients in the vHL group were younger, thinner, less likely to smoke, more likely to have a history of brain tumor or spinal surgery, and more often affected by motor dysfunction than those in the sporadic group.
The detailed radiological characteristics and preoperative functional statuses of the patients in each group are presented in Table 2. More than 90% of the tumors in both groups were found in the cervical and thoracic spine, and the distribution of tumor locations showed no statistically significant difference between the 2 groups (p= 0.36). The most common morphological type on MRI was the solid type in both groups, and there was no statistically significant difference in morphological type between the groups (p= 0.57). The tumor length was 16.4± 11.3 mm in the sporadic group and 14.0± 7.3 mm in the vHL group, with no statistically significant difference between the groups (p = 0.10). The distribution of the preoperative modified McCormick scale in each group demonstrated that the preoperative functional status tended to be worse in the vHL group than in the sporadic group, but the difference was not statistically significant (p= 0.24).
In the evaluation of all patients, the mean follow-up period was 53.6 ± 37.7 months, and GTR and recurrence rates were 92.9% and 3.6%, respectively. The surgical details and results of the 2 groups are presented in Table 3. GTR was performed in 94.1% and 91.0% of patients in the sporadic and vHL groups, respectively; however, there was no statistical difference in the degree of tumor removal between the groups (p = 0.15). The posterior approach was used in most patients in both groups. The operative time tended to be longer in the vHL group than in the sporadic group, but the difference was not statistically significant (p= 0.07). There were also no statistically significant differences in intraoperative blood loss (p= 0.51) or postoperative complications including cerebrospinal fluid leakage (p= 1.00), postoperative hematoma (p = 0.41), or surgical site infection (p= 0.22) between the 2 groups. There was one case in each group in which postoperative irradiation was performed for lesions that could not achieve GTR, without any statistical significance. The follow-up periods were not significantly different between the groups (p= 0.33). The recurrence rate tended to be higher in the vHL group (6.0%) than in the sporadic group (2.0%), but there was no significantly difference (p= 0.17).
The Kaplan-Meier plot of RFS after tumor resection stratified by the presence of vHL disease demonstrated no significant difference between the groups (p= 0.27) (Fig. 1A). In contrast, the Kaplan-Meier plot of RFS stratified by the extent of resection indicated that GTR significantly improved RFS (p< 0.01) (Fig. 1B).
Regarding short-term functional improvement after surgery, most of the patients in the sporadic group improved after the surgery (51.5%), whereas most of the patients in the vHL group remained unchanged (41.8%), indicating a lack of functional improvement between the preoperative period and discharge in the vHL group compared to the sporadic group, which was statistically significantly different (p= 0.02). Changes in the physical functional status during the perioperative period in each group are shown in Fig. 2. There were statistically significant differences in the distribution of modified McCormick grades between discharge and 6 months postoperatively (p< 0.01), and between the preoperative period and final follow-up (p< 0.01) (Fig. 2A). In other words, the sporadic group showed functional recovery from discharge to the sixth postoperative month and at the last follow-up compared with the preoperative period. In contrast, the vHL group showed no improvement (Fig. 2B).
From 6 months postoperatively to the final follow-up, the percentage of patients with modified McCormick grade was maintained in all grades I (42.6% to 45.5%), II (46.5% to 46.5%), III (7.9% to 5.0%), IV (1.0% to 1.0%), and V (2.0% to 3.0%) in the sporadic group. In contrast, in the vHL group, the percentage of patients with grade I remained the same (37.3% to 38.8%), those of grades II (32.8% to 25.4%) and III (16.4% to 13.4%) decreased, and those of grades IV (10.4% to 14.9%) and V (3.0% to 7.5%) increased, indicating that physical function tended to deteriorate without a significant difference (p= 0.54).
This study evaluated the relationship between recurrence and the extent of resection in surgery for sHB and its association with vHL disease. This study’s results showed that a high GTR rate of 92.9% resulted in a low recurrence rate of 3.0% during a mean follow-up of 53.6 months, and GTR tended to improve RFS in the vHL group, unlike in the sporadic group. An improvement in physical function up to 6 months after surgery was observed in the sporadic group but not in the vHL group.
The GTR rate of sHB is generally high [15] and partial resection is associated with tumor recurrence [16]. In a literature review, total, subtotal, and partial resection rates of 83.52%, 9.17%, and 3.5%, respectively [15]. There have been several reports illustrating the GTR rates and recurrence rate of sHB concurrently, but the relationship has been inconsistent. Prokopienko et al. [17] reported 12 cases and demonstrated that GTR was achieved in all patients with no tumor recurrence during a mean follow-up period of 5 years. Conversely, Yousef et al. [18] reported on 42 cases; GTR was achieved in 80.5%, and recurrence was observed in 41.4% of the case during the mean follow-up of 20.9 months. Herein, we analyzed data of the 168 cases and reported a GTR rate of 92.9% and a recurrence rate of 3.6%. This GTR rate is considerably higher than that reported in a recent literature review [15]. As a result, the recurrence rate was also found to be lower despite > 50 months of follow-up.
In previous studies, the GTR rate of sHB tended to be slightly lower in vHL cases than in sporadic cases, and it cannot be ruled out that this may also affect the recurrence rate. Siller et al. [19] reported the GTR rates of 100% for sporadic lesions and 90% for vHL lesions. Yousef et al. [18] reported the GTR rates achieved in 86.4% for sporadic lesions and 70.0% for vHL lesions, with no statistical difference. In a literature review, Jankovic et al. [15] also reported the recurrence rates of 7.9% in sporadic lesions and 22% in vHL lesions. However, few reports focused on the details of recurrence in sHB.
Only 2 reports investigating the impact of the presence of vHL disease and the degree of tumor removal using Kaplan-Meier plots of RFS have been published, but with slightly different results. Yousef et al. [18] found that RFS was markedly lower in the vHL group than in the sporadic group, unlike our results. They described in the article that local recurrence can occur in vHL-associated lesions regardless of the degree of removal, as there was no significant difference in GTR rates between sporadic lesions and vHL-associated lesions. Garcés-Ambrossi et al. [10] also revealed that GTR improved PFS. This trend is the same as the result of the present study but more marked in that previous report, and the reason for this is the small number of recurrence cases (3 of 15 cases).
In addition, we examined the association between RFS and GTR in each group, and the results indicated that the effect of GTR tended to be greater in the vHL group rather than in the sporadic group. These differences between the groups may be partly due to the high GTR rate, which maintains a low recurrence rate.
The differences in clinical presentation and radiological characteristics between sporadic and vHL diseases are controversial. Previous reports suggested that patients with vHL-associated lesions are younger at onset, which is consistent with the present study [16,18]. However, neurological symptoms are reported to be less severe than in patients with sporadic lesions [16] whereas more likely to present with multiple symptoms in patients with vHL-associated lesions [18]. Regarding the radiological characteristics of sHB, Yousef et al. [18] reported no differences between the 2 groups. Yet, Takai et al. [16] reported that lower spinal cord lesions were more frequently observed in patients with vHL disease than in those with sporadic disease with significant differences.
Although the radiological characteristics in the present study were not different between the 2 groups, there was more preoperative motor weakness and gait disturbance in the vHL group than in the sporadic group. Two hypotheses can explain these differences. One possibility is that preoperative motor dysfunction may have been significantly more frequent due to an overlying effect. Because the vHL group had a significantly more frequent history of brain tumor and spinal cord surgery than the sporadic group, it is highly likely that some patients had residual motor dysfunction due to previous treatment. Parker et al. [20] reported that 60% and 83% of sHB patients with vHL disease presented with multiple sHBs and extraspinal lesions, respectively. Another hypothesis is that the same sHB may potentially have slightly different surgical indications in patients with sporadic disease versus those with vHL disease. Some reports indicated that asymptomatic lesions were removed when radiologically detected enlargement was present [16,21], whereas others reported surgery after the lesion became symptomatic [11]. In an additional survey in this study about the surgical indications, 42.9% of the facilities indicated that they would extend the time to surgery for vHL cases more than for sporadic cases. This means that the time to surgery was intentionally postponed in 50.7% of the vHL cases in this study. Therefore, it is suggested that this may have influenced the difference in preoperative neurological dysfunction between the 2 groups.
In the present study, only the sporadic group showed significant functional improvement, both in the short- and long-term postoperatively. Although non-GTR in the vHL group was tended to be associated with recurrence, recurrence up to 6 months postoperatively was only observed in one patient, suggesting that other factors may be involved in this difference in functional improvement.
The first hypothesis is that an inferior preoperative functional status in patients with vHL disease leads to poor surgical outcomes. Among patients with spinal degenerative diseases, those with advanced myelopathy have poor surgical outcomes [22]. The vHL group had a significantly more frequent history of CNS disease and tended to have inferior preoperative functional status than the sporadic group, which may have resulted in little functional improvement from surgery in some cases. It is also possible that additional functional impairment due to the appearance of another lesion specific to vHL may have been the cause of this difference. In vHL cases, 42% of cases presented new lesions during a mean follow-up of 60 months [20], and spinal cord lesions are more frequent than in sporadic cases [23]. We additionally assessed the influence of past history of CNS tumor surgery and demonstrated that postoperative functional improvement was also shown on the vHL group without prior CNS tumor surgery like the sporadic group, indicating that patients with vHL disease do not necessarily have poor postoperative functional improvement (Supplementary Fig. 2).
Based on this study’s results, the surgical treatment of sHB in vHL disease remains challenging compared to that in sporadic disease. Patients with vHL disease are at high risks for neurological deterioration because they require successive surgical treatments for the newly developed lesions in the CNS over their lifetime [23]. Then, it is necessary to both increase the degree of removal to reduce recurrence and preserve functional status. In addition to more meticulous surgical techniques and optimal timing of surgery, GTR should be promoted using multimodal surgical support those have been developed in recent years, such as intraoperative neurophysiological monitoring [19], and indocyanine green (ICG) videoangiography [24] or additional technique of temporary feeder occlusion under ICG videoangiography [25], particularly in vHL cases.
This study has some limitations. First, this was a multicenter retrospective database study, and there are inevitable differences in the indications for surgery at each facility. Second, complete data on the appearance of other lesions are lacking. This is an essential endpoint in sHB, including vHL, when considering the long-term course of the disease. Third, there is no discussion of surgical strategy, such as when to remove the tumor or, in cases of multiple VHL, which tumor to remove first. Therefore, future studies on these points are warranted.
A high GTR rate may sufficiently decrease susceptibility to recurrence, especially in patients with sHB with vHL disease. In sporadic sHB, postoperative functional improvement can be expected, and the long-term functional prognosis is favorable. In vHL disease, short-term functional prognosis is expected to be adequate if there is no history of CNS tumor surgery, but thereafter the risk of functional deterioration increases with each surgery because of the tendency for new lesions to appear in the CNS. Therefore, the introduction of modern surgical assistance and optimal timing of surgery should be considered to achieve GTR to avoid local recurrence and preserve physical function.
Supplementary Materials
Supplementary Figs. 1-2 can be found via https://doi.org/10.14245/ns.2346368.184.
Supplementary Fig. 1.
Morphological classification of the tumor on magnetic resonance images. Cystic (A), solid (B), mixed (C) types. Mixed type is defined as a solid tumor with cysts at the margins of the tumor. T2WI, T2-weighted image; GdT1, gadolinium- enhanced T1-weighted image.
Supplementary Fig. 2.
Subanalysis of perioperative changes in functional status. (A) Sporadic cases without past history of CNS tumor surgery. (B) vHL cases without past history of CNS tumor surgery. (C) vHL cases with past history of CNS tumor surgery. The perioperative recovery trend seen in patients without prior CNS tumor surgery in both group is not seen in vHL group with prior CNS tumor surgery. CNS, central nervous system; vHL, von Hippel-Lindau; NS, not significant. Grade I, normal gait; grade II, mild gait disturbance not requiring support; grade III, gait with support; grade IV, assistance required; and grade V, wheelchair needed. *p<0.05.
NOTES
ACKNOWLEDGEMENTS
Portions of this work were presented in abstract at the thirty-sixth annual meeting of the Neurospinal Society of Japan (2021).
The authors thank investigators of intramedullary spinal cord tumors in the Neurospinal Society of Japan: Masahito Hara and Masahiro Aoyama; Aichi Medical University. Taku Sugawara; Akita Cerebrospinal and Cardiovascular Center. Hiroaki Shimizu; Akita University. Atsushi Sugawara; Iwate Medical University. Phyo Kim and Kazushige Itoki; Utsunomiya Brain and spinal cord center. Seiji Matsui and Seiji Shigekawa; Ehime University. Takao Yasuhara; Okayama University. Yasuyuki Miyoshi; Kawasaki Medical School. Daisuke Umebayashi; Kyoto Prefectural University of Medicine. Hisaaki Uchikado; Kurume University. Hitoshi Fukuda; Kochi University. Tomoaki Nakai, Hiroaki Nagashima and Takashi Sasayama; Kobe University. Shunsuke Yano and Toru Sasamori; Sapporo Azabu Neurosurgical Hospital. Nobuhiro Mikuni and Yukinori Akiyama; Sapporo Medical University. Takeshi Hara; Juntendo University. Mitsuhiro Yoshida; Yokkaichi Municipal Hospital. Hideki Komatani and Yuichi Takahashi; Shin Komonji Hospital. Kiyoshi Ito; Shinshu University. Hisaharu Goto, Node Yasuhiro and Kazuma Doi; Shinyurigaoka General Hospital. Yoshitaka Hirano; Southern TOHOKU Research Institute for Neuroscience. Teiji Tominaga; Tohoku University. Hiroki Ohashi; The Jikei University School of Medicine. Naoyuki Harada; Toho University. Ryu Kurokawa, Tetsuro Shingo and Satoshi Kawajiri; Dokkyo Medical University. Keisuke Takai and So Fujimoto; Tokyo Metropolitan Neurological Hospital. Hiroyuki Nakase; Nara Medical University. Akihiko Saito; Niigata City hospital. Daijiro Morimoto and Kyongsong Kim; Nippon Medical School. Tatsuya Ohtonari; Brain Attack Center, Ota Memorial Hospital. Hiroto Kageyama; Hyogo Medical University. Takafumi Mitsuhara; Hiroshima University. Toshiyuki Takahashi and Ryo Kanematsu; Fujieda Heisei Memorial Hospital. Toshitaka Seki, Motoyuki Iwasaki and Kazuyoshi Yamazaki; Hokkaido University. Izumi Koyanagi; Hokkaido Neurosurgical Memorial Hospital. Masashi Fujimoto; Mie University. Misao Nishikawa; Moriguchi-Ikuno Memorial Hospital. Takashi Yagi and Hiroyuki Kinouchi; University of Yamanashi. Hidetoshi Murata; Yokohama City University. Mari Kitayama; Wakayama Medical University. The authors also thank Yasukazu Hijikata for his suggestions on statistical analysis.
Fig. 1.
Kaplan-Meier plot demonstrating recurrence-free survival (RFS) after resection of intramedullary hemangioblastoma. Stratified analysis by the presence of von Hippel-Lindau (vHL) disease (A) and extent of resection (B) in all patients. No significant difference is observed between the 2 groups in terms of RFS (p=0.27). In contrast, gross total resection (GTR) improved RFS (p<0.01). Stratification according to the extent of resection in the sporadic (C) and vHL groups (D). As opposed to the sporadic group, non-GTR tended to shorten the RFS in the vHL group. Patients at risk are indicated.
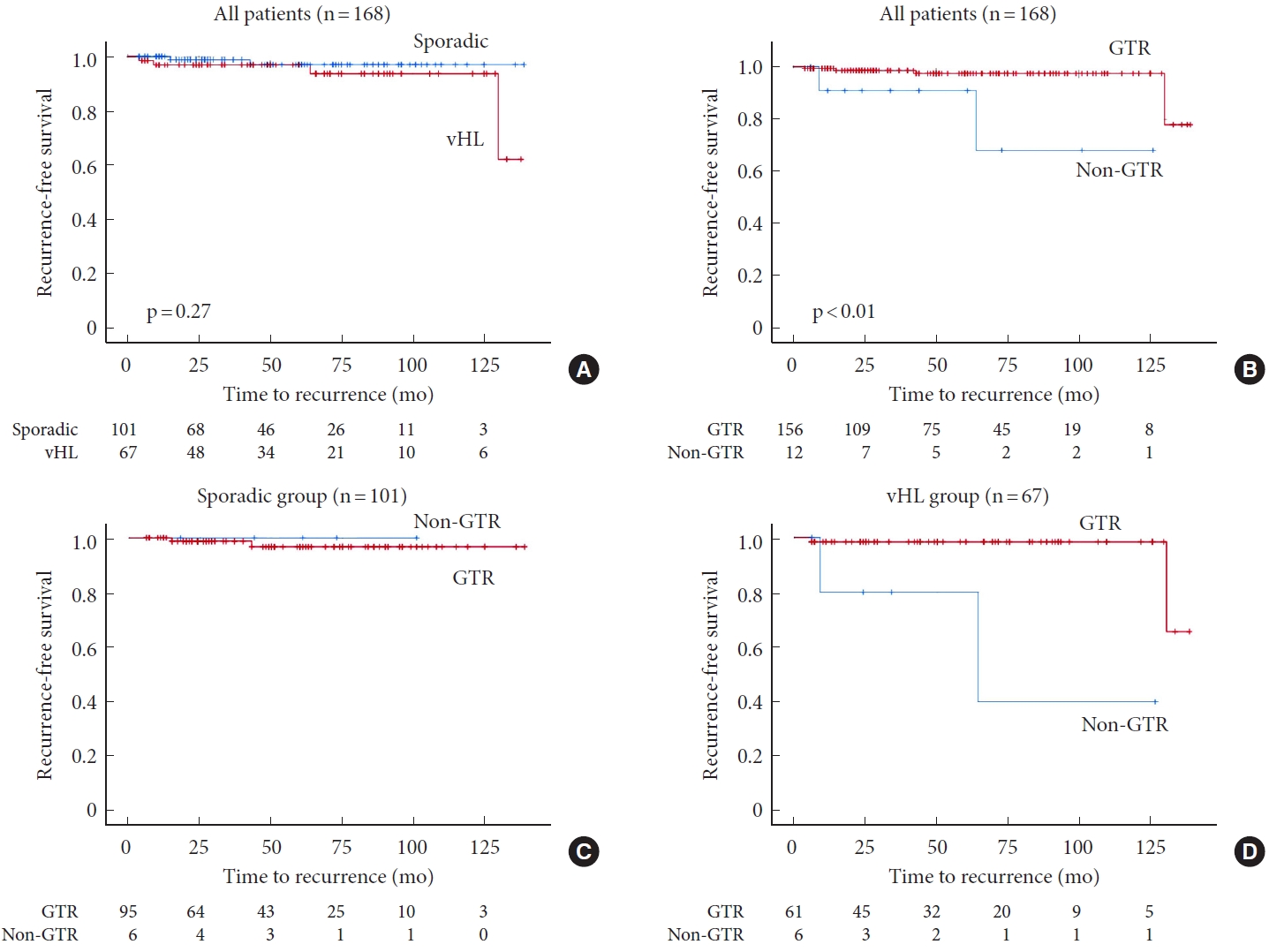
Fig. 2.
Perioperative changes in the functional status in the sporadic (A) and von Hippel-Lindau (vHL) group (B). There are significant differences in the distribution of the modified McCormick scale between discharge and 6 months postoperatively (p<0.01), and between the preoperative period and final follow-up (p<0.01) in the sporadic group. However, there is no significant difference in any assessment interval in the vHL group. NS, not significant. Grade I, normal gait; grade II, mild gait disturbance not requiring support; grade III, gait with support; grade IV, assistance required; and grade V, wheelchair needed. *p<0.05.
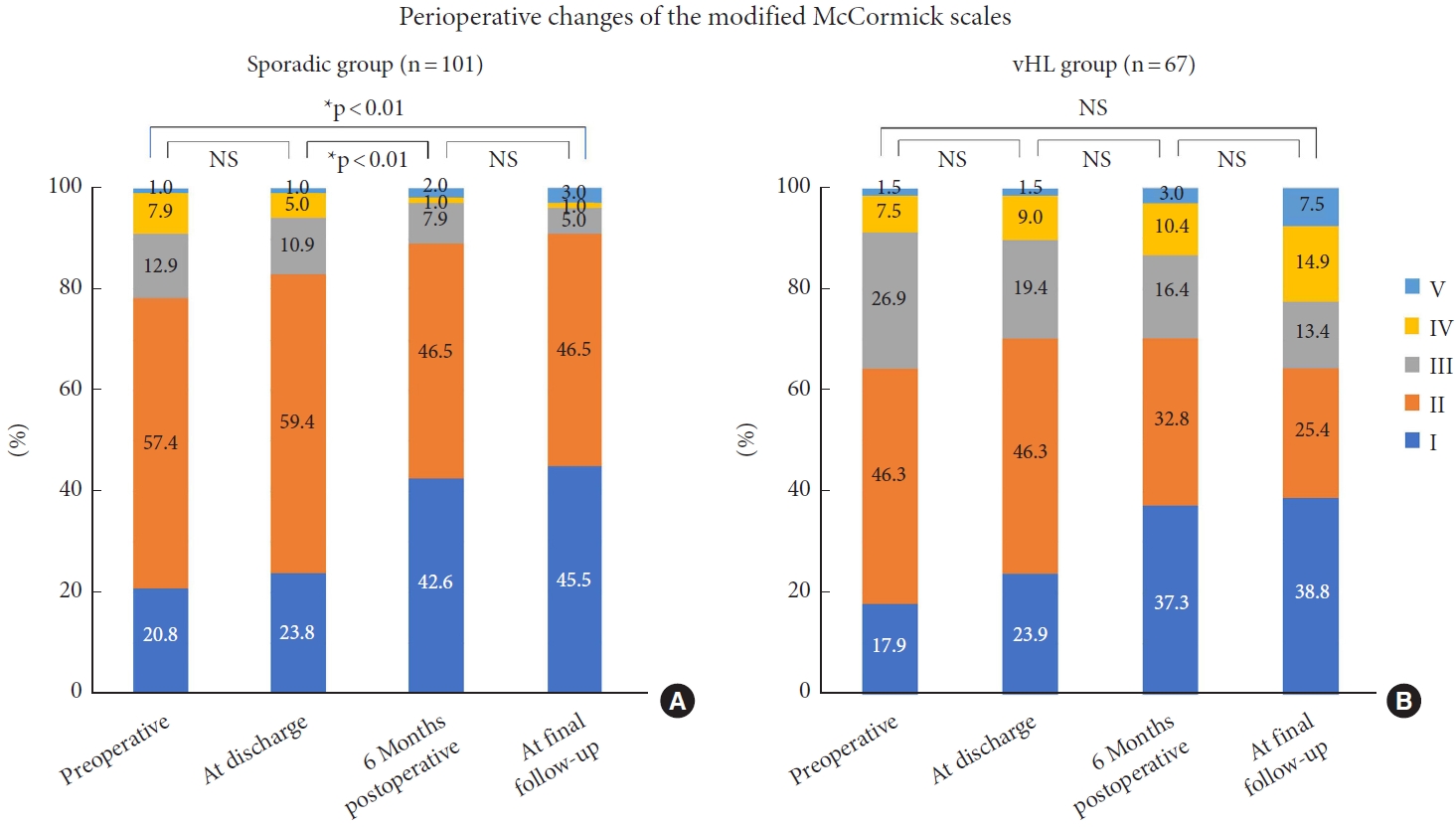
Table 1.
Clinical characteristics of the 2 groups
| Variable | Sporadic group (n=101) | vHL group (n=67) | p-value | |
|---|---|---|---|---|
| General characteristic | ||||
| Age (yr) | 45.4 ± 16.8 | 39.6 ± 14.1 | 0.02*,a) | |
| Male sex | 50 (49.5) | 25 (37.3) | 0.12b) | |
| Body mass index (kg/m2) | 22.8 ± 3.8 | 21.4 ± 3.1 | 0.02*,a) | |
| Hypertension | 14 (13.9) | 7 (10.0) | 0.52b) | |
| Diabetes | 5 (5.0) | 6 (9.0) | 0.31b) | |
| Malignancy | 8 (7.9) | 12 (17.9) | 0.05b) | |
| Cigarette smoking | 32 (31.7) | 10 (14.9) | 0.02*,b) | |
| Past history; brain tumor | 1 (1.0) | 47 (70.1) | < 0.01*,b) | |
| Past history; spinal surgery | 3 (3.0) | 20 (29.9) | < 0.01*,b) | |
| Duration of illness (mo) | 20.3 ± 47.9 | 33.1 ± 74.6 | 0.22a) | |
| Clinical presentation | ||||
| Headache | 5 (5.0) | 9 (13.4) | 0.05b) | |
| Back pain | 49 (48.5) | 31 (46.3) | 0.78b) | |
| Pain in the extremities | 39 (38.6) | 30 (44.8) | 0.43b) | |
| Numbness | 88 (87.1) | 61 (91.0) | 0.43b) | |
| Motor weakness | 48 (47.5) | 46 (68.7) | < 0.01*,b) | |
| Gait disturbance | 38 (37.6) | 41 (61.2) | < 0.01*,b) | |
| Bladder and bowel dysfunction | 22 (21.8) | 20 (29.9) | 0.24b) | |
Table 2.
Radiological characteristics and preoperative functional status of the 2 groups
| Variable | Sporadic group (n=101) | vHL group (n=67) | p-value | |
|---|---|---|---|---|
| Tumor location | 0.36b) | |||
| Cervical | 48 (47.5) | 35 (52.2) | ||
| Cervicothoracic | 3 (3.0) | 6 (9.0) | ||
| Thoracic | 43 (42.6) | 23 (34.3) | ||
| Thoracolumbar | 3 (3.0) | 2 (3.0) | ||
| Lumbosacral | 4 (4.0) | 1 (1.5) | ||
| Morphological type on MRI | 0.57b) | |||
| Cystic | 15 (14.9) | 9 (13.4) | ||
| Solid | 49 (48.5) | 38 (56.7) | ||
| Mixed | 37 (36.6) | 20 (29.9) | ||
| Length of the tumor (mm) | 16.4 ± 11.3 | 14.0 ± 7.3 | 0.10a) | |
| Preoperative modified McCormick scale | 0.24b) | |||
| I | 21 (19.8) | 12 (17.9) | ||
| II | 58 (57.4) | 31 (46.3) | ||
| III | 13 (12.9) | 18 (26.9) | ||
| IV | 8 (7.9) | 5 (7.5) | ||
| V | 1 (1.0) | 1 (1.5) | ||
Table 3.
Surgical details and results of the 2 groups
| Variable | Sporadic group (n=101) | vHL group (n=67) | p-value | |
|---|---|---|---|---|
| Tumor removal | 0.15b) | |||
| Gross total removal | 95 (94.1) | 61 (91.0) | ||
| Subtotal | 2 (2.0) | 5 (7.5) | ||
| Partial | 4 (4.0) | 1 (1.5) | ||
| Surgical approach | 0.34b) | |||
| Anterior | 1 (1.0) | 2 (3.0) | ||
| Posterior | 100 (99.0) | 65 (97.0) | ||
| Operative time (min) | 365.6 ± 174.9 | 415.9 ± 170.7 | 0.07c) | |
| Intraoperative blood loss (mL) | 197.3 ± 340.4 | 166.1 ± 167.4 | 0.51a) | |
| Surgical complication | ||||
| Cerebrospinal fluid leakage | 3 (3.0) | 2 (3.0) | 1.00b) | |
| Postoperative hematoma | 1 (1.0) | 0 (0) | 0.41b) | |
| Surgical site infection | 0 (0) | 1 (1.5) | 0.22b) | |
| Short-term functional improvement after surgery | 0.02*,b) | |||
| Improved | 52 (51.5) | 22 (32.8) | ||
| Unchanged | 23 (22.8) | 28 (41.8) | ||
| Deteriorated | 26 (25.4) | 17 (25.4) | ||
| Postoperative irradiation | 1 (1.0) | 1 (1.5) | 0.77b) | |
| Duration to the final follow-up (mo) | 51.2 ± 35.7 | 57.1 ± 40.5 | 0.33a) | |
| Recurrence | 2 (2.0) | 4 (6.0) | 0.17b) | |
| After the gross total removal | 2 (100) | 2 (50.0) | ||
| After the non-gross total removal | 0 (0) | 2 (50.0) | ||
REFERENCES
1. Deng X, Wang K, Wu L, et al. Intraspinal hemangioblastomas: analysis of 92 cases in a single institution: clinical article. J Neurosurg Spine 2014;21:260-9.

2. Hijikata Y, Ueda S, Yasuhara T, et al. Description of the diversity in surgical indication and surgical strategies for primary spinal cord tumors: a nationwide survey by the Neurospinal Society of Japan. Neurospine 2022;19:1122-9.




3. Jecko V, Roblot P, Mongardi L, et al. Intramedullary spinal cord lesions: a single-center experience. Neurospine 2022;19:108-17.




4. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 2021;23:1231-51.




5. Tsuchiya T, Takami H, Yoshimoto S, et al. Chronological progression and management of syringobulbia caused by spinal hemangioblastoma: a case series and review of the literature. World Neurosurg 2022;167:e127-36.


6. Glenn GM, Linehan WM, Hosoe S, et al. Screening for von Hippel-Lindau diseas by DNA polymorphism analysis. JAMA 1992;267:1226-21.


7. Han B, Zhang L, Jia W. Pediatric spinal hemangioblastomas: clinical features and surgical outcomes of 39 cases. Neurospine 2023;20:343-52.




8. Lonser RR, Weil RJ, Wanebo JE, et al. Surgical management of spinal cord hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg 2003;98:106-16.


9. Maher ER, Webster AR, Moore AT. Clinical features and molecular genetics of von Hippel-Lindau disease. Opthalmic Genet 1995;16:79-84.

10. Garcés-Ambrossi GL, McGift MJ, Mehta VA, et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine 2009;11:591-9.


11. Sadashivam S, Abraham M, Kesavapisharady K, et al. Longterm outcome and prognostic factors of intramedullary spinal hemangioblastomas. Neurosurg Rev 2020;43:169-75.



12. Takami H, Graffeo CS, Perry A, et al. Presentation, imaging, patterns of care, grouth, and outcome in sporadic and von Hippel-Lindau-associated central nervous system hemangioblastomas. J Neurooncol 2022;159:221-31.



13. Endo T, Inoue T, Mizuno M, et al. Current trends in the surgical management of intramedullary tumors: a multicenter study of 1,033 patients by the Neurospinal Society of Japan. Neurospine 2022;19:441-52.




14. McCormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg 1990;72:523-32.


15. Jankovic D, Hanissian A, Rotim K, et al. Novel clinical insights into spinal hemangioblastoma in adults: a systematic review. World Neurosurg 2022;158:1-10.


16. Takai K, Taniguchi M, Takahashi H, et al. Comparative analysis of spinal hemangioblastomas in sporadic disease and Von Hippel-Lindau syndrome. Neurol Med Chir (Tokyo) 2010;50:560-7.


17. Prokopienko M, Kunert P, Podgórska A, et al. Surgical treatment of sporadic and von Hippel-Lindau syndrome-associated intramedullary hemangioblastoma. Neurol Neurochir Pol 2016;50:349-55.

18. Yousef A, Rutkowski MJ, Yalcin CE, et al. Sporadic and von Hippel-Lindau disease-associated spinal hemangioblastomas: institutional experience on their similarities and differences. J Neurooncol 2019;143:547-52.



19. Siller S, Szelényi A, Herlitz L, et al. Spinal cord hemangioblastomas: significance of intraoperative neurophysiological monitoring for resection and long-term outcome. J Neurosurg Spine 2017;26:483-93.


20. Parker F, Ducati LG, et al. Results of microsurgical treatment of medulla oblongata and spinal cord hemangioblastomas: a comparison of two distinct clinical patient groups. J Neurooncol 2009;93:133-7.



21. Boström A, Hans FJ, Reinacher PC, et al. Intramedullary hemangioblastomas: timing of surgery, microsurgical technique and follow-up in 23 patients. Eur Spine J 2008;17:882-6.




22. Uchida K, Nakajima H, Sato R, et al. Multivariate analysis of the neurological outcome of 327 surgery for cervical compressive myelopathy. J Orthop Sci 2005;10:564-73.

23. Conway JE, Chou D, Clatterbuck RE, et al. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery 2001;48:55-62. discussion 62-3.



- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























